CircCEP85L对牛肌肉干细胞增殖、成肌分化的调控
2023-10-23韦瑶张瑞门安强王乐怡张永旺邹超霞张尔康莫碧云石德顺杨素芳邓彦飞韦英明
韦瑶,张瑞门,安强,王乐怡,张永旺,邹超霞,张尔康,莫碧云,石德顺,杨素芳,邓彦飞,韦英明
CircCEP85L对牛肌肉干细胞增殖、成肌分化的调控
韦瑶1,张瑞门1,安强1,王乐怡1,张永旺1,邹超霞1,张尔康1,莫碧云2,石德顺1,杨素芳1,邓彦飞,韦英明
1广西大学动物科学技术学院/广西畜禽繁育与疾病防控重点实验室,南宁 530004;2钦州市动物疫病预防控制中心,广西钦州 535099;3广西大学农牧产业发展研究院,南宁 530004
【目的】目前,越来越多的研究证明环状RNA(circRNA)在牛肌肉发育过程中扮演着重要的角色,但其分子调控机理尚未完善。通过挖掘与牛肌肉发育相关的circRNA的作用机制,为进一步阐明其调控牛肌肉发育的分子机制奠定基础。【方法】以前期分析的增殖期(GM)与成肌分化期(DM)黄牛肌肉干细胞(MuSCs)的RNA-seq测序结果为基础,筛选出显著差异表达的circRNA,circCEP85L。采集新鲜黄牛胎牛的心脏、肝脏、脾脏、肺脏、肾脏、肌肉、肠和胃的组织样本,分离培养黄牛肌肉干细胞并诱导成肌分化,收集黄牛体外培养的GM与DM细胞,分别提取RNA并反转录为cDNA。通过实时荧光定量PCR(quantitative real time PCR, qRT-PCR)检测circCEP85L在不同组织及不同细胞状态的表达规律。同时,设计特异性引物扩增circCEP85L全长,构建过表达载体p-circCEP85L,质粒转染MuSCs后收集过表达circCEP85L细胞样本。以过表达质粒pCD5-ciR细胞样本为对照,采用qRT-PCR、流式细胞术、Western Blot及免疫荧光等技术检测过表达circCEP85L对黄牛MuSCs增殖、凋亡及成肌分化的影响。【结果】PCR电泳结果证明circCEP85L环化位点真实存在。CircCEP85L在多种组织中表达,并在DM期的表达量显著高于GM期(<0.001);为了进一步探究对circCEP85L黄牛MuSCs的影响。将过表达载体p-circCEP85L与对照载体pCD5-ciR转染体外培养的黄牛MuSCs,继续培养24 h之后,EdU结果表明过表达circCEP85L能显著降低EdU阳性细胞比例(<0.001);流式周期检测结果表明,过表达circCEP85L增加了G0/G1期细胞比例,显著减少S期细胞比例(<0.001);流式凋亡结果表明,过表达circCEP85L显著抑制MuSCs的凋亡率(<0.05)。QRT-PCR及Western blot检测黄牛MuSCs增殖及凋亡相关基因的表达情况,结果表明过表达circCEP85L后显著降低了黄牛MuSCs增殖及凋亡相关基因的mRNA表达水平(<0.001),凋亡蛋白BAX的表达量也显著降低(<0.01)。此外,为了检测过表达circCEP85L对黄牛MuSCs成肌分化的影响,在转染24h后更换分化培养基诱导细胞分化,Western blot及免疫荧光结果显示过表达circCEP85L后显著促进分化标志基因的表达水平(<0.001),细胞融合形成肌管的数量和大小均显著高于对照组。【结论】研究表明,circCEP85L通过抑制黄牛MuSCs增殖和凋亡、促进细胞成肌分化,从而影响黄牛骨骼肌的生长发育过程,为circRNA调控黄牛骨骼肌的生长发育机制研究奠定基础。
circCEP85L;肌肉干细胞;细胞增殖;成肌分化
0 引言
【研究意义】骨骼肌的生长发育与牲畜的产肉量密切相关,它的发育受许多基因、转录因子及一些非编码RNA(ncRNA)的调控[1-4]。最新的研究表明circRNA在骨骼肌细胞增殖、成肌分化等过程中发挥重要作用。因此,研究动物骨骼肌生长发育相关circRNA的调控机制对于促进畜牧业发展具有重要意义。【前人研究进展】circRNA是不具有5'末端帽子和3'末端poly(A)尾巴的共价闭合环状结构的内源性非编码RNA分子[5]。circRNA最初是通过电子显微镜在植物类病毒中发现的[6],长时间内被认为是没有功能的RNA分子。随着生物学的快速发展,circRNA在脑、心脏、骨骼肌等许多组织中被发现[7],具有高度的保守性和稳定性。近年来的研究发现circRNA以多种方式参与调控动物骨骼肌的发育。首先,作为竞争性内源RNA(ceRNA)发挥作用,如circZfp609通过吸附miR-194-5p调节小鼠成肌细胞分化[8];CDR1as通过竞争性结合miR-7促进山羊骨骼肌的发育[9];circHIPK3、circFGFR2和circSVIL通过吸附miRNA促进鸡骨骼肌的发育[10-12];circTUT7可作为miR-30a海绵调节猪骨骼肌的发育[13]; circMYBPC1、circRILPL1和circTTN等[14-18]结合miRNA参与牛骨骼肌的发育。其次,circRNA能与特定的RNA结合蛋白(RBP)结合进而调控RBP及目的分子的作用,如circ-FOXO3可以和细胞周期蛋白依赖性激酶2()与形成circ-FOXO3-CDK2-P21三元复合物,从而抑制细胞周期[19]。此外,circRNA可编码蛋白质,如circ- ZNF609可翻译成微肽并在肌生成中发挥作用[20]。以上研究表明,circRNA在调控肌肉发育方面发挥至关重要的作用。【本研究切入点】目前circRNA在癌症、肿瘤和其他医学方面的研究较为广泛,但在黄牛肌肉发育方面的研究鲜见报道,其调控机制尚不完善。在之前的一项研究中,黄牛肌肉干细胞(MuSCs)增殖、成肌分化的circRNA表达谱已经被揭示[17],本研究从中发现了一个新的circRNA,circCEP85L可能对MuSCs有关键的调控作用。【拟解决的关键问题】进一步探究circCEP85L的表达特征及其对黄牛MuSCs的功能影响,为后续深入研究circCEP85L在黄牛肌肉生长发育中的分子机制提供参考。
1 材料与方法
1.1 试验时间和地点
试验于2022年4—9月在广西大学广西畜禽繁育与疾病防控重点实验室进行。
1.2 试验材料
胚胎期(3月龄)的黄牛胎儿来源于广西南宁市屠宰场、钦州市钦北区嘉德富肉牛养殖专业合作社。收集肌肉、肝脏、心脏、肺脏、肠、肾脏、脾脏和胃组织,并立即投入液氮中冷冻,运回到实验室再将各组织整理后置-80℃保存备用。大肠杆菌Trans-T1感受态细胞、环状RNA过表达载体pCD5-ciR、内切酶(RI和HI)及连接酶均由广西大学广西畜禽繁育与疾病防控重点实验室提供。
1.3 试验方法
1.3.1 黄牛MuSCs的分离、培养和成肌分化 黄牛MuSCs的分离、培养:胚胎期(3月龄)的黄牛胎儿从体内取出后,用一次性无菌袋包装后在恒温条件下于2—3 h内送达实验室。将黄牛胎儿用含双抗(1%青霉素和链霉素)的PBS缓冲液冲洗,并用75%酒精擦拭胎儿皮肤表面,眼科剪和镊子分离出背最长肌肌肉组织。将组织转移至超净工作台中,用含双抗的PBS缓冲液漂洗组织块3次,再放入75%酒精中漂洗20—30 s,随即将组织块用含双抗的PBS缓冲液漂洗3—5次。剔除筋膜后将组织放到小皿中剪碎至1 mm3,采用两步酶消化法(先0.2%I型胶原蛋白酶消化1 h, 再0.25%胰蛋白酶消化0.5 h)消化后[21],加入2倍体积的含10%胎牛血清(FBS; Thermo Fisher Scientific, HyClone, Logan, UT, USA)的高糖DMEM(Gibco, Logan, UT, USA)终止消化;使用70 µm细胞滤器进行过滤,除去多余的组织块和其他杂质,离心并弃上清;将细胞铺至100 mm的无菌培养皿中,于37 ℃、5%CO2培养箱中培养;2 h后取上清液至另一新的培养皿进行贴壁培养。原代细胞用含10%FBS和双抗的高糖DMEM培养基进行培养。
黄牛MuSCs传代培养及成肌分化诱导:当细胞汇合度达90%左右时,即可进行细胞的传代培养。经过传代培养后,处于增殖状态的MuSCs标记为GM样本(n=3),并收集备用;当MuSCs长至90%时将培养基更换为成肌分化培养基(含2%HS的高糖DMEM)用于细胞诱导分化,每天更换分化培养基,4 d后收集成肌分化的细胞并标记为DM样本(n=3)。
1.3.2 总RNA的提取及cDNA的合成 采用Trizol试剂(Vazyme, 南京)提取各样本的总RNA,并使用ND-100超微量紫外可见分光光度计(Miulab, 杭州)测定RNA浓度及纯度。将质量和浓度检测合格的RNA样品根据HiScript® Ⅲ RT SuperMix for qPCR试剂盒(Vazyme, 南京)逆转录为cDNA。
1.3.3 实时荧光定量PCR(qRT-PCR)引物设计及合成 根据GenBank基因序列,使用Oligo7.0软件设计特异性扩增引物,具体引物信息如表1所示,均由上海生工生物工程有限公司合成。使用ChamQ Universal SYBR qPCR Master Mix(Vazyme, Nanjing, China)进行qRT-PCR试验。作为内参,qRT-PCR采用2-ΔΔt方法计算。
1.3.4 qRT-PCR反应 反应体系为20 µL:2×ChamQ Universal SYBR qPCR Master Mix 10 µL,上下游引物(10 µmol·L-1)各0.4 µL,cDNA模板(100 ng·µL-1)1 µL,加ddH2O至20 µL。反应程序为95 ℃预变性30 s,95 ℃变性10 s,60 ℃退火30 s,40个循环。每个样本设置3个重复[22]。
1.3.5 circCEP85L的鉴定及过表达载体构建 bta_ circ_0003271是来源于其宿主基因CEP85L,因此命名为circCEP85L,转录本长度为657 nt的环状RNA。设计并合成circCEP85L的全长序列引物(circCEP85L F:5′CGGAATTCTAATACTTTCAGTGATGTGCAGAG TCAGAGT-3′; circCEP85L R:5′CGGGATCCAGTTGTTCTTACTCAAGAGTTGGAAGATCAG-3′)。扩增circCEP85L全长序列。使用RI和HI酶切PCR扩增产物及pCD5-ciR载体,酶切产物纯化后利用同源重组酶进行连接。取10 µl连接产物转化至Trans-T1感受态中,转化完成后在37℃摇床培养1 h,将菌液于含氨苄抗性的LB营养琼脂板上进行培养,随机挑取10个独立的阳性克隆进行菌液扩增,菌液PCR完成后,将能扩出circCEP85L的菌液送至上海生工生物工程股份有限公司测序。把正确连接的单克隆菌液进行扩大培养,用无内毒素质粒抽提试剂盒(天根, 北京)抽提质粒,并将载体命名为p-circCEP85L。

表1 qRT-PCR基因引物合成序列
1.3.6 黄牛MuSCs的转染试验 将细胞接种于孔板中,细胞汇合度达70%左右时,利用脂质体转染法(转染试剂ExFect Transfection Reagent(Vazyme, 南京))分别将过表达质粒p-circCEP85L和对照质粒pCD5- ciR与转染试剂ExFect Transfection Reagent(Vazyme, 南京)加入高糖DMEM基础培养基中(每组3个重复),室温孵育15 min,将孵育好的混合物分别加到MuSCs细胞孔板中并轻轻混匀,12 h后更换完全培养液继续培养。待细胞生长密度为90%以上时,使用分化培养基诱导黄牛MuSCs分化。
1.3.7 EdU试验检测细胞增殖 将细胞接种于96孔板,当细胞密度达到70%左右,分别用过表达质粒p-circCEP85L和对照质粒pCD5-ciR转染MuSCs细胞,12 h后换液继续培养。转染48 h后参照锐博EdU试剂盒(RiboBio, 广州)说明书,对细胞进行孵育、固定、通透等处理,染色完成后加PBS缓冲液避光保存待用。每个样品随机选择3个视野,用荧光显微镜观察细胞。增殖率=(新增殖的细胞数目/总细胞数目)×100%。
1.3.8 流式细胞仪检测细胞周期与凋亡 在12孔板中接种细胞,分别用过表达质粒p-circCEP85L和对照质粒pCD5-ciR转染黄牛MuSCs细胞,48h后收集细胞,Cell Cycle Staining Kit(Multi Sciences, 杭州)试剂盒用来检测不同试验组的细胞周期分布情况。AnneXin V-FITC/PI Staining Kit(Multi Sciences)试剂盒用来检测黄牛MuSCs细胞凋亡率。在试剂盒孵育细胞完成后使用Attune™ NxT声波聚焦流式细胞仪(Invitrogen, Singapore)检测细胞周期以及凋亡率。每个样品3个重复,试验结果使用Flow Jo v10(Tree Star, Inc, Ashland, OR)软件分析。
1.3.9 免疫印迹(Western blot, WB)检测 收集细胞,用含1% PMSF(GenStar, 北京)的RIPA(Beyotime, 上海)裂解液冰上裂解细胞30 min,用BCA试剂盒(Beyotime, 上海)测定蛋白浓度,并经4×SDS上样缓冲液(Solarbio® Life Sciences, 北京)变性。提取到的蛋白经SDS聚丙烯酰胺凝胶电泳(SDS-PAGE)分离蛋白质并转移到PVDF膜上。用5%脱脂奶粉处理膜,室温封闭2 h。稀释一抗PCNA、MyH6、CDK2、BAX、β-Actin(1﹕2 000 Dilution;ABclonal Technology,武汉),稀释后的一抗与PVDF膜共同在4 ℃孵育过夜。用1×TPST清洗膜后,再次将PVDF膜与山羊抗兔二抗(1﹕5 000 Dilution;ABclonal Technology)共同室温孵育2 h。用超敏ECL化学发光试剂盒处理PVDF膜,进行曝光和显影。作为对照样本,用ImageJ软件(National Institutes of Health, Baltimore, MD, USA)分析蛋白灰度值。相对蛋白表达量以目的条带灰度值与条带的比值表示。
1.3.10 免疫荧光试验 使用4%多聚甲醛固定处理的细胞30 min,去除4%多聚甲醛后用1%TritonX-100通透处理10 min,1%BSA用来封闭细胞,1h后加入兔抗MyH6(1﹕200 Dilution, ABclonal Technology)、PAX7(1﹕200 Dilution, ABclonal Technology),4℃孵育过夜。PBS清洗后加入山羊抗兔抗体(1﹕100 Dilution, ABclonal Technology),避光室温孵育1.5 h,最后用10 µg·mL-1DAPI染色10 min,加入少量PBS缓冲液覆盖细胞,在荧光显微镜下随机选择3 个视野进行拍照观察。
1.3.11 统计分析 qRT-PCR数据使用t检验评估组间差异,并使用Prism8.0版(GraphPad Software, USA)进行统计分析。≤0.05被认为是有统计学意义的。每个试验重复3次,*<0.05; **<0.01;***<0.001。
2 结果
2.1 黄牛MuSCs的分离、培养和鉴定
3月龄黄牛胎儿用于分离MuSCs(图1-A);贴壁培养24 h的P1代黄牛MuSCs,呈纺锤形,形态均一性较好(图1-B);对培养的MuSCs进行分化诱导,结果显示诱导4 d细胞融合产生大量肌管(图1-C)。细胞免疫荧光试验检测P1代黄牛MuSCs中PAX7的表达情况,结果显示MuSCs能表达其特异性蛋白PAX7(图1-D),由此说明,本研究所分离获得的细胞为高纯度黄牛MuSCs,可作为后续研究的试验材料。

A:3月龄黄牛胎儿;B:黄牛MuSCs的增殖期(GM)生长状态;C:黄牛MuSCs的分化期(DM)生长状态;D:细胞免疫荧光显示黄牛MuSCs表达其特异性蛋白PAX7。标尺=100/400 µm
2.2 circCEP85L的表达谱特征
circCEP85L在黄牛胎牛组织中的表达分析显示(图2-A),circCEP85L在各组织中均有表达,在脾脏中表达最高,其次是心脏、肌肉、肠、胃、肺脏和肾脏,在肝脏中表达最低。对培养的增殖期黄牛MuSCs进行分化诱导4 d后收样,qRT-PCR检测结果显示(图2-B),circCEP85L在DM期的表达量极显著高于GM期(<0.001)。

A:circCEP85L的组织表达谱;B:circCEP85L在黄牛MuSCs分化前后的表达情况。图表‘abcd’表示差异的显著性,首先按照平均值从大到小排列,最大的标为'a',任何不显著的差异标为'a',直到与之有显著差异的标为'b'(P<0.05),以此类推;*P<0.05,**P<0.01,***P<0.001, 无*表示差异不显著(P<0.05)。下同
2.3 circCEP85L的鉴定及过表达载体的构建
根据测序所得的circCEP85L基因序列,扩增circCEP85L全长序列为657 bp,将circCEP85L全长克隆到pCD5-ciR,获得其过表达质粒p-circCEP85L。circCEP85L基因序列PCR电泳结果和质粒p-circCEP85L电泳鉴定结果如图3所示。
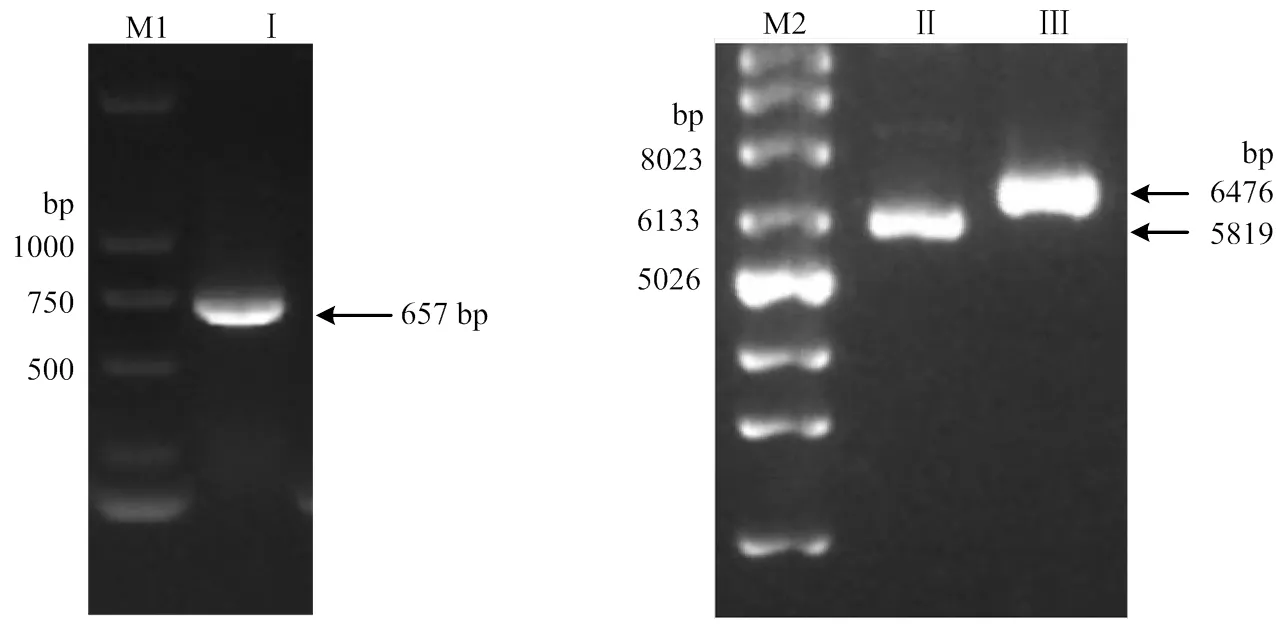
M1:Supercoiled DNA ladder marker;Ⅰ:cirCEP85L的PCR扩增产物;M2:DL2000 Plus DNA marker;Ⅱ:pCD5-ciR质粒;Ⅲ:p-circCEP85L质粒
2.4 过表达circCEP85L对黄牛MuSCs增殖的影响
将过表达质粒p-circCEP85L和空白对照质粒pCD5-ciR分别转染黄牛MuSCs,转染48 h后通过qRT-PCR验证circCEP85L的mRNA相对表达量。结果显示(图4-A),转染了p-circCEP85L的细胞中circCEP85L的表达水平显著升高(<0.01)。EdU检测结果显示(图4-B、C),过表达circCEP85L极显著降低EdU阳性细胞比例(<0.001);过表达circCEP85L在mRNA水平上和蛋白水平上显著抑制了增殖标记基因、的表达(图4-D—F)(<0.001)。此外,通过流式细胞术检测试验进一步评估过表达circCEP85L对黄牛MuSCs周期分布的影响,结果显示(图4-G、H),过表达circCEP85L增加G0/G1期细胞比例,显著减少S期细胞比例(<0.001)。表明,过表达circCEP85L能够抑制黄牛MuSCs的增殖。

A:circCEP85L的过表达效率;B、C:EdU法检测细胞增殖并统计阳性细胞数;D:细胞增殖相关基因mRNA的表达情况检测;E、F:增殖相关基因蛋白的表达情况检测;G、H:使用流式细胞术分析评估过表达circCEP85L对细胞周期分布的影响。标尺=400 µm
2.5 过表达circCEP85L对黄牛MuSCs凋亡的影响
检测过表达circCEP85L对黄牛MuSCs中凋亡相关标记基因表达的影响,结果显示(图5-A—C),过表达circCEP85L后使凋亡标记基因(、)的mRNA表达水平显著降低(<0.001),同时,凋亡标记基因()的蛋白表达水平也显著降低(<0.01)。此外,通过细胞凋亡试剂盒进一步评价过表达circCEP85L对MuSCs凋亡的影响,流式凋亡结果显示(图5-D、E),过表达circCEP85L在黄牛MuSCs中具有显著抑制细胞凋亡的特性(<0.05)。表明,过表达circCEP85L能够抑制黄牛MuSCs细胞的凋亡。

A:细胞凋亡相关基因mRNA的表达情况检测;B、C:凋亡标记基因BAX蛋白的表达情况检测;D、E:使用流式细胞术分析评估过表达circCEP85L对细胞凋亡的影响
2.6 过表达circCEP85L对黄牛MuSCs分化的影响
对转染pCD5-ciR与p-circCEP85L的黄牛MuSCs进行分化诱导,结果发现转染p-circCEP85L组的肌管数量及大小高于pCD5-ciR组(图6-A),且免疫荧光和Western Blot实验结果显示诱导分化后分化标志基因的蛋白表达水平显著升高(<0.001)(图6-B—D)。以上结果表明,过表达circCEP85L能够促进黄牛MuSCs的分化。

A:诱导分化4 d后黄牛MuSCs的形态;B:免疫荧光显示诱导分化后MyH6的表达情况;C、D:Western Blot检测诱导分化后分化标志基因MyH6蛋白表达情况检测,标尺=100/400 µm
3 讨论
3.1 CircRNA调控骨骼肌的生长发育
MuSCs作为骨骼肌的基本形成单位,它通过成肌分化、相互融合形成肌管以影响骨骼肌的发育过程,这个过程受到肌肉发育调节因子和调控通路多层次的精细调节。CircRNA作为一类新型的非编码RNA已被大量研究证实在生物过程中发挥重要的作用,同样也被证实作为调控因子参与骨骼肌的发育过程[23]。与线性RNA不同,circRNA由于其独特的环状结构而具有更高的稳定性、抗RNase R和更长的半衰期等特性,同时表现出动态和组织特异性表达的特征,在不同的组织中表现出不同的功能[24-25]。在肌肉发育过程中,虽然已经报道了许多功能性circRNA,但仍有许多未知的circRNA有待发现,其调控机制尚不明确。笔者根据前期的测序数据,筛选出了一个新的circRNA ——circCEP85L,在MuSCs的DM细胞中的表达量显著高于GM细胞,表明circCEP85L在DM细胞中可能发挥重要的作用。此外,circCEP85L在黄牛不同组织中均有表达,这提示circCEP85L在黄牛骨骼肌发育过程具有潜在的调控作用。
3.2 CircCEP85L调控MuSCs的增殖与凋亡
有研究表明circRNA在细胞增殖及凋亡过程中发挥重要的作用,如circSNX29经过表达后可以抑制牛成肌细胞的增殖[26];circEch1过表达促进牛成肌细胞的增殖[27];circLMO7增加了成肌细胞S期的细胞的数量,降低了G0/G1期细胞的比例[28];circHUWE1和circINSR可以促进牛成肌细胞增殖并抑制细胞凋亡[29-30];circ-13267通过let-7-19/ERBB4通路调控蛋鸭卵泡颗粒细胞凋亡[31]。为了探究circCEP85L在MuSCs中的作用,笔者使用EdU初步证实了过表达circCEP85L抑制黄牛MuSCs;通过qRT-PCR、Western Blot技术从mRNA、蛋白水平上检测增殖、凋亡相关基因的表达;流式细胞术分析细胞周期及凋亡情况进一步证实了过表达circCEP85L抑制黄牛MuSCs增殖和凋亡。
3.3 CircCEP85L对MuSCs成肌分化的影响
CircRNA除了调控肌肉细胞增殖和凋亡,它对细胞的分化调控也发挥重要作用。circ-CDR1as可能通过竞争性结合miR-7阻碍下调来促进山羊骨骼肌卫星细胞(SMSCs)分化[9]。小鼠成肌细胞细胞系(C2C12)中,circZfp609通过吸附miR-194-5p以解除对下游靶基因的抑制作用,并通过影响和的表达间接抑制成肌细胞的分化[8]。此外,circFGFR4是形成的环状RNA,它在牛肌肉中高表达并竞争性结合miR-107调控Wnt3a的表达,间接促进牛成肌细胞的分化[32]。本试验表明过表达circCEP85L后,黄牛MuSCs成肌分化融合形成明显的肌管,且肌管数量显著高于对照组;分化标记基因的蛋白表达水平也上调。这表明过表达circCEP85L对黄牛MuSCs的成肌分化具有显著促进作用。
3.4 CircCEP85L可能调控的下游机制
CircRNA有多种调控机制,包括:调控其宿主基因的表达;吸附miRNA;调节结合蛋白的活性;翻译短肽发挥功能;结合基因启动子影响其转录。CEP85L基因是circCEP85L的亲本基因,circCEP85L可以通过影响CEP85L基因的转录从而对肌肉干细胞进行调控。同时,circCEP85L还可以通过吸附miRNA对下游基因进行调控。另外,circCEP85L也可通过RNA结合蛋白进而影响下游基因的活性。尽管circCEP85L对黄牛肌肉干细胞的作用已明确,但是其作用机制还有待进一步研究。
4 结论
成功分离了黄牛肌肉干细胞,根据之前已有的黄牛circRNA测序结果,筛选得到可能存在关键作用的circRNA——circCEP85L,对其在黄牛胎牛以及黄牛肌肉干细胞中的表达特征进行了分析,并且证明了circCEP85L是调控黄牛MuSCs增殖和成肌分化的关键分子,为研究circRNA的调控机制奠定了基础。同时,为肉牛的分子育种新增候选分子靶标。
[1] BASSEL-DUBY R, OLSON E N. Signaling pathways in skeletal muscle remodeling. Annual Review of Biochemistry, 2006, 75: 19-37.
[2] BUCKINGHAM M, BAJARD L, CHANG T, DAUBAS P, HADCHOUEL J, MEILHAC S, MONTARRAS D, ROCANCOURT D, RELAIX F. The formation of skeletal muscle: from somite to limb. Journal of Anatomy, 2003, 202(1): 59-68.
[3] NEGUEMBOR M V, JOTHI M, GABELLINI D. Long noncoding RNAs, emerging players in muscle differentiation and disease. Skeletal Muscle, 2014, 4(1): 8.
[4] VAN ROOIJ E, LIU N, OLSON E N. microRNAs flex their muscles. Trends in Genetics, 2008, 24(4): 159-166.
[5] MENG S J, ZHOU H C, FENG Z Y, XU Z H, TANG Y, LI P Y, WU M H. CircRNA: functions and properties of a novel potential biomarker for cancer. Molecular Cancer, 2017, 16(1): 94.
[6] SANGER H L, KLOTZ G, RIESNER D, GROSS H J, KLEINSCHMIDT A K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proceedings of the National Academy of Sciences of the United States of America, 1976, 73(11): 3852-3856.
[7] JECK W R, SORRENTINO J A, WANG K, SLEVIN M K, BURD C E, LIU J Z, MARZLUFF W F, SHARPLESS N E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA, 2013, 19(2): 141-157.
[8] WANG Y H, LI M L, WANG Y H, LIU J, ZHANG M L, FANG X T, CHEN H, ZHANG C L. A Zfp609 circular RNA regulates myoblast differentiation by sponging miR-194-5p. International Journal of Biological Macromolecules, 2019, 121: 1308-1313.
[9] 聂露. 环状RNA CDR1as调控山羊骨骼肌卫星细胞分化的机制研究[D]. 雅安: 四川农业大学, 2018.
NIE L. Mechanism of circ-CDR1as regulating goat skeletal muscle satellite cells differentiation[D]. Yaan: Sichuan Agricultural University, 2018. (in Chinese)
[10] CHEN B A, YU J A, GUO L J, BYERS M, WANG Z J, CHEN X L, XU H P, NIE Q H. Circular RNA circHIPK3 promotes the proliferation and differentiation of chicken myoblast cells by sponging miR-30a-3p. Cells, 2019, 8(2): 177.
[11] CHEN X L, OUYANG H J, WANG Z J, CHEN B A, NIE Q H. A novel circular RNA generated bygene promotes myoblast proliferation and differentiation by sponging miR-133a-5p and miR-29b-1-5p. Cells, 2018, 7(11): 199.
[12] OUYANG H J, CHEN X L, LI W M, LI Z H, NIE Q H, ZHANG X Q. Circular RNA circSVIL promotes myoblast proliferation and differentiation by sponging miR-203 in chicken. Frontiers in Genetics, 2018, 9: 172.
[13] HONG L J, GU T, HE Y J, ZHOU C, HU Q, WANG X W, ZHENG E Q, HUANG S X, XU Z, YANG J E, YANG H Q, LI Z C, LIU D W, CAI G Y, WU Z F. Genome-wide analysis of circular RNAs mediated ceRNA regulation in porcine embryonic muscle development. Frontiers in Cell and Developmental Biology, 2019, 7: 289.
[14] CHEN M J, WEI X F, SONG M M, JIANG R, HUANG K W, DENG Y F, LIU Q Y, SHI D S, LI H. Circular RNA circMYBPC1 promotes skeletal muscle differentiation by targeting MyHC. Molecular Therapy - Nucleic Acids, 2021, 24: 352-368.
[15] SHEN X M, TANG J A, JIANG R, WANG X G, YANG Z X, HUANG Y Z, LAN X Y, LEI C Z, CHEN H. CircRILPL1 promotes muscle proliferation and differentiation via binding miR-145 to activate IGF1R/PI3K/AKT pathway. Cell Death & Disease, 2021, 12(2): 142.
[16] WANG X G, CAO X K, DONG D, SHEN X M, CHENG J, JIANG R, YANG Z X, PENG S J, HUANG Y Z, LAN X Y, ELNOUR I E, LEI C Z, CHEN H. Circular RNA TTN acts As a miR-432 sponge to facilitate proliferation and differentiation of myoblasts via the IGF2/PI3K/AKT signaling pathway. Molecular Therapy-Nucleic Acids, 2019, 18: 966-980.
[17] ZHANG R M, PAN Y, ZOU C, AN Q, CHENG J R, LI P J, ZHENG Z H, PAN Y, FENG W Y, YANG S F, SHI D, WEI Y M, DENG Y F. CircUBE2Q2 promotes differentiation of cattle muscle stem cells and is a potential regulatory molecule of skeletal muscle development. BMCGenomics, 2022, 23:267.
[18] YANG Z X, SONG C C, JIANG R, HUANG Y Z, LAN X Y, LEI C Z, QI X L, ZHANG C L, HUANG B Z, CHEN H.regulates bovine myoblasts proliferation and differentiation via the miR-411a/axis. Journal of Agricultural and Food Chemistry, 2022, 70(32): 10044-10057.
[19] DU W W, YANG W N, LIU E, YANG Z G, DHALIWAL P, YANG B B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Research, 2016, 44(6): 2846-2858.
[20] LEGNINI I, DI TIMOTEO G, ROSSI F, MORLANDO M, BRIGANTI F, STHANDIER O, FATICA A, SANTINI T, ANDRONACHE A, WADE M, LANEVE P, RAJEWSKY N, BOZZONI I. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Molecular Cell, 2017, 66(1): 22-37.e9.
[21] 李静怡, 徐小艾, 晁雨竹, 李向东, 沈红, 张永红. 地鳖肽对H2O2刺激鸡骨骼肌卫星细胞增殖的影响. 北京农学院学报, 2019, 34(1): 70-75.
LI J Y, XU X A, CHAO Y Z, LI X D, SHEN H, ZHANG Y H. The effects of ESW Walker polypeptides on the proliferation of chicken muscle satellite cells stimulated with H2O2. Journal of Beijing University of Agriculture, 2019, 34(1): 70-75. (in Chinese)
[22] 杨闯, 吴龙飞, 柳广斌, 李耀坤, 刘德武, 孙宝丽. 雷琼牛与陆丰牛背最长肌lncRNA表达特点及其相关ceRNA网络分析[J/OL]. 畜牧兽医学报: 1-14[2023-03-21].
YANG C, WU L F, LIU G B, LI Y K, LIU D W, SUN B L. ression profile and bioinformatics analysis of lncRNA and its associated CeRNA networks in longissimus dorsi from Lufeng Cattle and Leiqiong Cattle. Acta Veterinaria et Zootechnica Sinica, 1-14[2023- 03-21]. (in Chinese)
[23] 石田培, 王欣悦, 侯浩宾, 赵志达, 尚明玉, 张莉. 基于全转录组测序的绵羊胚胎不同发育阶段骨骼肌circRNA的分析与鉴定. 中国农业科学, 2020, 53(3): 642-657.
SHI T P, WANG X Y, HOU H B, ZHAO Z D, SHANG M Y, ZHANG L. Analysis and identification of circRNAs of skeletal muscle at different stages of sheep embryos based on whole transcriptome sequencing. Scientia Agricultura Sinica, 2020, 53(3): 642-657. (in Chinese)
[24] SALZMAN J, CHEN R E, OLSEN M N, WANG P L, BROWN P O. Cell-type specific features of circular RNA expression. PLoS Genetics, 2013, 9(9): e1003777.
[25] WESTHOLM J O, MIURA P, OLSON S, SHENKER S, JOSEPH B, SANFILIPPO P, CELNIKER S E, GRAVELEY B R, LAI E C. Genome-wide analysis ofcircular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Reports, 2014, 9(5): 1966-1980.
[26] PENG S J, SONG C C, LI H, CAO X K, MA Y L, WANG X G, HUANG Y Z, LAN X Y, LEI C Z, CHAOGETU B, CHEN H. Circular RNA SNX29 sponges miR-744 to regulate proliferation and differentiation of myoblasts by activating the Wnt5a/Ca2+signaling pathway. Molecular Therapy - Nucleic Acids, 2019, 16: 481-493.
[27] HUANG K W, CHEN M J, ZHONG D D, LUO X E, FENG T, SONG M M, CHEN Y L, WEI X F, SHI D S, LIU Q Y, LI H. Circular RNA profiling reveals an abundant circEch1 that promotes myogenesis and differentiation of bovine skeletal muscle. Journal of Agricultural and Food Chemistry, 2021, 69(1): 592-601.
[28] WEI X F, LI H, YANG J M, HAO D, DONG D, HUANG Y Z, LAN X Y, PLATH M, LEI C Z, LIN F P, BAI Y Y, CHEN H. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death & Disease, 2017, 8(10): e3153.
[29] SHEN X M, TANG J A, RU W X, ZHANG X Y, HUANG Y Z, LEI C Z, CAO H, LAN X Y, CHEN H. CircINSR regulates fetal bovine muscle and fat development. Frontiers in Cell and Developmental Biology, 2021, 8: 615638.
[30] YUE B L, WANG J, RU W X, WU J Y, CAO X K, YANG H Y, HUANG Y Z, LAN X Y, LEI C Z, HUANG B Z, CHEN H. The circular RNA circsponges the miR-29b-axis to regulate myoblast development. Molecular Therapy - Nucleic Acids, 2020, 19: 1086-1097.
[31] 吴艳, 张昊, 梁振华, 潘爱銮, 申杰, 蒲跃进, 黄涛, 皮劲松, 杜金平. circ-13267通过let-7-19/ERBB4通路调控蛋鸭卵泡颗粒细胞凋亡. 中国农业科学, 2022, 55(8): 1657-1666.
WU Y, ZHANG H, LIANG Z H, PAN A L, SHEN J, PU Y J, HUANG T, PI J S, DU J P. Circ-13267 regulates egg duck granulosa cells apoptosis through let-7-19/ERBB4 pathway. Scientia Agricultura Sinica, 2022, 55(8): 1657-1666. (in Chinese)
[32] LI H, WEI X F, YANG J M, DONG D, HAO D, HUANG Y Z, LAN X Y, PLATH M, LEI C Z, MA Y, LIN F P, BAI Y Y, CHEN H. circFGFR4 promotes differentiation of myoblasts via binding miR-107 to relieve its inhibition of Wnt3a. Molecular Therapy - Nucleic Acids, 2018, 11: 272-283.
CircCEP85L Regulates the Proliferation and Myogenic Differentiation of Bovine MuSCs

1State Key Laborator for Conservation and Untilization of Subtropical Agro-bioresources /College of Animal Science and Technology, Guangxi University, Nanning 530004;2Qinzhou Center for Animal Disease Control and Prevention, Qinzhou 535099, Guangxi;3Research Institute of Agriculture and Animal Husbandry Industry Development, Guangxi University, Nanning 530004
【Objective】At present, studies have proved that circRNA plays important roles in the development of bovine muscle, but its molecular regulation mechanism remain elusive.Screening circRNAs related to bovine muscle development can lay a foundation for further elucidating the molecular mechanism of bovine muscle development.【Method】In this study, RNA-seq sequencing results of proliferating (GM) and myogenic differentiation (DM) yellow bovine muscle stem cells (MuSCs) analyzed in the previous stage were used to screen for significantly differentially expressed circRNA, circCEP85L. Tissue samples of heart, liver, spleen, lung, kidney, muscle, intestine and stomach were collected aseptically from fresh yellow fetal calves, and yellow muscle stem cells were isolated and cultured and induced into myogenic differentiation. GM and DM cells cultured in vitro from yellow calves were collected, RNA was extracted and reverse transcribed into cDNA, respectively. Quantitative real time PCR (qRT-PCR) was used to detect the expression of circCEP85L in different tissues and different cell states. Meanwhile, specific primers were designed to amplify the full length of circCEP85L, and the overexpression vector p-circCEP85L was constructed. The plasmid was transfected into MuSCs and overexpressed circCEP85L cell samples were collected. Using overexpression plasmid pCD5-ciR cell samples as control, qRT-PCR, flow cytometry, Western Blot and immunofluorescence were used to detect the effects of overexpression of circCEP85L on proliferation, apoptosis and myogenic differentiation of yellow bovine MuSCs.【Result】The electrophoresis of PCR product proved the existence of circCEP85L. CircCEP85L was expressed in various tissues, and the expression level in DM stage was significantly higher than that in GM stage (<0.001). To further investigate the effect on circCEP85L scallion MuSCs. The overexpression vector p-circCEP85L was transfected with the control vector pCD5-ciR in vitro cultured yellow bovine MuSCs and the EdU results showed that overexpression of circCEP85L significantly reduced the proportion of EdU positive cells (<0.001) after continuing the culture for 24 h. Flow cycle analysis showed that overexpression of circCEP85L increased the proportion of cells in G0/G1 phase and significantly decreased the proportion of cells in S phase (<0.001). Flow cytometry showed that overexpression of circCEP85L significantly inhibited the apoptosis rate of MuSCs (<0.05). qRT-PCR and western blot were used to detect the expression of proliferation and apoptosis-related genes in MuSCs, respectively. The results showed that overexpression of circCEP85L significantly reduced the mRNA expression levels of proliferation and apoptosis-related genes in bovine MuSCs (<0.001), and the expression of apoptotic protein BAX was also significantly reduced (<0.01). In addition, in order to detect the effect of circCEP85L overexpression on myogenic differentiation of cattle MuSCs, the differentiation medium was replaced 24 hours after transfection to induce cell differentiation. Western blot and immunofluorescence results showed that overexpression of circCEP85L significantly promoted the expression level of differentiation marker gene MyH6 (<0.001), and the number and size of myotubes formed by cell fusion were significantly higher than those of the control group.【Conclusion】The results of this study indicate that circCEP85L affects the growth and development process of skeletal muscle in cattle by inhibiting the proliferation and apoptosis of MuSCs and promoting myogenic differentiation of cells, which is expected to be a key circRNA for subsequent mechanistic studies to regulate the growth and development process of skeletal muscle in cattle.
circCEP85L; muscle stem cells; cell proliferation; myogenic differentiation
2022-11-01;
2023-05-16
国家现代农业产业技术体系广西创新团队建设项目(nycytxgxcxtd-09-01);国家重点研发计划(2021YFD110010)、广西自然科学基金重点项目(2021JJD130097)、巴马县人才科技计划项目(202101263)
韦瑶,E-mail:1612985655@qq.com。通信作者韦英明,E-mail:dkywym@163.com。通信作者邓彦飞,E-mail:yanfei-dun@163.com
10.3864/j.issn.0578-1752.2023.18.014
(责任编辑 林鉴非)
