Prognosis after splenectomy plus pericardial devascularization vs transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding
2023-10-21WeiLiQiJunWenTianFuWenWeiPengXiaoYunZhangJunYiShenXiaoLiChuanLi
Wei-Li Qi, Jun Wen, Tian-Fu Wen, Wei Peng, Xiao-Yun Zhang, Jun-Yi Shen, Xiao Li, Chuan Li
Abstract
Key Words: Portal hypertension; Liver cirrhosis; Esophagogastric variceal bleeding; Splenectomy; Pericardial devascularization; Transjugular intrahepatic portosystemic shunt
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is an important cause of liver cirrhosis in China. Patients with cirrhosis that progresses to the portal hypertension stage will face a series of complications, including esophagogastric variceal bleeding (EGVB), ascites, splenomegaly, hypersplenism, primary bacterial peritonitis, hepatic encephalopathy, and hepatorenal syndrome[1]. EGVB is one of the most serious emergency complications of cirrhosis. The mortality rate for the first bleeding is as high as approximately 20%[2,3]. Moreover, the rate of variceal rebleeding (VRB) within two years is nearly 60%, and the mortality rate is 30%[4].
The management strategy for EGVB is oriented toward prevention of the first EGVB (primary prophylaxis), control of acute EGVB, and prevention of VRB (secondary prophylaxis). Endoscopic treatment, including endoscopic variceal ligation and endoscopic injection sclerotherapy, and nonselective beta-blockers are the mainstay of primary and secondary prophylaxis for EGVB[5]. Similarly, endoscopic therapy is also recommended by the major clinical practice guidelines as a first-line treatment option for patients with acute EGVB[6-8]. In China, transjugular intrahepatic portosystemic shunt (TIPS) and splenectomy plus pericardial devascularization (SPD) are recommended as salvage therapies for patients with acute EGVB who failed endoscopic treatment or as secondary prevention of EGVB[9]. TIPS results in rapid control of acute EGVB and a significant reduction in VRB rates. Especially for patients at high risk of EGVB, early TIPS has been shown to significantly reduce VRB rates and improve prognosis in these patients[10,11]. SPD not only has a high hemostasis rate and low VRB rate, but can also improve liver function and has a relatively low incidence of hepatic encephalopathy[12,13]. However, the effectiveness and safety of SPDvsTIPS in the management of acute EGVB and as secondary prophylaxis for VRB are unknown.
The purpose of this study was to compare the prognosis after SPDvsTIPS for acute EGVB after failure of endoscopic therapy or secondary prophylaxis of VRB in patients with HBV-related cirrhosis combined with portal hypertension. We compared the differences in VRB, abnormal liver function, and incidence of hepatocellular carcinoma (HCC) between patients treated with SPD and TIPS.
MATERIALS AND METHODS
Study population
This was a retrospective cohort study. We retrospectively collected clinical data from 823 consecutive patients with portal hypertension combined with EGVB who received SPD or TIPS as a treatment for bleeding uncontrolled by endoscopic therapy or as secondary prophylaxis for VRB at West China Hospital of Sichuan University from January 1, 2009 to December 31, 2013. According to the inclusion and exclusion criteria, 318 patients were finally included in the analysis.Patients were divided into either an SPD group (n= 162) or a TIPS group (n= 156) based on treatment modality(Figure 1). All participants were diagnosed with portal hypertension with esophagogastric varices by endoscopy.
The inclusion criteria included the following: (1) Age 18-70 years; (2) clinical diagnosis of HBV-related cirrhosis combined with EGVB; (3) presence of acute EGVB uncontrolled by endoscopic therapy or VRB after secondary prophylaxis; (4) treatment with SPD or TIPS; (5) good liver function (Child-Pugh class A or B); and (6) good other organ function. The exclusion criteria included the following: (1) Cirrhosis due to other etiologies, such as alcoholic cirrhosis,schistosomal cirrhosis, and primary biliary cirrhosis; (2) gastrointestinal bleeding due to other causes, such as peptic ulcer bleeding; (3) coexistence of serious infectious or hematological diseases; (4) coexistence of serious organ impairment, such as cardiopulmonary and renal diseases, thus indicating patients who cannot tolerate surgery; (5) coexistence of malignancy; (6) poor liver function (Child-Pugh class C); (7) no history of EGVB; (8) coexistence of portal vein thrombosis or portal vein cavernous lesions; and (9) history of previous relevant surgical procedures, such as liver transplantation or TIPS.
Baseline patient data were obtained from electronic medical records and included demographic data, degree of esophagogastric varices, length of bleeding history, liver function tests, renal function tests, blood cell counts, coagulation tests, Child-Pugh classification, HBV markers, and HBV-DNA levels.
The study complied with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (No. 2023-354). The ethics committee waived the requirement for informed consent due to the retrospective nature of this research.
Surgical procedures
All procedures were performed by specialists with more than 10 years of experience. The SPD procedure was performed routinely by splenectomy, complete dissection of at least 6 cm of the lower esophagus and all vessels of the upper plasma layer of the stomach, and preservation of the gastric coronary vein and the main trunk of the paraesophageal vein. The surgeon performed the procedure with a common monopolar electric knife, ultrasonic knife, or Ligasure, depending on his personal preference. The splenic hilum was closed with suture ligation, hemo-lock or titanium clips, or off-segment closure with a vascular closure device, depending on the situation. According to the American Association for the Study of Liver Diseases guidelines[14], the TIPS procedure was performed by ultrasound-guided puncture of the right internal jugular vein, insertion of a catheter into a branch of the hepatic vein and venography, and placement of a stent from the hepatic vein through the portal vein to create an artificial shunt. The application of polytetrafluoroethylene (PTFE)-covered stents (Viatorr stents) has greatly reduced the rate of stenosis and occlusion of the shunt and the incidence of hepatic encephalopathy[15,16]. The Director of the Interventional Center supervised and controlled the quality of the TIPS procedure.
Outcomes and follow-up evaluation
The primary outcome measure in this study was overall survival (OS), and secondary outcomes were VRB, abnormal liver function, and the occurrence of HCC. All included patients were followed up to the last follow-up date (December 31, 2016) or until they died. The OS, rate of VRB, rate of abnormal liver function, and rate of HCC were calculated for all patients. Abnormal liver function was defined as total bilirubin (TB) > 28.2 µmol/L, albumin (ALB) < 35 g/L, or alanine aminotransferase or aspartate aminotransferase more than twice the reference value for a duration of more than 3 mo.
All patients were followed at months 1, 3, 6, and 12 after surgery and every 6 mo thereafter. The follow-up protocol included physical examination, multiphase enhanced computed tomography (CT), blood cell and differential counts, liver function tests, alpha-fetoprotein (AFP) levels, HBV markers, and HBV-DNA levels. During the follow-up period, patients presenting with VRB underwent endoscopy.
Statistical analysis
Continuous variables were tested by thettest, categorical variables by the chi-square test or Fisher's exact probability method, and ordered categorical variables by the rank sum test. Survival analysis was performed using the Kaplan-Meier method with the occurrence of death, VRB, abnormal liver function, and HCC as endpoint events. The log-rank test was used to compare the differences between the two groups for each outcome event. Univariate and multivariate COX regression analyses were used to identify risk factors associated with outcome indicators. Univariate Cox regression analysis was used to assess the significance of the variables to investigate the risk factors associated with outcome indicators. All variables with significant associations with death (P< 0.1) were further included in the multivariate COX regression analysis. Nearest neighbor 1:1 propensity score matching (PSM) with a caliper size of 0.02 was used to reduce the effect of selection bias and potential confounding between the SPD group and the TIPS group.

Figure 1 Flowchart of the process for patient selection. SPD: Splenectomy plus pericardial devascularization; TIPS: Transjugular intrahepatic portosystemic shunt; HBV: Hepatitis B virus.
R, version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria), was used to conduct all statistical analyses.The threshold for statistical significance was set at 0.05 for all two-sided statistical tests.
RESULTS
Patient characteristics
Baseline data for all patients are shown in Table 1. The median age was 45.0 years, and 226 (71.1%) of patients were male.The median follow-up duration of this study was 43 mo. There were statistically significant differences in the variables of age (P< 0.001), ALB (P< 0.001), hemoglobin (HGB) (P< 0.001), red blood cells (P< 0.001), and international normalized ratio (P= 0.02) between the two groups of patients.
To minimize the effect of potential confounders, we generated 90 pairs of patients by PSM. After PSM, there were no significant differences in baseline characteristics between the two groups of patients.
Overall patient survival
During the study period, 18 (11.1%) patients died in the SPD group, and 33 (21.2%) patients died in the TIPS group.Patient survival rates at 1, 3, and 5 years were 98.1%, 90.5%, and 86.5% in the SPD group and 94.8%, 81.0%, and 74.7% in the TIPS group, respectively. The mean survival time was 84.7 mo for patients in the SPD group and 73.6 mo in the TIPS group. In comparison to the TIPS group, the OS was significantly longer in the SPD group (P= 0.004; Figure 2A). After PSM, the SPD group still showed significantly better OS than the TIPS group (P= 0.01; Figure 2B).
Multivariate Cox regression analysis of 318 patients showed that the SPD group had a significantly lower risk of death than the TIPS group [hazard ratio (HR), 0.47; 95% confidence interval (CI): 0.25-0.90;P= 0.02; Table 2], which was independent of other predictors. Other significant factors associated with death were age (HR, 1.03; 95%CI: 1.01-1.06;P=0.02), albumin/globulin ratio (A/G; HR, 0.11; 95%CI: 0.03-0.48;P= 0.003), and prothrombin time (PT; HR, 1.09; 95%CI:1.02-1.16;P= 0.01; Table 2).
Cumulative incidence of variceal rebleeding, abnormal liver function, and hepatocellular carcinoma
For the duration of the study, VRB occurred in 40 (24.7%) patients in the SPD group and 59 (37.8%) patients in the TIPS group. The 1-, 3-, and 5-year cumulative VRB rates were 8.6%, 19.1%, and 24.1% in the SPD group and 20.5%, 34.6%, and 37.8% in the TIPS group, respectively (P= 0.001, Table 3). After PSM, the 1-, 3-, and 5-year cumulative VRB rates were 7.8%, 15.6%, and 23.3% in the SPD group and 11.1%, 27.8%, and 31.1% in the TIPS group, respectively (P= 0.09, Table 3).Multivariate COX regression analysis of 318 patients showed that the independent influential factors associated with VRB were treatment strategy (SPDvsTIPS; HR, 0.58; 95%CI: 0.37-0.89;P= 0.01), gamma-glutamyl transpeptidase (GGT; HR,1.005; 95%CI: 1.001-1.008;P= 0.01), and HGB (HR, 0.99; 95%CI: 0.98-1.00;P= 0.01; Table 4).

Table 1 Baseline features of the study population
A total of 40 (24.7%) patients in the surgical group and 92 (60.9%) patients in the TIPS group experienced persistent abnormal liver function. The rates of abnormal liver function at 1, 3, and 5 years were 13.6%, 19.8%, and 22.8% in the SPD group and 48.7%, 57.7%, and 60.3% in the TIPS group, respectively (P< 0.001; Table 3). After PSM, the rates of abnormal liver function at 1, 3, and 5 years were 13.3%, 18.9%, and 20.0% in the SPD group and 46.7%, 53.3%, and 56.7% in the TIPS group, respectively (P< 0.001, Table 3). Multivariate COX regression analysis of 318 patients showed that the independent influential factors associated with abnormal liver function were treatment strategy (SPDvsTIPS; HR, 0.26;95%CI: 0.17-0.39; P<0.001), TB (HR, 1.03; 95%CI: 1.02-1.05;P< 0.001), alkaline phosphatase (ALP) (HR, 1.004; 95%CI:1.000-1.007;P= 0.03) and PT (HR, 1.06; 95%CI: 1.00-1.13;P= 0.045; Table 4).
There were 11 (6.8%) patients in the SPD group and 18 (11.5%) patients in the TISP group who developed HCC. The proportions of progression to HCC at 1, 3, and 5 years were 2.5%, 3.7%, and 4.9% in the SPD group and 3.8%, 9.0%, and 11.5% in the TIPS group, respectively (P= 0.03, Table 3). Following adjustment by PSM, the proportions of progression to HCC at 1, 3, and 5 years were 2.2%, 2.2%, and 2.2% in the SPD group and 4.4%, 10.0%, and 12.2% in the TIPS group,respectively (P= 0.02, Table 3). In a multifactorial COX regression analysis of 318 patients, the independent influential factors associated with HCC were treatment strategy (SPDvsTIPS; HR, 0.43; 95%CI: 0.20-0.93;P= 0.03), TB (HR, 1.03;95%CI: 1.00-1.07;P= 0.045), and ALP (HR, 1.006; 95%CI: 1.000-1.012;P= 0.043; Table 4).
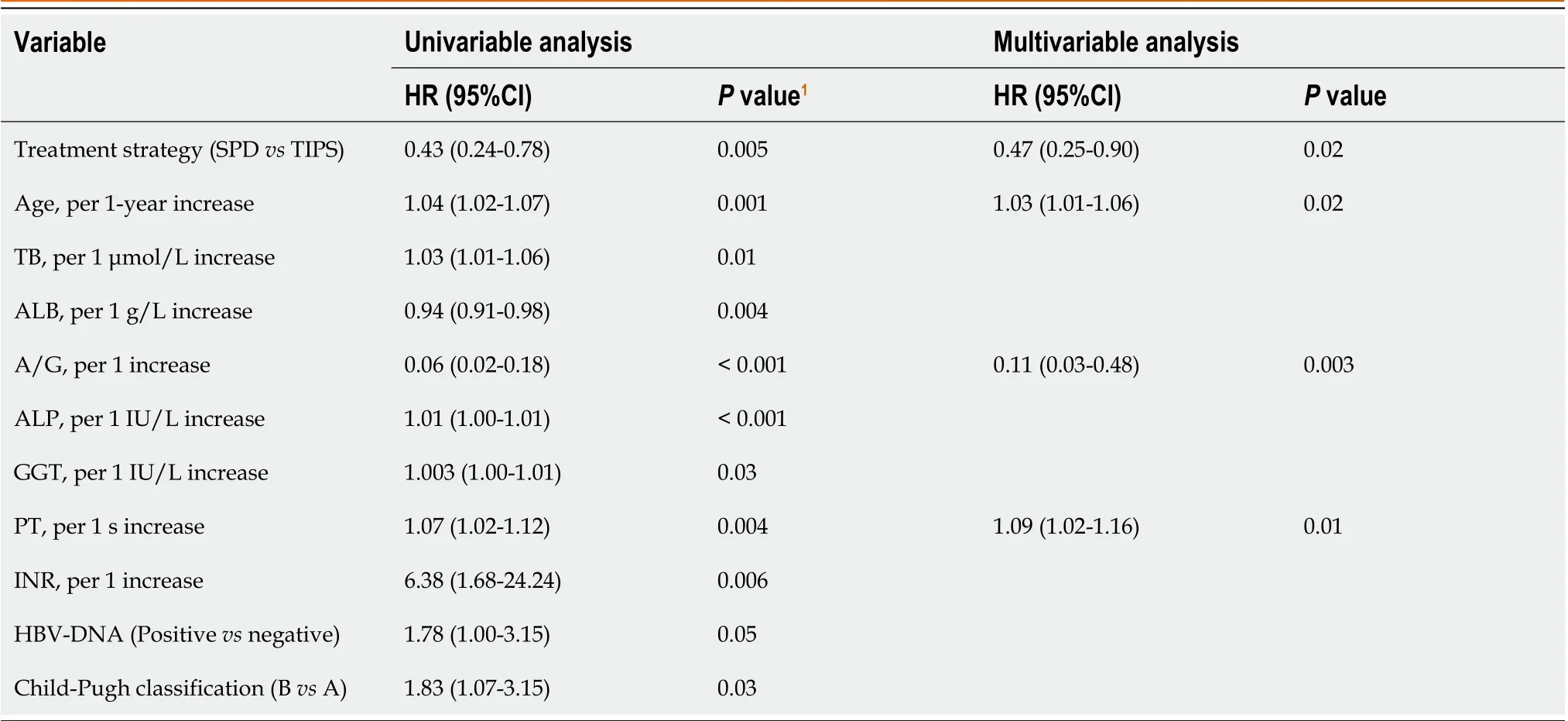
Table 2 Univariate and multivariate Cox regression analyses of factors associated with death
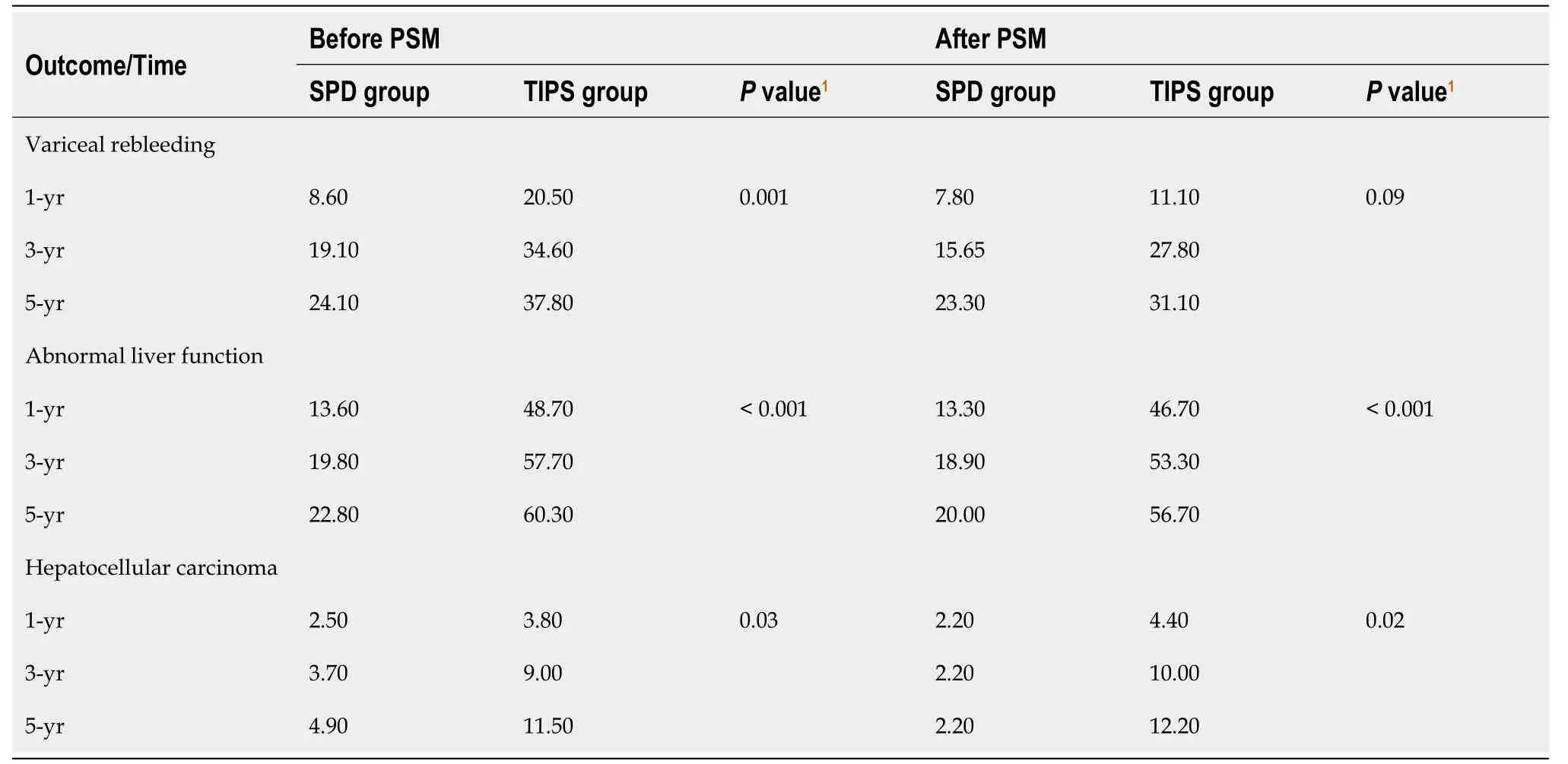
Table 3 Proportions of 1-, 3-, and 5-year cumulative variceal rebleeding, abnormal liver function, and hepatocellular carcinoma in the two groups before and after propensity score matching, %
Comparison of postoperative hospital days and number of reoperations and adverse events between the two groups
The mean postoperative hospital stay was 9.5 d in the SPD group, which was significantly longer than the 6.6 d in the TIPS group (P< 0.001; Table 5). In the SPD group, 162 patients had a total of five reoperations, and in the TIPS group, 156 patients had a total of 92 reoperations. The reoperation rate of patients in the SPD group was significantly lower than that in the TIPS group (P< 0.001; Table 5). In the SPD group, one patient died during hospitalization due to abdominal hemorrhage, and in the TIPS group, one patient died during hospitalization due to liver failure. Each group one patient who died during hospitalization, with no significant difference (P= 0.98, Table 5). The 90-d mortality was one patient in the SPD group and four patients in the TIPS group, with no statistically significant difference (P= 0.21; Table 5). Hepatic encephalopathy occurred in one patient in the SPD group and 25 patients in the TIPS group, which was significantlylower in the SPD group than in the TIPS group (P< 0.001; Table 5). Within 30 d, one patient was readmitted in the SPD group and 22 patients in the TIPS group, and the 30-d readmission was significantly lower in the SPD group than in the TIPS group (P< 0.001; Table 5).

Table 4 Multivariate Cox regression analysis of factors associated with variceal rebleeding, abnormal liver function, and hepatocellular carcinoma
DISCUSSION
Cirrhotic portal hypertension is very common in China due to the high prevalence of HBV infection. SPD and TIPS are commonly used to treat portal hypertension combined with EGVB. This study compared the two treatment modalities in terms of long-term survival, postoperative VRB rate, postoperative liver function status, HCC incidence, quality of life,and safety. It can provide a reference for clinicians in the selection of the protocol for the treatment of EGVB.
In this study, we found that the SPD group had higher long-term survival rate, sustained normal liver function rate,and no-HCC rate than the TIPS group. Moreover, compared to the TIPS group, the SPD group also showed a noninferiority trend before and after PSM in terms of VRB. In addition, the incidence of hepatic encephalopathy, 30-d readmission rate, and reoperation rate were significantly lower in the SPD group than in the TIPS group, and there was no significant difference between the two groups in terms of in-hospital mortality and 90-d mortality. These results suggest that SPD treatment is no less safe and effective than TIPS treatment and that SPD treatment is even better than TIPS treatment in some aspects.
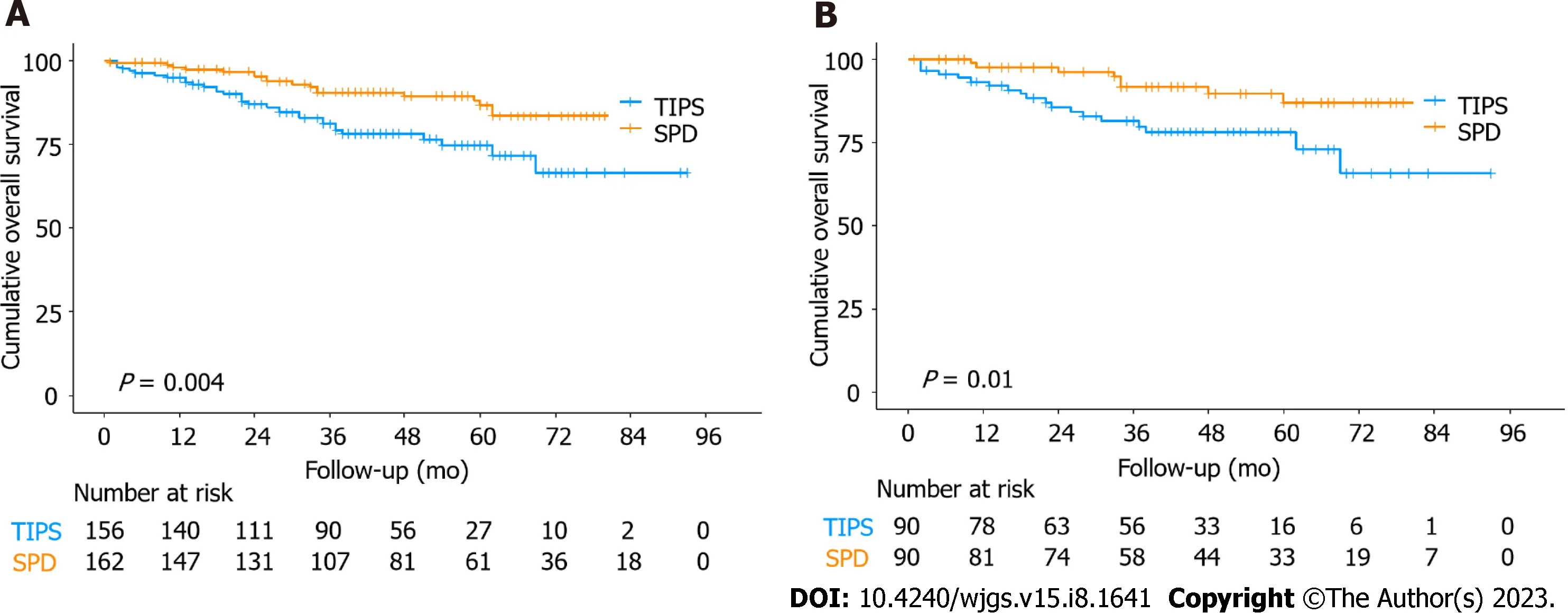
Figure 2 Overall patient survival. A: Kaplan-Meier analysis of overall survival (OS) in the splenectomy plus pericardial devascularization (SPD) group and transjugular intrahepatic portosystemic shunt (TIPS) group before propensity score-matched analysis (PSM); B: Kaplan-Meier analysis of OS in the SPD group and TIPS group after PSM. SPD: Splenectomy plus pericardial devascularization; TIPS: Transjugular intrahepatic portosystemic shunt.
High portal vein pressure is the direct cause of EGVB; therefore, reducing portal vein pressure plays a critical role in the treatment of EGVB[17,18]. TIPS significantly reduces portal vein resistance by creating a direct shunt between the hepatic and portal veins[19,20]. However, decreased portal perfusion after TIPS can lead to deterioration of liver function[21]. Moreover, shunts can allow portal blood flow to bypass hepatocytes and enter the circulation directly, resulting in the failure to metabolize toxic substances such as ammonia and γ-aminobutyric acid, which can lead to hepatic encephalopathy[22-24]. SPD is also effective in reducing portal vein pressure. Several studies have shown that the loss of splenic vein blood flow after splenectomy subsequently leads to a reduction in portal flow and thus a reduction in portal pressure[25,26]. However, blood flow in the hepatic artery increased after splenectomy and thus facilitated hepatocyte regeneration and improved liver function[25,27].
Whether to perform splenectomy is a controversial aspect of SPD. First, proponents of preserving the spleen argue that splenectomy increases the risk of thrombosis and infection. However, many previous studies have found that TGF-β1 endothelin and platelet-derived growth factors produced by splenic macrophages exacerbate liver fibrosis and inhibit liver regeneration in cirrhotic conditions[28,29]. A recent study by Zhanget al[30] showed that CD11b(+)CD43(high)Ly6C-(low) splenic monocytes migrating to the liver and transforming into macrophages can aggravate the liver fibrosis process. This further suggests that splenectomy may slow the process of liver fibrosis and promote liver regeneration. In addition, the traditional view is that splenectomy may impair the immunity of the body and may have a detrimental effect on resistance to tumorigenesis. However, Wanget al[31] reported that patients with cirrhosis secondary to hypersplenism and HCC who underwent simultaneous splenectomy and hepatectomy improved their tumor immunity in the long term. McKennaet al[32] reported that splenectomy promotes intraocular tumor elimination by affecting tumorassociated cell subsets. Nomuraet al[33] reported that splenectomy not only improved liver fibrosis but also increased the CD8+ cell percentage and decreased the CD4+/CD8+ ratio, which helped to improve antitumor immunity. Stöthet al[34]found that splenectomy reduced the number of tumor-associated macrophages (TAMs), tumor-associated neutrophils(TANs), and tumor-infiltrating dendritic cells (TIDCs), which in turn affected tumor growth and metastasis. This suggests that splenectomy not only does not decrease the immunity of the body but also may improve the potential antitumor ability. Finally, splenectomy is also effective in improving hypersplenism in patients with cirrhosis. Takahashiet al[35]reported that in ten patients with biliary atresia combined with hypersplenism who underwent splenectomy prior to liver transplantation, the patients' hematocrit recovered to normal levels 1 mo after surgery, and the mean Model for End-stage Liver Disease (MELD) score improved significantly.
There are some limitations to this study. First, although we applied PSM to reduce selection bias and potential confounding, unmeasured bias and confounding in this retrospective study might still exist. Second, due to the retrospective nature of this study, hepatic venous pressure gradient testing was not performed in both groups to more accurately assess portal vein pressure between the two groups of patients. Third, this study failed to collect data related to the occurrence of postoperative venous thrombosis in both groups of patients for analysis. Fourth, the medications(including nonselective beta-blockers and antiviral therapy) and endoscopic treatment of patients were not studied in detail in this study.
CONCLUSION
In conclusion, compared with TIPS, SPD is associated with higher postoperative OS rates, lower rates of abnormal liver function and HCC, and better quality of survival as acute EGVB treatment after failed endoscopic therapy or as secondary prophylaxis of VRB in patients with HBV-related cirrhosis combined with portal hypertension. There is no significant difference in the VRB rates between the two groups.
ARTICLE HIGHLIGHTS
Research background
The primary goals of the portal hypertension management program are prevention of first esophagogastric variceal bleeding (EGVB), control of acute EGVB, and prevention of variceal rebleeding (VRB). Splenectomy combined with pericardial devascularization (SPD) and transjugular intrahepatic portosystemic shunt (TIPS) are suggested in China as salvage therapies for patients with acute EGVB who have failed endoscopic treatment or as secondary prophylaxis of VRB. However, it is unclear whether SPD or TIPS is more effective and safe in the treatment of acute EGVB and as secondary prevention of VRB.
Research motivation
Both SPD and TIPS are effective treatments for EGVB, but the effectiveness and safety of both methods are currently controversial.
Research objectives
To compare the prognosis after SPD vs TIPS for acute EGVB after failure of endoscopic therapy or secondary prophylaxis of VRB in patients with HBV-related cirrhosis combined with portal hypertension.
Research methods
This was a retrospective study. We used propensity score matching analysis (PSM), Kaplan-Meier method, and multivariate Cox regression analysis to compare the effectiveness and safety of the two treatment modalities for comparative analysis.
Research results
We found that SPD was significantly associated with better overall survival (OS) (P = 0.01), lower rates of liver function abnormalities (P < 0.001), and a lower incidence of HCC (P = 0.02) than TIPS. There was no significant difference in VRB rates between the two groups (P = 0.09).
Research conclusions
Compared with TIPS, SPD is associated with higher postoperative OS rates, lower rates of abnormal liver function and HCC, and better quality of survival as acute EGVB treatment after failed endoscopic therapy or as secondary prophylaxis of VRB in patients with HBV-related cirrhosis combined with portal hypertension. There is no significant between-group difference in VRB rates.
Research perspectives
This study may provide a clinical basis for the treatment of patients with portal hypertension combined with EGVB.
FOOTNOTES
Author contributions:Wen TF, Li X, and Li C conceptualized and designed the study, and provided the study materials or patients; Wen TF provided administrative support; Qi WL, Wen J, Li C, Peng W, and Zhang XY collected and assembled the data; Qi WL, Wen J, and Shen JY performed data analysis and interpretation; all authors participated in manuscript writing and approved the final manuscript.Qi WL and Wen J contributed equally to this work.
Supported bythe National Key R&D Program of China, No. 2022YFC2503701; the Science and Technological Supports Project of Sichuan Province, No. 2022YFS0255; and the National Natural Science Foundation of China, No. 81800449.
Institutional review board statement:The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (No. 2023-354).
Informed consent statement:The ethics committee approved the waiver of informed consent because the study was retrospective in nature.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement - checklist of items, and the manuscript was prepared and revised according to the STROBE Statement - checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Wei-Li Qi 0000-0001-8899-4836; Jun Wen 0000-0002-5553-8890; Tianfu Wen 0000-0002-8260-7601; Wei Peng 0000-0002-5780-2157; Xiao Li 0000-0001-9420-9558; Chuan Li 0000-0001-5450-6961.
S-Editor:Liu JH
L-Editor:Wang TQ
P-Editor:Ji MX
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Initial suction drainage decreases severe postoperative complications after pancreatic trauma: A cohort study
- Vascular complications of chronic pancreatitis and its management
- Historical changes in surgical strategy and complication management for hepatic cystic echinococcosis
- Post-transplant biliary complications using liver grafts from deceased donors older than 70 years:Retrospective case-control study
- Goldilocks principle of minimally invasive surgery for gastric subepithelial tumors
- Prognostic scores in primary biliary cholangitis patients with advanced disease
