一系列萘羧酸膦酸镧系配合物的合成、结构和荧光性质
2023-10-19李素芝李新星
徐 艳 李素芝 李新星
(宿迁学院信息工程学院材料工程系,宿迁 223800)
Nowadays,metal-organic frameworks (MOFs) are rapidly emerging and being developed because of their unique structural characteristics and attractive application prospects[1-6].MOFs are a family of crystalline porous materials with metal centers and organic linkers,with inherent advantages of ordered and designable structures[7-8]and wide applications[9-10].Therefore,it is vital to rationally select organic ligands and create secondary building units for building MOFs with the required functions and properties[11-12],even if directed synthesis is still a challenge[13].
In particular,lanthanide complexes have provoked great interest.Lanthanide-based MOF (Ln-MOF)materials have an unusual and interesting porous crystalline structure[15-16]and luminous characteristics[17-19]because of their coordination number and the flexible variability of coordination modes of rare-earth ions.During the last two decades,great effort has been devoted to the design and synthesis of metal phosphonates with novel open frameworks or microporous structures due to their potential applications in electrooptical,ion exchange,catalysis,and sensors[20-26].The synthesis of lanthanide phosphonates has drawn the scientist′s attention for their possible optical and magnetic properties.However,reports on lanthanide phosphonates are rather limited[27-29],because lanthanide phosphonates normally have low solubility in water and organic solvents,hence it is difficult to obtain single crystals suitable for X-ray structural analysis.Nevertheless,the elucidation of the structures of lanthanide phosphonates is very important since these complexes may exhibit useful luminescent properties in both the visible and near IR regions.In particular,reports on the structure and photoluminescence properties of lanthanide carboxylate-phosphonates are still scarce[30-33]although this kind of ligand may enhance the fluorescence of the lanthanide ions,via the so-called antenna effect.
The solvothermal reaction of lanthanide(Ⅲ)nitrate hexahydrate with (5-carboxynaphthalen-1-yl)phosphonic acid (5-pncH3),afforded a series of new isostructural complexes,with 3D open-framework architectures formulated as [Pr(5-pnc)(H2O)]·2H2O (1),[Sm(5-pnc)(H2O)] ·H2O (2),and [Eu(5-pnc) (H2O)] ·H2O (3)(Scheme 1).Herein we report their synthesis,crystal structure,thermal behavior,and luminescent properties.
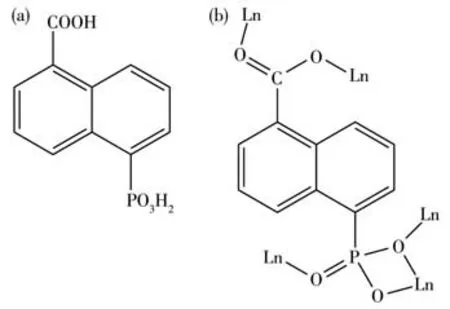
Scheme 1 (a)Molecular structures of 5-pncH3 and(b)its coordination mode with Ln(Ⅲ)ions in complexes 1-3
1 Experimental
1.1 Materials and physical measurements
All reagents and solvents employed in this work were commercially available and used without further purification.5-pncH3was synthesized following a previous procedure[34].Elemental analyses (C,H,and N)were performed on a Perkin-Elmer 240C elemental analyzer.IR spectra were recorded on a Bruker Tensor 27 spectrometer in a range of 400-4 000 cm-1using KBr pellets.Thermogravimetric analysis (TGA) was performed using a Mettler Toledo TGA/DSC thermo analyzer in a temperature range of 25-500 ℃in N2flow(20 mL·min-1) at a heating rate of 10 ℃·min-1.Powder X-ray diffraction (PXRD) data were recorded on a Bruker D8 ADVANCE X-ray powder diffractometer(CuKα,λ=0.154 06 nm) operating at 45 kV and 40 mA over a 2θrange of 5° to 50° at room temperature.The UV-Vis spectra were measured on a Perkin Elmer Lambda 950 UV/VIS/NIR spectrometer using powder samples.The steady fluorescence spectra were attained at Bruker Spectrofluorimeter LS55.
1.2 Synthesis
1.2.1 Synthesis of complex 1
A mixture of Pr(NO3)3·6H2O (0.1 mmol,0.044 5 g),5-pncH3(0.1 mmol,0.025 6 g),and 4 mL of a mixed solution ofN,N-dimethylformamide (DMF) and deionized water (H2O) (1∶1,volume ratio),adding 1 mL 0.5 mol·L-1HCl,was kept in a Teflon-lined autoclave at 140 ℃ for 2 d.Colorless rod - like crystals were obtained as a pure phase,confirmed by the PXRD measurements.Yield:43.9%based on Pr.Elemental analysis Calcd.for C11H12O8PPr(%): C 29.75,H 2.72; Found(%):C,29.59;H,2.78.
1.2.2 Synthesis of complex 2
Complex 2 was synthesized following a similar procedure to complex 1 except that the Pr(NO3)3·6H2O was replaced by Sm(NO3)3·6H2O.Colorless rod-like crystals were obtained as a pure phase,confirmed by the PXRD measurements.Yield: 35.7% based on Sm.Elemental analysis Calcd.for C11H10O7PSm(%):C 30.33,H 2.31;Found(%):C,29.79;H,2.74.
1.2.3 Synthesis of complex 3
Complex 3 was synthesized following a similar procedure to complex 1 except that the Pr(NO3)3·6H2O was replaced by Eu(NO3)3·6H2O.Colorless rod-like crystals were obtained as a pure phase,confirmed by the PXRD measurements.Yield: 34.4% based on Eu.Elemental analysis Calcd.for C11H10O7PEu(%):C 30.22,H 2.31;Found(%):C,29.93;H,2.23.
1.3 Crystallographic data collection and refinement
Single crystals with sizes of 0.12 mm× 0.12 mm×0.11 mm for 1,0.06 mm× 0.06 mm× 0.05 mm for 2,and 0.16 mm× 0.12 mm× 0.10 mm for 3 were used for structural determination on a Bruker D8 Venture diffractometer using graphite-monochromated (CuKα,λ=0.154 184 nm) at 293 K for 1 and 3,(GaKα,λ=0.134 139 nm) at 173 K for 2.A hemisphere of data was collected in the 2θranges of 4.150° to 67.151° for 1,12.762° to 109.748° for 2,4.328° to 65.081° for 3.The numbers of observed and unique reflections were 6 144 and 2 389 (Rint=0.032 1) for 1,8 500 and 2 425(Rint=0.063 5) for 2,33 130 and 6 926 (Rint=0.075) for 3.The data were integrated using the Siemens SAINT program,with the intensities corrected for Lorentz factor,polarization,air absorption,and absorption due to variation in the path length through the detector faceplate.The structures were solved by direct methods and refined onF2by full matrix least squares using SHELXTL.For 2 and 3,the solvent molecules inside pores were highly disordered,which have been removed by the SQUEEZE routine in the PLATON software package.All the non-H atoms were located from the Fourier maps and were refined anisotropically.All H atoms were refined isotropically,with the isotropic vibration parameters related to the non - H atom to which they are bonded.Details of the crystal data and refinements of 1-3 are summarized in Table 1,and selected bond lengths and angles of 1-3 are in Table 2.
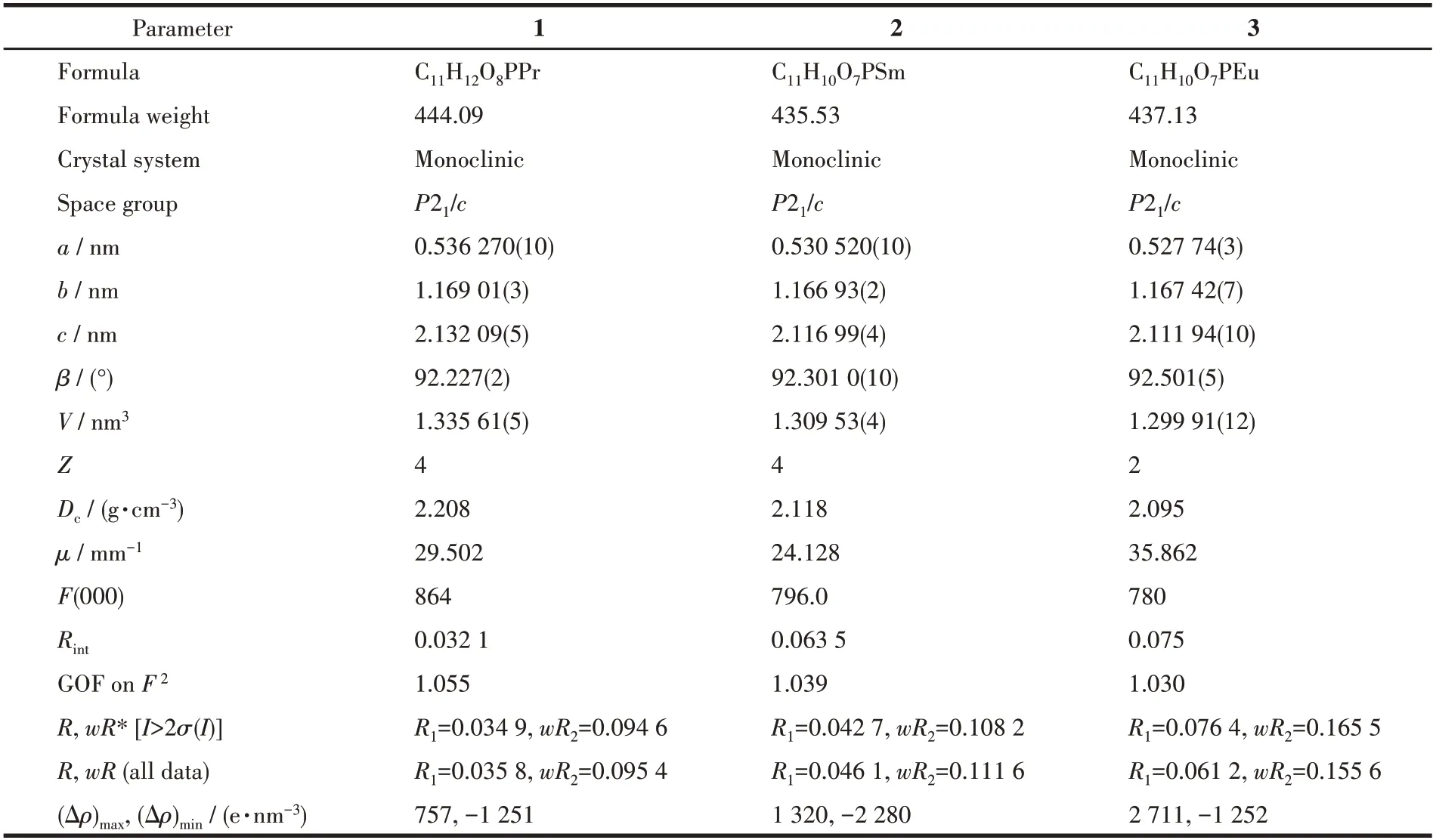
Table 1 Crystallographic data and structure refinement details for complexes 1-3
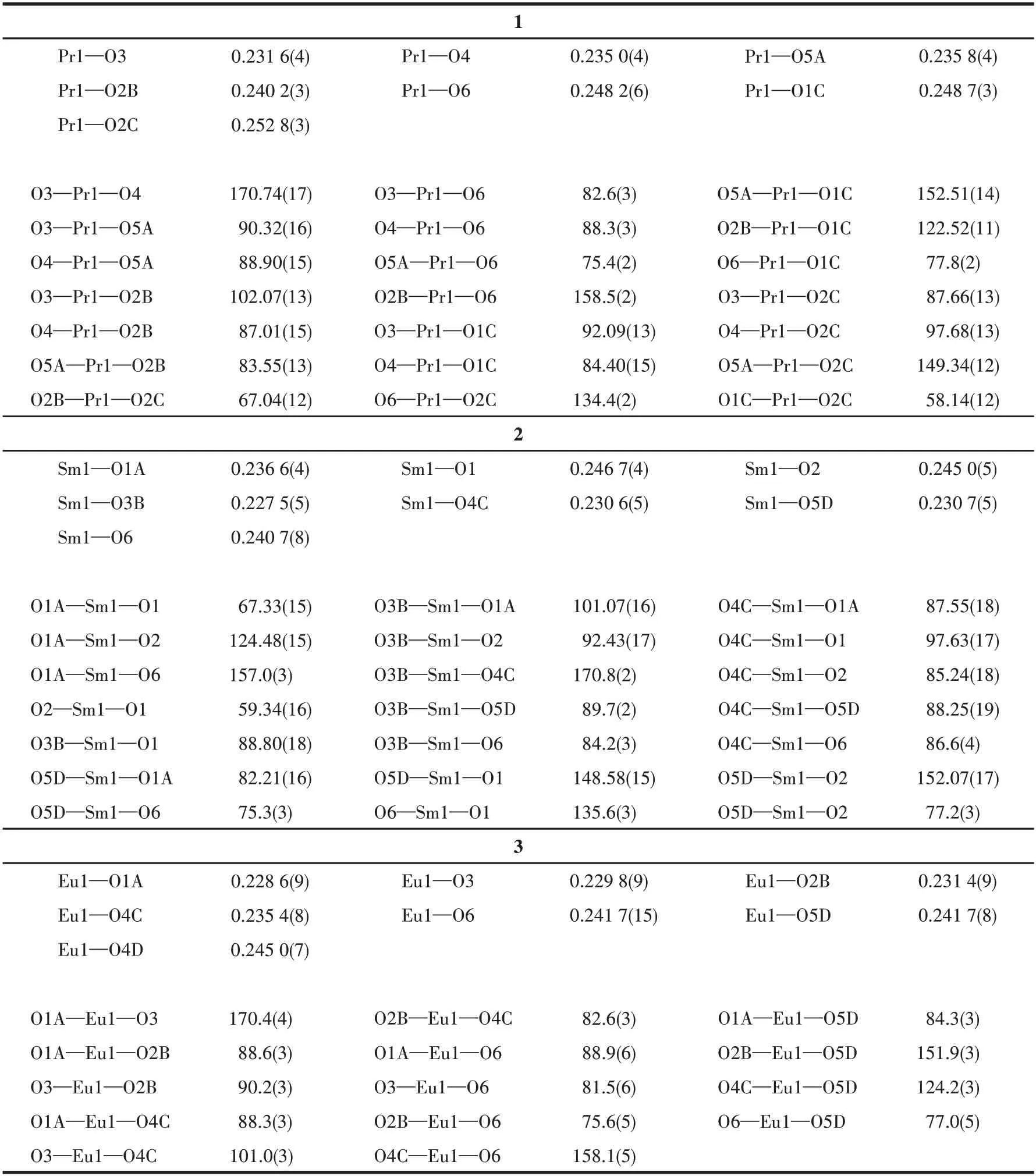
Table 2 Selected bond lengths(nm)and angles(°)of complexes 1-3
CCDC:2258142,1;2258143,2;2006506,3.
2 Results and discussion
2.1 Synthesis
In the reaction system,it is particularly important to find the optimal reaction conditions for the 5-pncH3ligand and rare-earth ions.We attempted to change the pH of the reaction system.After doing experiments several times in which we only adjusted the pH of the reaction system to 1-2 (a strong acid condition) by adding 1-1.5 mL 0.5 mol·L-1HCl and the other reaction factors remained constant,well-formed single crystals of complexes 1-3 with the desired yields were obtained.The other members of the family,Nd (Ⅲ),Gd (Ⅲ),and Tb(Ⅲ)complexes also have been obtained as microcrystalline powders and confirmed by IR and PXRD.Unfortunately,we did not produce crystals suitable for singlecrystal XRD.In addition,the effect of temperature change on the reaction system was studied.We experimented with reaction temperatures of 100,120,140,and 160 ℃,respectively.These experimental results show that the best single crystals for suitable singlecrystal XRD could only be achieved at 140 ℃.The effect of the solvent on the experiment was also studied.The results show that only the mixed solution of DMF and H2O (1∶1) could yield the title complexes,while pure DMF or pure H2O,as the solvent,leads to unknown white powders.The above experimental results indicated that the pH,reaction temperature,and solvent of the reaction system may be the main factors affecting the desired complexes.
2.2 IR and PXRD characterizations
The IR spectra of free 5-pncH3ligand and complexes 1-3 are shown in Fig.1.The free 5-pncH3ligand showed absorption peaks in a spectral range between 1 707 and 1 273 cm-1arising from stretching and bending vibrational modes associated with C=O,C—O,and C—H bonds.The IR spectra of complexes 1-3 were similar to each other.Complex 1 is selected as a representative for a detailed description.For complex 1,a broad absorption band appearing at 3 200 cm-1should be attributed to the stretching vibrations of the unassociated O—H in the water molecules.The peaks at 1 508,1 418,and 1 389 cm-1are the characteristic absorptions for the asymmetric and symmetric stretching vibrations of the C=O bond.Compared with the free 5-pncH3ligand,the characteristic peak of the asymmetric stretching vibration of C=O was shifted from 1 707 to 1 508 cm-1.The significant red shift indicates that the metal ion is coordinated by the ligand 5-pncH3[33].The peak at 1 094,1 009,and 963 cm-1is the characteristic stretching vibration of P—O.The PXRD patterns of complexes 1-3 were compared with the calculated one (Fig.2),indicating that the products have been successfully obtained as isostructural pure crystalline phases.

Fig.1 IR spectra of 5-pncH3 and complexes 1-3
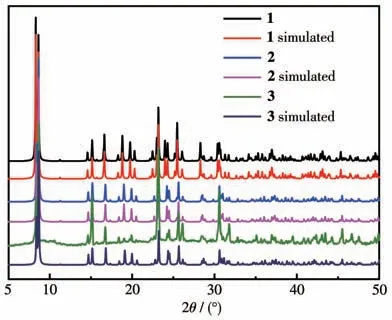
Fig.2 PXRD patterns of complexes 1-3
2.3 Structure description
Single-crystal XRD measurements revealed that complexes 1-3 are isostructural.Complex 1 is selected as a representative for a detailed structure description.Complex 1 crystallizes in the monoclinic system with theP21/cspace group.The asymmetric unit is relatively simple containing one Pr3+ion,one 5-pnc3-ligand,one coordinated water molecule,and two lattice water molecules as displayed in Fig.3a.The Pr3+cation is bonded to seven oxygen atoms,in which six O atoms(Pr—O 0.231 6(4)-0.252 8(3)nm)are from five crystallographically equivalent phosphonate groups and the other one O atom (Pr—O 0.248 2(6) nm) comes from the coordinated water molecule (Fig.3b).The Pr—O(phosphonate) distance is slightly shorter than that of Pr—O (water).The bond angles of O—Pr—O fluctuate in a range of 58.14(2)°-170.74(17)°.The geometry of the [PrO7] (Fig.3d) center is best described as a slightly distorted pentagon-bipyramidal geometry.
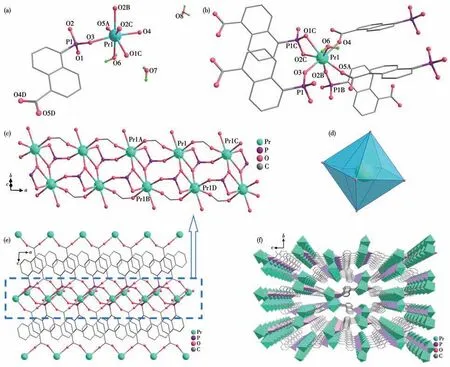
Fig.3 (a)Asymmetric unit of complex 1;(b)Perspective view of the coordination environment of Pr3+;(c)Inorganic double metal chain in 1 running along the a-axis;(d)Geometry of the{PrO7};(e)2D network structure;(f)3D supramolecular open-framework structures
The phosphonate and carboxylate groups of 5-pnc3-ligands are fully deprotonated and each serves as a pentadentate ligand,binding and chelating five Pr ions (Scheme 1b).There are three types of bridging between adjacent Pr atoms: (1) one O—C—O and one O—P—O units (for Pr1…Pr1A,Pr1…Pr1C); (2) two O—P—O units (for Pr1…Pr1B);(3)twoμ-O (phosphonate) and one O—P—O units.As a consequence,the building blocks [PrO7] of complex 1 are linked into a 1D double metal chain structures running along theaaxis(Fig.3c).The Pr…Pr distances are 0.536 27 nm for Pr1…Pr1A,0.507 20 nm for Pr1…Pr1B and 0.411 01 nm for Pr1…Pr1D.The neighboring double metal chains are further cross-linked by the organic groups of 5-pnc3-leading to a 3D framework with open rhombic channels with sizes of 1.92 nm×0.82 nm for 1,along thea-axis (Fig.3f).Two lattice water molecules fill in the channels.Hydrogen bonds exist between the lattice water molecules and coordinated water molecules(O8…O6 0.277 9(4)nm;O7…O6 0.289 9(8)nm,O7…O8 0.236 7(4) nm),and between the phosphonate oxygen atoms and lattice water molecules (O8…O1 0.272 1(7)nm)along thea-axis.
Complexes 2 and 3 also crystallize in theP21/cspace group of the monoclinic system (Table 1).The cell volume follows the sequence: 1 > 2 > 3,attributed to the lanthanide contraction effect.The Sm—O and Eu—O distances are in the ranges of 0.227 5(5) -0.246 7(4) nm and 0.228 6(9)-0.245 0(7) nm,respectively,with the Ln—O (phosphonate) distance slightly shorter than that of Ln—O (water).The O—Sm—O and O—Eu—O angles are 67.33(15)°-170.8(2)° and 75.6(5)°-170.4(4)°,respectively (Table 2).Compared to 1,two aspects are distinct: (a) lattice solvents in 2 and 3 are heavily disordered but not in 1; (b) two lattice water molecules are found in 1,while one in 2 and 3,respectively.These structural differences may be reflected in their luminescent properties.
It is worth mentioning that complexes 1-3 are 3D open-framework architectures based on naphthyl carboxylate-phosphonate ligands which,as far as we are aware,have not been reported before.The structures of 1-3 are remarkably different from those of Ln(HPMIDA)(H2O)2·H2O (Ln=Gd,Tb,Dy,Y,Er,Yb,Lu),where the phenyl carboxylate - phosphonate is involved[30].The latter features a 3D network with helical tunnels,in which the nine-coordinate La3+ions are bridged by phosphonate groups of the ligands.The carboxylate group of the phosphonate ligand remains protonated and is involved in the interlayer hydrogen bonding.In the previously reported complexes,the phosphonate group is singly protonated,whereas the complexes in the present study show complete deprotonation of the acidic oxygen atoms and all of the oxygen atoms coordinate to Ln3+ions.It is worth noting that complexes 1-3 show open-framework structures with rhombic channels (sizes of 1.92 nm×0.82 nm for 1)filled with lattice water molecules.
2.4 Thermal stability of the complexes
To verify the thermal stability of the complexes,TGA was performed at a heating rate of 10 ℃·min-1under a N2atmosphere within a temperature range from 30 to 600 ℃.The TGA curves of complexes 1-3 were similar to each other and exhibited three steps of weight loss (Fig.4).Complex 3 was used as an example.The first step was observed below 200 ℃with a weight loss of 4.12% in agreement with the release of one lattice water molecule (Calcd.4.21%).In the second step,there was a weight loss of 4.05% on heating to 250 ℃,attributed to the release of the coordination water molecule (Calcd.4.21%).It is thermally stable up to about 500 ℃,above which a weight loss was observed with the collapse of the structure.The step began at 450 ℃and was completed at 550 ℃,during which the organic groups were burnt,and the final products were not identified.However,we suspect they are mainly EuPO4.

Fig.4 TGA curves for complexes 1-3
2.5 Luminescent properties of the complexes
The solid-state luminescent properties of the free 5-pncH3ligand and complexes 1-3 were investigated at room temperature.The emission spectra are shown in Fig.5.The 5-pncH3ligand shows the strongest emission at 396 nm under an excitation of 330 nm.Under the excitation of 330 nm,the emission spectrum of complex 1 exhibited a very broad band (350 to 450 nm)with a peak at 420 nm,and several strong and sharp peaks at 468 (strong),482 (weak),492 (weak),which may be assigned to the intraligandπ-π* fluorescence,while the sharp peak 561 nm(middle) could be assigned to3P0to7FJtransition for 1 (Pr),and4G5/2to6H5/2transition for 2(Sm)[35-36](Fig.5a).Complex 3 exhibited strong characteristic emission bands for the Eu(Ⅲ)ion in the visible region under excitation at 330 nm(Fig.5b).The profile of 3 is characteristic of5D0to7FJtransitions (J=1-4) at 594,615,654,and 705 nm,respectively,of Eu with discernible (400 - 550 nm)organic emission.
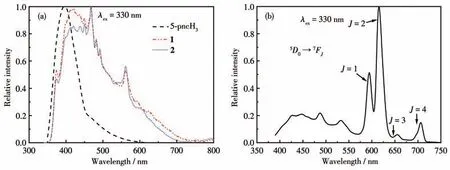
Fig.5 (a)Solid-state emission spectra of 5-pncH3,1,and 2 at room temperature;(b)Solid-state emission spectrum of 3 at room temperature
3 Conclusions
By using the naphthyl carboxylate-phosphonate moiety,5-pncH3,as a metal linker,we have solvothermally synthesized three lanthanide carboxylatephosphonates formulated as [Pr(5-pnc)(H2O)]·2H2O(1),[Sm(5-pnc)(H2O)]·H2O (2),and [Eu(5-pnc)(H2O)]·H2O(3)(5-pncH3=5-carboxynaphthalen-1-yl)phosphonic acid).Their structures feature 3D open-framework structures with rhombic channels filled with lattice water molecules.Complexes 1 and 2 displayed very broad intraligand emission bands in the blue light region,whereas complex 3 exhibited strong luminescence in the red light region.Efforts are underway to synthesize the complete series of lanthanide (Ⅲ)complexes with this particular ligand to elucidate their crystal structures and magnetic and luminescent properties.
