Anti-inflammatory Mechanism of Coregonus peled Oil via the Inhibition of Nuclear Factor Kappa-B (NF-κB), Signal Transducer and Activator of Transcription 1 (STAT1), and Succinate/Hypoxia-Inducible Factor-1α (HIF-1α) Signaling Pathways
2023-10-17LIUHongxuSONGGuokuWANGZhenXIAXiaodongQINNingbo
LIU Hongxu, SONG Guoku, WANG Zhen, XIA Xiaodong, QIN Ningbo*
(College of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China)
Abstract: Objective: To investigate the chemical composition and anti-inflammatory mechanism of Coregonus peled oil(CPO).Methods: CPO was extracted by ultrasonic homogenization and its major chemical components were detected.In addition, its fatty acid (FA) composition was analyzed by gas chromatography-mass spectrometry (GC-MS).Macrophage RAW264.7 cells were cultured as an inflammation model and stimulated with lipopolysaccharides (LPS).Cytotoxicity was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.The accumulation of succinate was detected by liquid chromatography tandem mass spectrometry (LC-MS/MS).The concentration of nitric oxide (NO) and the activity of inducible nitric oxide synthase (iNOS) in the supernatant were measured by commercial kits.Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the supernatant.The expression levels of CD68, CD86, Arg1, CD206, YM1 and HIF-1α were detected by quantitative real-time polymerase chain reaction (qPCR), and their protein expression levels were determined by Western blotting.Results: The major chemical constituents of CPO were palmitoleic acid, oleic acid, eicosapntemacnioc acid (EPA), and docosahexaenoic acid (DHA).CPO significantly inhibited the production of NO, iNOS, IL-6, TNF-α, and IL-1β as well as their gene expression levels.CPO blocked NF-κB activation by inhibiting the degradation of inhibitor α of NF-κB (IκBα) and decreasing p65 nuclear translocation in LPS-induced RAW264.7 cells.In addition, CPO promoted macrophage polarization from M1 to M2 types by inhibiting the phosphorylation of signal transducer and activator of transcription 1 (STAT1).The content of succinate and the expression levels of hypoxia-inducible factor-1α (HIF-1α)gene and protein indicated that CPO could inhibit the production of IL-1β by inhibiting the accumulation of succinate.Conclusion: The anti-inflammatory mechanism of CPO may be due to the inhibition of the NF-κB, STAT1, and succinate/HIF-1α signaling pathways.
Keywords: Coregonus peled; fish oil; inflammation; polarization; succinate/hypoxia-inducible factor-1α
During inflammatory processes, macrophages play an important role in inflammatory and immune responses by acting as an internal defense against microbial infections[1-3].Macrophages are a diverse group of immune cells that initiate an innate immune response through phagocytosis and cytokine release.Despite the fact that macrophage phenotypes in our bodies are plastic and adaptable, they can be simply divided into two extremes: classically activated macrophages(M1) associated with pro-inflammatory responses and alternatively activated macrophages (M2) associated with anti-inflammatory responses[4].M1 macrophages contribute to the pro-inflammatory process by activating the signal transducer and activator of transcription 1 (STAT1) and nuclear factor kappa-B (NF-κB) signaling pathways, as well as regulating the expression of cytokines[5-6].M2 macrophages, on the other hand, play an important role in the resolution of inflammation by activating STAT3 and STAT6 and regulating the expression of interleukin-10 (IL-10),interleukin-4 (IL-4) and mannose receptor (CD206)[7-8].
Once activated, macrophages could rapidly respond by producing cytokines and chemokines to clear the foreign material, indicating that dramatic changes in cell metabolism influence macrophage phenotypic and functional changes.When macrophages are stimulated, oxidative phosphorylation(OXPHOS) decreases and glycolysis and pentose-phosphate pathways increase.The rapid increase in glycolysis is linked to macrophage inflammatory phenotypes[9-10].Numerous studies have revealed that M1 macrophages rely on glycolysis to produce adenosine triphosphate (ATP) and contribute to the accumulation of tricarboxylic acid (TCA) cycle metabolites.M2 macrophages, on the other hand, rely primarily on OXPHOS and the TCA cycle is intact[11-12].Succinate, as a TCA cycle intermediate, is essential for ATP generation in mitochondria[13-14].Succinate’s new role as a metabolic signal in inflammation has recently emerged[13].Succinate,accumulated in activated macrophages, has been shown to stimulate the production of pro-inflammatory cytokines and stabilize the transcription factor hypoxia-inducible factor-1α(HIF-1α) during inflammation[10-15].Furthermore, glycolysis in inflammatory macrophages is strongly linked to HIF-1α.As a result, succinate has spreaded beyond metabolism into inflammatory signaling.
Natural products have gained popularity as antiinflammatory agents with few side effects[16-18].Previous researches indicated fish oil is well known for their antiinflammatory properties in sever chronic diseases[19-21].Coregonus peledis a freshwater species from cold regimes that belongs to the Salmonidae family and is widespread in Europe and Asia.C.peledis currently cultured in Sayram Lake (Xinjiang, China) and primarily processed into fillets for market sales in China.The fish oil fromC.peledis frequently used as low-value materials for animal feed or is directly removed before processing, resulting in a loss of valuable nutrients.We previously investigated fish oil’s anti-obesity activity[22], but the immunomodulatory activity of fish oil fromC.peledrelated to metabolic syndrome has not been studied.
We investigate the hypothesis that fish oil fromC.peledpolarizes macrophages away from M1 to M2 phenotype via inhibition of NF-κB, STAT1, and succinate/HIF-1α signaling pathways in this study to shed light on the immunomodulatory activity of fish oil from freshwater.The current findings will shed light on the immunomodulatory mechanism of fish oil and pave the way for the use of fish oil fromC.peledas a functional food.
1 Materials and Methods
1.1 Materials and reagents
C.peledwere kindly provided by Xinjiang Saihu Fishery Technology Develop.Co., Ltd.(China).A specimen (CP2019)had been deposited at the National Engineering Research Center of Seafood, Dalian Polytechnic University, China.
High performance liquid chromatography (HPLC) grade methanol, acetonitrile, chloroform, isopropanol, acetic acid,trimethylamine, tetrahydrofuran, and ammonium formate were purchased from Spectrum Chemical Mfg.Corp.(Gardena, CA, USA).Dulbecco’s modified Eagle medium(DMEM) was obtained from Invitrogen-Gibco (Grand Island,NY, USA).Fetal bovine serum (FBS) was purchased from Biological Industries (Shanghai, China).Lipopolysaccharide(LPS) fromEscherichia coliO55:B5, interferon γ (IFN-γ) and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide(MTT) were purchased from Sigma-Aldrich, Inc.(St, Louis,MO, USA).Mouse IL-1β and Tumor Necrosis Factor-α(TNF-α) Enzyme-Linked Immunosorbent Assay (ELISA)Kits and Nitric Oxide Synthase (NOS) Typed Assay Kit were purchased from Nanjing Jiancheng Bioengineering Co., Ltd.(Nanjing, China).Mouse IL-6 ELISA Kit was purchased from Bio-Tech China Co., Ltd.(Shanghai, China).The Nitric Oxide (NO) Assay Kit and BCA Protein Assay Kit were purchased from Beyotime Biotechnology Co., Ltd.(Shanghai,China).Antibodies againstβ-actin, STAT1, p-STAT1, NF-κB p65, p-NF-κB p65, inhibitor a of NF-κB (IκBα) and p-IκBα were purchased from Cell Signaling Technology (Beverly,MA, USA).Antibody against HIF-1α was obtained from Abcam (Branford, CT, USA).Fluorescein isothiocyanate(FITC) AffiniPure Goat Anti-Rabbit Immunoglobulin G (IgG)was purchased from Zen-Bio, Inc.(Chengdu, China).
1.2 Instruments and equipment
T 25 digital ULTRA TURRAX was purchased from IKA-Werke GmbH & Co.(KG, Staufen, Germany).SB-800DT ultrasonic bath (40 kHz) was purchased from Ningbo Scientz Biotechology Co., Ltd.(China).7890B gas chromatography was purchased from Agilent Technologies(USA).5977A gas chromatography mass spectrometer(GC-MS) system was purchased from Palo Alto (CA, USA).
1.3 Methods
1.3.1 Fish oil extraction
Fresh fish oil fromC.peledwere extracted by using homogenizer extraction plus sonication (HES)[23].Briefly,10 g of fish fillets fromC.peledwas mixed with 100 mL ofn-hexane and homogenized by T 25 digital ULTRA TURRAX at 4 000 r/min for 4 min at room temperature.Later, the solution was treated in an ultrasonic bath (40 kHz) for 90 min at cold temperature.After extraction, the mixture was centrifuged at 7 800 ×gfor 10 min at 4 ℃.The supernatant organic layer was dried by nitrogen.The fish oil obtained was stored at -20 ℃ for further analysis.
1.3.2 Analysis of fatty acid (FA) compostion
The assay was performed according to previous described method with minor modification[24].A total of 100 mg of fish oil was mixed and reacted with 2.5 mL of 50% KOH and 5 mL of anhydrous methanol at 60 ℃ for 2 h.After cooling, the mixture was extracted for three times with 9 mL ofn-hexane.The remaining water layer was extracted withn-hexane after being adjusted to pH 2 with 6 mol/L HCl.The hexane layers were combined and dried by rotary evaporator.The residue was dissolved withn-hexane to 10 mg/mL, and then 2.5 mL of solution was reacted with 2.0 mL of fresh methylation reagent at 60 ℃ for 1 h.The vial was shaken with distilled water and the hexane layer was dried with anhydrous Na2SO4, filtered through a 0.22 μm filter membrane, and analyzed with a GC-MS system equipped with an HP-5-MS capillary column (30 m × 0.25 mm,0.25 μm) according to a previous study[25].The sample injection volume was 1 μL.The determination of FA composition was conducted by comparison of relative retention times and NIST02 mass spectral database.
1.3.3 Cell culture and viability assay
Mouse macrophage RAW264.7 cells were cultured with DMEM, which contained 10% (V/V) FBS and 1% penicillinstreptomycin solution, at 37 ℃ in a humidified incubator with 5% CO2.
The cytotoxicity was determined by MTT method.Briefly, RAW264.7 cells were planted into a 96-well plate(2.5 × 104cells/mL) and cultured with various does of fish oil (0-200 μg/mL) for 24 h.Subsequently, the MTT was co-cultured with cells at 37 ℃ for 4 h.Finally, dimethyl sulfoxide (DMSO) (200 μL) was added to cells to dissolve the crystals.The absorbance at a wavelength of 570 nm was recorded with a qTOWER 2.2 microplate reader.All tests were carried out in 6 independent experiments.
1.3.4 Determination of inflammatory factors
RAW264.7 cells were seeded into 96-well plates with 2.5 × 104cells/mL for 24 h, and then co-cultured by 500 ng/mL LPS with or without fish oil (25, 50, 100 μg/mL)for an additional 16 h.Following the NO assay kit’s instructions, the nitrate content of the cell-free supernatant was assessed.Pro-inflammatory cytokines were quantified by ELISA kits for IL-1β, TNF-α, and IL-6.
1.3.5 Real-time quantitative PCR (qPCR) analysis
After incubating the cells with LPS and fish oil for 16 h,the total RNA was extracted by the RNAeasy™ Animal RNA Isolation Kit with Spin Column.The concentration and purity of RNA were measured with Nanodrop ND-1000 spectrophotometer.Then total RNA was reversely transcribed into cDNA using PrimeScript™ RT Reagent Kit with a gDNA Eraser.The primer sequences are shown in Table 1.Finally, qPCR was performed with TB Green™Premix ExTaq™ II and Infinite M200.The thermal cycling parameters were 95 ℃ for 30 s, 45 cycles of 95 ℃for 15 s, and 60 ℃ for 15 s.

Table 1 Primer sequences used in the experiment
1.3.6 Western blotting assay
Cells were collected and resuspended in radioimmunoprecipitation assay (RIPA) buffer supplemented with 0.1 mmol/L phenylmethanesulfonyl fluoride (PMSF)protease inhibitor.The cell suspension was placed on ice for 15 min, and then centrifuged at 13 000 ×gto obtain the supernatant.The supernatant was collected and the protein concentration was determined by the BCA Protein Quantification Kit.10 μL of protein in each sample was loaded into a pre-made 10% SDS-PAGE for electrophoresis.After 1 h of blocking with 5% skimmed milk buffer at room temperature, the membrane was incubated with the corresponding primary antibody overnight at 4 ℃, and then washed with TBST three times for 15 min each time.The membrane was then incubated with the corresponding secondary antibody at room temperature for 1 h before being washed again as described above.Finally, the premixed liquid of liquid A and liquid B in the BeyoECL Moon system were laid flat on the membrane for 1 min, the liquid was discarded,and the membrane was placed in a darkened chamber for exposure.
1.3.7 Immunofluorescence analysis
Under an inverted microscope, the cells were counted and the cell concentration was adjusted to 3 × 104cells/mL.A total of 1 mL of cell suspension was inoculated in each well of the 24-well plates containing an 8 mm glass coverslip, and cultured overnight in an incubator at 37 ℃ with 5% CO2.Following the above-mentioned processing, the cells were fixed with 4% paraformaldehyde for 30 min and washed 3 times with phosphate buffered saline (PBS).The coverslips were then slightly dried before being permeabilized with 0.1%Triton X-100 at room temperature for 10 min and washed 3 times with PBS.After 1 h incubation with blocking buffer(3% BSA in PBS), cells were incubated at 4 ℃ overnight with NF-κB p65 (D14E12) XP®Rabbit mAb (1:500) in 1% BSA.The cells were washed 3 times with PBS.After removing the PBS, the coverslips were incubated for 1 h at room temperature in the dark with a FITC AffiniPure Goat Anti-Rabbit IgG corresponding to the primary antibody.PBS was used to wash the cells 3 times.After slightly drying,the slides were stained with antifade mounting medium containing DAPI and visualized with RVL-100 (ECHO).ImageJ 1.53q software was used for image analysis.
1.3.8 Measurement of succinate by LC-MS
The RAW264.7 cells were stimulated with LPS for 12 h with or without fish oil, and the succinate was analyzed.Following stimulation, an 80% methanol solution stored at-80 ℃ was added to the cells and kept at -80 ℃ for 30 min.The cells were then quickly scraped off on ice and placed in a centrifuge tube.After the cells were broken by the ultrasonic cell disruptor, the protein concentration of each sample was measured and normalized to the same concentration.Subsequently, the liquid with the same protein concentration was centrifuged at 11 000 ×gfor 10 min at 4 ℃.The supernatant was then nitrogen-dried.Following drying, the pellet was dissolved in 300 μL of water, filtered through a 0.22 μm filter membrane, and loaded into a sample bottle for LC-MS testing[10].
1.4 Statistical analysis
GraphPad Prism 7.0 was used for statistical analysis.Data were expressed as mean ± standard deviation (SD), and one-way analysis of variance (ANOVA) was used to evaluate the statistical significance of differences between treatment groups, which was followed by Tukey’s test for multiple comparisons.Significance was expressed as *P< 0.05, **P<0.01, and ***P< 0.001.
2 Results and Analysis
2.1 FA composition analysis
As shown in Table 2, GC-MS analysis indicated that myristic acid (C14:0) and palmitic acid were primary saturated fatty acids (SFA), palmitoleic acid (C16:0), oleic acid (C18:0),eicosapntemacnioc acid (EPA, C20:5,n-3,6,9,12,15), and docosahexaenoic acid (DHA, C22:6,n-3,6,9,12,15,18) were dominant unsaturated fatty acids (UFA) in fish oil fromC.peled.SFA, UFA, and polyunsaturated fatty acid (PUFA)accounted for 28.12%, 71.58%, and 27.28% of the total FA in fish oil, respectively.In addition, the levels of EPA and DHA in fish oil were (11.56 ± 0.32)% and (3.86 ± 0.24)%,respectively.Then-3 long-chain PUFA, in particular EPA and DHA from seafood, has received a lot of attention in recent decades.Moreover, previous studies have also indicated that some marine fish and seafood including salmosalar, thunnus alalonga, sea cucumber, and sea urchins, were rich in EPA and DHA[26-27].Nevertheless,C.peled, as a freshwater fish,is rich in PUFA, especially EPA and DHA, which is unusual and interesting.
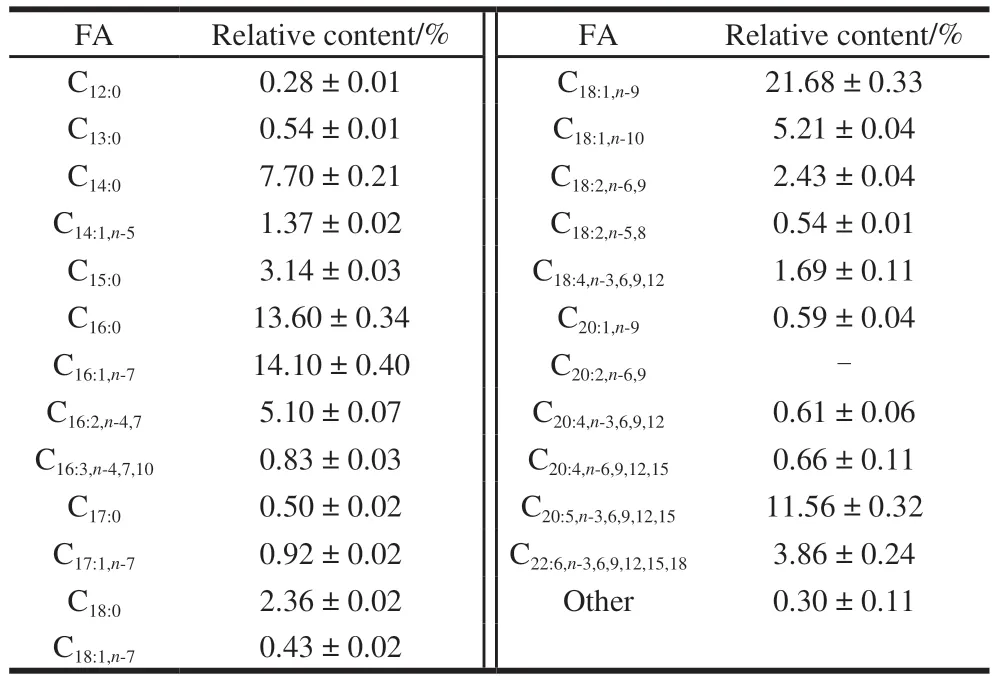
Table 2 FA composition of lipids from C. peled
2.2 Effects of fish oil on cell viability and NO production
The viability assay was used to assess the cytotoxic effect of fish oil on RAW264.7 cells.As shown in Fig.1A,there was no significant difference in cell viability when the concentration of fish oil was less than 200 μg/mL.But the cell viability was significantly decreased with 400 μg/mL of fish oil compared to control.Additionally, the cell viability was lower at 200 μg/mL of fish oil compared to the other 3 concentrations (25, 50, 100 μg/mL).Thus,we chose three concentrations (25, 50, 100 μg/mL) for subsequent experiments.

Fig.1 Effect of fish oil treatment on cell viability (A), NO secretion (B),iNOS activity (C) and mRNA expression of iNOS (D) in LPS-induced macrophages
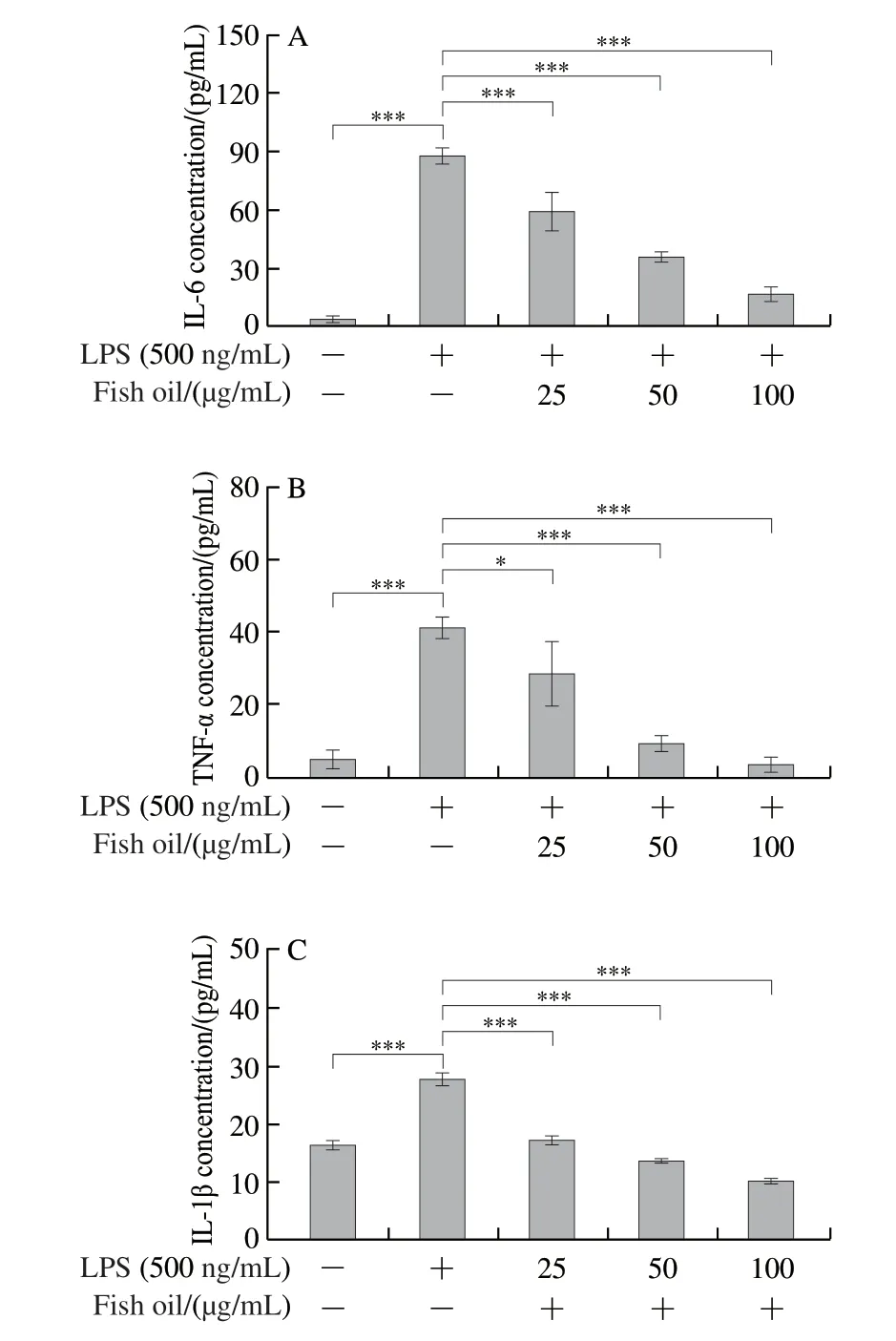
Fig.2 Secretion of pro-inflammatory cytokines IL-6 (A), TNF-α (B)and IL-1β (C) and mRNA expression levels of IL-6 (D), TNF-α (E) and IL-1β (F) in RAW264.7 cells
Gas signals can also boost the resolution of inflammation.For example, NO is dissolved in the extracellular fluid and can diffuse through the cell membrane to affect cell function.As shown in Fig.1B, the concentration of NO was remarkably increased in RAW264.7 cells treated with LPS compared to control cells not treated with LPS.However, fish oil inhibited NO production in LPS-treated RAW264.7 cells in a dose-dependent manner.Because NO production in macrophages was closely related to the expression level of inducible nitric oxide synthase (iNOS)[28],the iNOS expression was determined.As shown in Fig.1C,the LPS stimulation significantly increased iNOS activity,whereas fish oil treatment significantly reduced iNOS activity.Moreover, qPCR results showed that the expression ofiNOSmRNA was significantly inhibited by fish oil in LPS-induced macrophages (Fig.1D).
2.3 Effect of fish oil on inflammatory cytokines
Inflammatory cytokines play an indispensable role in the immune response, and identifying inflammatory cytokines can help to better understand the anti-inflammatory mechanism[29].To assess the effect of fish oil on inflammatory cytokines, ELISA and qPCR were used to measure the levels of IL-6, TNF-α and IL-1β in LPS-stimulated RAW264.7 cells.As shown in Figs.2A-C, LPS significantly promoted the secretion of IL-1β, IL-6, and TNF-α compared to control cells.The secretion of IL-1β, IL-6, and TNF-α was potently suppressed by fish oil in a dose-dependent manner.Additionally, the relative gene expression ofIL-6,TNF-αandIL-1βwas extraordinarily up-regulated, whereas it was significantly down-regulated with fish oil treatment in LPS-stimulated RAW264.7 cells (Figs.2D-F).Though fish oil at 25 μg/mL did not significantly inhibit the expression ofTNF-αmRNA in cells stimulated with LPS, high concentration of fish oil could dramatically inhibit the mRNA expression.In summary, these data further indicated that fish oil has a significant anti-inflammatory effect, which prompted us to explore the possible mechanism.
2.4 Effect of fish oil on the NF-κB signaling pathway
NF-κB signaling pathway makes a pivotal impact in the pathogenesis of inflammation.Therefore, the effect of fish oil on transduction proteins involved in the LPS-induced NF-κB signaling cascade was evaluated.Compared to control cells,LPS dramatically enhanced the expression level of IκBα, but there was no change administrated with fish oil.It is worth noting that the expression level of p-IκBα was up-regulated with LPS, while that was remarkably down-regulated with fish oil administration (Fig.3).The data indicated that fish oil could significantly suppress the phosphorylation of IκBα in RAW264.7 cell.
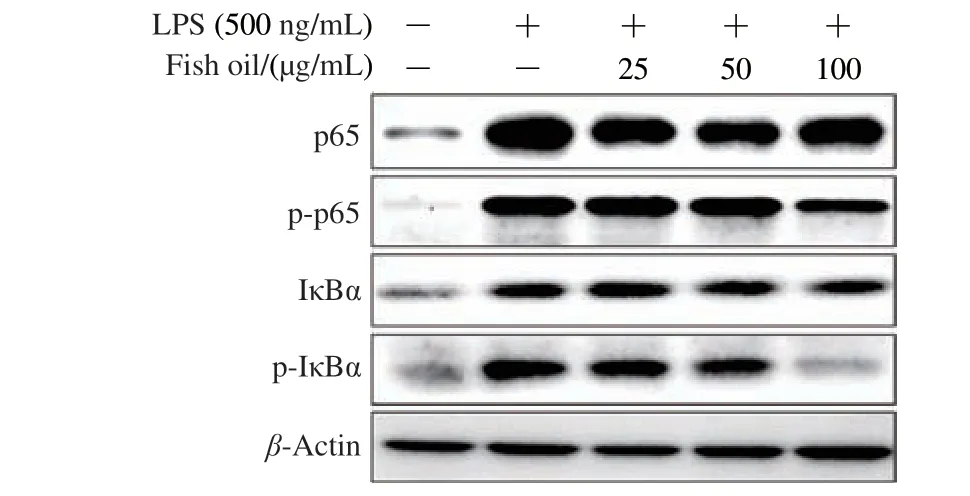
Fig.3 Effect of fish oil on p65, p-p65, IκBα and p-IκBα in LPS-induced RAW264.7 cells
Additionally, the immunofluorescence analysis of NF-κB p65 was introduced to better evaluate the effect of fish oil on the NF-κB signaling pathway.The results demonstrated that fish oil could significantly suppress the translocation of NF-κB p65 from cytosol to nucleus, which were also confirmed by Western blot analysis.As shown in Fig.4, the high expression of p-p65 with LPS treatment was remarkably suppressed with fish oil administration, whereas p65 was not affected.
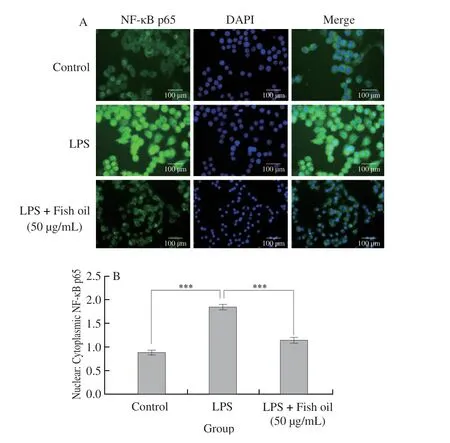
Fig.4 Immunofluorescence staining of NF-κB p65 (A) and quantification of nuclear-cytoplasmic ratio of NF-κB p65 (B) in RAW264.7 cells
2.5 Effect of fish oil on polarization of macrophages
Some surface markers of macrophages were evaluated by qPCR to investigate the effect of fish oil on polarization of macrophages.We firstly determined the effect of fish oil on the levels ofCD68andCD86, which are M1 surface markers.The mRNA expression ofCD68andCD86was significantly decreased with fish oil in RAW264.7 cells compared to that with IFN-γ and LPS (Figs.5A and B).M1 macrophages are associated with activating and maintaining inflammation,whereas M2 macrophages are associated with settlement of chronic inflammation[30].Therefore, we also analyzed the mRNA expression of M2 markers by qPCR.As observed,the fish oil could remarkably promote the expression level ofArg1,YM1andCD206genes (Figs.5C-E).
STAT1 and p-STAT1 play critical roles in macrophage polarization[31].The phosphorylated STAT1 (p-STAT1)dimerizes translocate to the nucleus, where it initiates the transcription of inflammatory cytokines and genes associated with M1 macrophage activation.Then the effect of fish oil on the expression of STAT1 and p-STAT1 proteins was assessed.As shown in Fig.5F, there was no distinct difference on the expression levels of STAT1 in absence or presence of fish oil.However, the expression levels of p-STAT1 were significantly down-regulated after treatment with fish oil at different concentration, especially at 100 μg/mL.


Fig.5 mRNA expression levels of CD68 (A), CD86 (B), Arg1 (C),YM-1 (D) and CD206 (E) markers in RAW264.7 cells and effect of fish oil on STAT1 and p-STAT1 protein expression (F)
2.6 Effect of fish oil on the succinate/HIF-1α signaling pathway
Succinate, a Krebs cycle metabolite, is important in inflammatory, hypoxic, and metabolic signaling.LC-MS was used to examine the metabolic profile of succinate.The results indicated that LPS caused a 4-fold increase in succinate compared to control cells.However, the fish oil administration caused 2- to 4-fold decrease in succinate compared to LPS-induced cells (Figs.6C and 7A-E).Succinate stabilizes the transcription factor HIF-1α in activated macrophages.Thus, the expression of HIF-1α was analyzed by qPCR and Western blot.The qPCR analysis revealed that LPS significantly increased the expression level ofHIF-1αmRNA, whereas the fish oil treatment remarkably decreased the expression ofHIF-1αmRNA (Fig.6A), which was confirmed by Western blot analysis (Fig.6B).
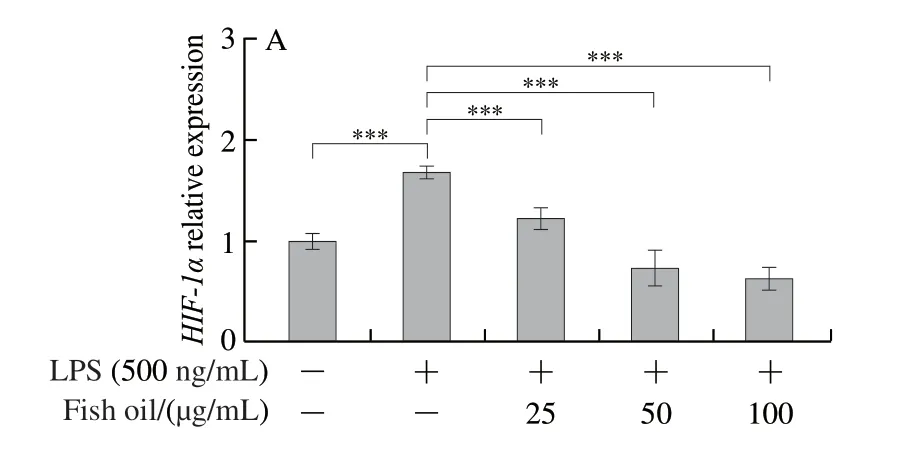
Fig.6 Effect of fish oil on HIF-1α mRNA expression (A), HIF-1α protein expression (B) and succinate concentration (C)
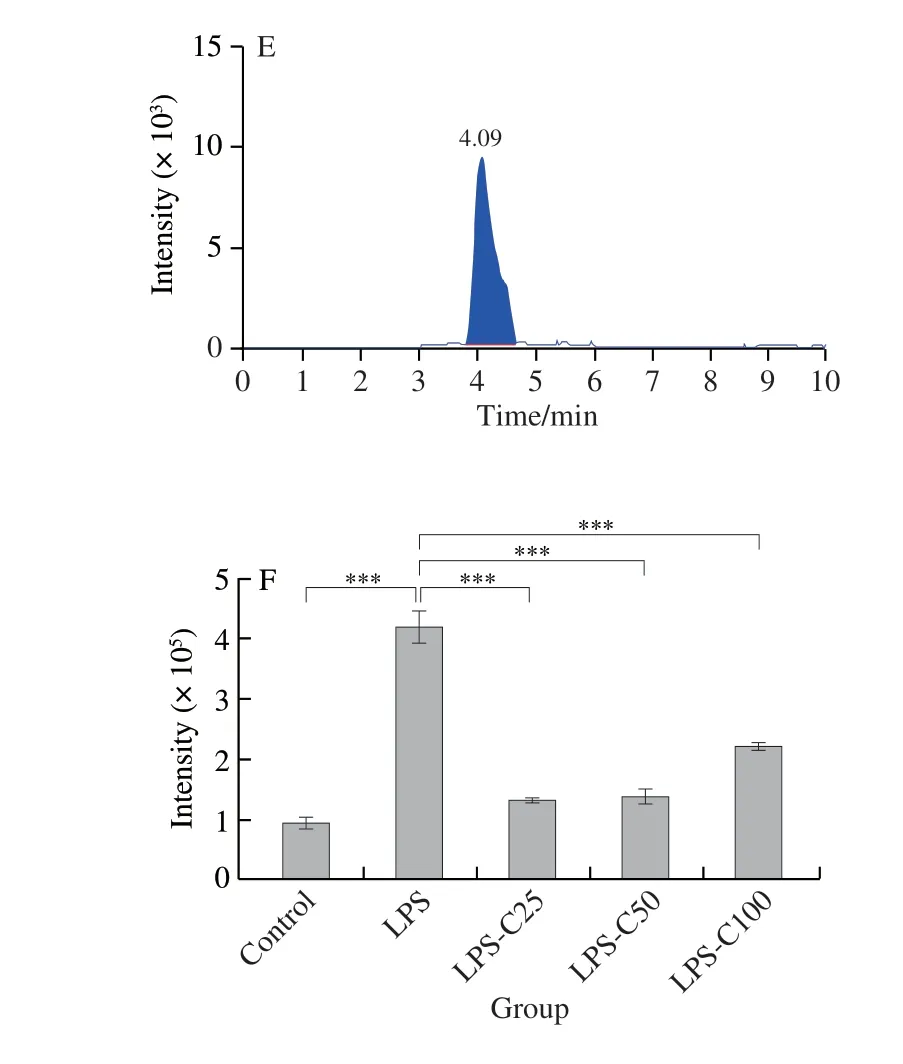
Fig.7 LC-MS chromatograms of the TCA cycle intermediate succinate in mouse macrophages
3 Discussion
It is well known that macrophages are important mediators of the innate immune response to microorganism invasion or damage, as well as a key player in inflammatory responses that can lead to a variety of diseases[32-33].Previous research had shown that M1 and M2 macrophages could express distinct markers[34].Boer et al.[35]found that macrophages treated with fish oil had decreased lipid uptake and mRNA expression of M1 markers in the acute or chronic inflammation model.Kawano et al.[36]reported that DHA from fish oil resulted in upregulation of M2 macrophage markers and increased secretion of anti-inflammatory cytokines by U937 cells.In addition, DHA enhanced the efferocytosis of RAW264.7 cells and inhibited LPS-induced production of pro-inflammatory cytokines, which suggested that DHA altered the macrophage phenotype in favor of M2 while it suppressed M1 polarization[36-37].Consistent with previous studies, the present results also revealed fish oil treatment reduced the expression of M1 phenotypic markers while increasing the expression of M2 phenotypic markers,resulting in the resolution of the inflammatory process.The significance of NF-κB signaling in inflammation had been established and received considerable attention.When activated by LPS, macrophages produce large amounts of cytokines, chemokines, and antiapoptotic proteins[38].The activation of the NF-κB signaling pathway plays an important role in the polarization of M1 and M2 macrophages[39-40].NF-κB activation in M1 macrophages has been shown to drive the initial significant inflammatory phase, whereas at the late stage of endotoxin tolerance, macrophages were polarized into an anti-inflammatory M2 phenotype and NF-κB activation was inhibited[41].Our findings suggest that fish oil can polarize macrophages from M1 to M2 and exert antiinflammatory effects by inhibiting the NF-κB signaling pathway.
According to some studies, macrophage polarization is mediated by a variety of transcription factors, with STAT1 playing a critical role in modulating macrophage M1/M2 polarization[42-43].Bonilla et al.[44]reported that DHA from fish oil could downmodulate the IFN-γ-mediated STAT1 signaling pathway by decreasing STAT1 phosphorylation in murine-macrophage-like J774.A1 cells.Wang Xiaofeng et al.[45]found thatn-3 PUFAs could significantly suppress the expression of STAT1.In our study, we discovered that the level of p-STAT1 protein and the ratio of p-STAT1 to STAT1 were reduced, despite the fact that the level of STAT1 showed no obvious changes with fish oil treatment in IFN-γ and LPS-induced M1 macrophages, which was consistent with previous reports[46-47].However, the upstream mechanisms of STAT1 regulation in macrophage polarization remain unknown.
Succinate, as an intermediate in the TCA cycle, has received increased attention in the field of signal transduction in recent years[25].Succinate was found to be elevated in LPS-induced inflammation, leading to the stabilization of HIF-1α and the high expression of IL-10, which was consistent with our findings.Additionally, we found that succinate content was decreased together with lower expression of HIF-1α and IL-1β with fish oil treatment in LPS-induced RAW264.7 cells compared to LPS group.Studies have reported that NF-κB is the key transcriptional regulator of HIF-1α, and NF-κB activation is critical in the induction of HIF-1α and the expression of target genes[48].As a result, the succinate/HIF-1α signaling pathway may play an important role in the anti-inflammatory process of fish oil, which was partly mediated by inhibiting the NF-κB signaling pathway.
Previous studies have shown that natural compound could polarize macrophages toward M1 or M2 phenotype in metabolic inflammation[49-53].In present study, it was found that fish oil inhibited the activation of M1 macrophages and promoted macrophages toward M2 phenotypes, thereby alleviating LPS-induced inflammation[54].Nevertheless,we mostly focused on how the mixed FA in fish oil fromC.peledsynergistically polarized macrophages to show anti-inflammatory effect.The content and composition of FA from fish oil were analyzed in detail, though the activity-responsible FA is not clarified.The results showed that FA were dominantly composed of myristic acid, palmitic acid, palmitoleic acid, oleic acid, EPA, and DHA in fish oil fromC.peled.Previous studies had shown these different FA possessed anti-inflammatory and immunomodulatory effect[55-58].Moreover, we just think it is unusual and interesting thatC.peled, as a freshwater fish, is rich in EPA and DHA and do not think EPA and DHA are directly responsible to inflammation in LPS-induced macrophages.Therefore, in this study, we only showed that the mixed FA in fish oil may shift macrophage polarization away from the M1 to M2 phenotype by inhibiting NF-κB, STAT1, and succinate/HIF-1α signaling pathways to exhibit its anti-inflammatory activity, but we did not determine which constituents were contributed to immunomodulatory activity in fish oil fromC.peled.Further investigations need to be made to identify which constituents are contributed to immunomodulatory activity and why in future study.
In summary, in this study, we investigated the immunomodulatory effect of fish oil fromC.peledon LPSinduced RAW264.7 cells.LPS induced macrophages to produce a large number of pro-inflammatory factors such as TNF-α, IL-1β, IL-6, and NO, which was interfered by fish oil.Fish oil might shift macrophage polarization away from the M1 to M2 phenotype by inhibiting NF-κB, STAT1,and succinate/HIF-1α signaling pathways to exhibit its antiinflammatory activity.
