Preparation of antibacterial non-woven AgNCs@PP-g-PAA via radiation method
2023-10-13FeiHanWenRuiWangDanYiLiSiYiXuYingSuLinLinManLiLuJiHaoLiLinFanLi
Fei Han · Wen-Rui Wang · Dan-Yi Li · Si-Yi Xu · Ying Su · Lin Lin · Man-Li Lu · Ji-Hao Li,3 ·Lin-Fan Li,3
Abstract With the growing threat of airborne epidemics, there has been an increasing emphasis on personal protection.Masks serve as our primary external defense against bacteria and viruses that might enter the respiratory tract.Hence, it’s crucial to develop a polypropylene (PP) nonwoven fabric with quick antibacterial capabilities as a key component for masks.This study introduces silver nanoclusters (AgNCs) into non-woven PP using radiation technology to infuse antibacterial properties.Initially, a solid ligand (PP-g-PAA) was procured via radiation grafting of the ligand polyacrylic acid (PAA), which was incorporated into the nonwoven PP with the aid of a crosslinking agent at a lower absorbed dosage.Subsequently, AgNCs were synthesized in situ on PP-g-PAA via an interaction between PAA and AgNCs, leading directly to the formation of AgNCs@PP-g-PAA composites.Owing to the hydrophilicity of PAA, AgNCs@PP-g-PAA maintains good moisture permeability even when the voids are heavily saturated with PAA gel, preventing droplet aggregation by diffusing droplets on the surface of the material.This feature enhances the comfort of the masks.Most importantly, due to the incorporation of AgNCs, AgNCs@PP-g-PAA demonstrates outstanding antibacterial effects against Escherichia coli and Staphylococcus aureus, nearly achieving an instant“touch and kill” outcome.In conclusion, we synthesized a modified nonwoven fabric with significant antibacterial activity using a simple synthetic route, offering a promising material that provides improved personal protection.
Keywords Silver nanoclusters · Radiation technology · Antibacterial
1 Introduction
Many infectious diseases, particularly respiratory ones, are often spread via droplets or airborne particles, leading to extensive transmission [1, 2].The airborne transmission of infectious respiratory droplets is widely recognized as a source of infection in diseases prone to causing pandemics,such as SARS, influenza A, and Middle East respiratory syndrome [3–5].This realization has prompted individuals to place greater emphasis on personal protection to safeguard themselves from illnesses.Therefore, public health experts recommend the use of surgical masks as a barrier to prevent viruses from entering the respiratory tract, subsequently reducing infection risk [6].Individuals wear medical masks as a protective measure [7, 8].However, with increasing health awareness, there is also a rising demand for antibacterial properties in protective materials.
Silver has been used as a bactericidal agent long before the advent of antibiotics [9].There is no single bacterium that has developed a universal resistance to silver, underscoring the broad-spectrum antibacterial properties of silverbased materials [10].With advancements in nanotechnology, diversely structured and purposed silver nanoparticles(AgNPs) have been successfully synthesized [11–13].Due to their small size and high specific surface area, AgNPs have been extensively employed in creating various bactericidal and antibacterial materials [14–16].For instance,Shi et al.embedded silver nanoparticles into delaminated hectorite, resulting in a composite material that realized swift bactericidal activity by disrupting bacterial cell walls[17].Sandu et al.used AgNPs to treat materials, which displayed antibacterial properties and impressive resistance to washing [18].Silver nanoclusters (AgNCs) are a novel type of nanomaterial with smaller particle sizes and unique physicochemical properties such as photoluminescence [19].Compared to AgNPs, AgNCs exhibit smaller sizes, thereby offering a higher specific surface area and stronger bactericidal activity [21].The bactericidal effect of AgNCs onE.colisurpasses that of AgNPs at the same silver ion concentration [9, 21].This suggests that fewer AgNCs are required to realize effective antibacterial results.Therefore, compared to AgNPs, AgNCs exhibit more potent bactericidal activity and, more significantly, lower cytotoxicity [22].Shang et al.developed a silver nanocluster hydrogel with high biocompatibility and enhanced synergistic antibacterial capabilities, providing a fresh, universal strategy for designing highperformance antibacterial dressings to treat a wide array of infectious diseases [23].As noted, AgNCs have emerged as outstanding candidates for bactericidal agents due to their broad-spectrum properties and high bactericidal efficiency.
Polypropylene (PP) is a thermoplastic polymer material with outstanding properties, widely employed in the field of air filtration [22].Polypropylene non-woven material exhibits a random network of intersecting fibers and multi-connected pore structure.The material is known for its excellent thermal and chemical stability as well as low cost, and it is used in filtration devices and masks [24].The fiber network structure of non-woven PP can block virus-carrying droplets and other particles due to the smaller gaps in the fabric, thus playing a role in preventing viral contact.However, physical isolation alone does not entirely prevent viral infiltration.Furthermore, given that PP lacks antibacterial activity, bacteria can easily proliferate during the use of non-woven PP.This limitation affects the lifespan of medical masks and could potentially increase the risk of infection for users [25].To counter this, many researchers have adopted different surface treatments or modifications of nonwoven PP to realize effective antibacterial properties [26, 27].Currently, the production process of PP nonwoven fabrics is quite mature,making it a viable approach to enhance the antibacterial properties of these fabrics.
Loading silver nanoclusters (AgNCs) onto non-woven PP imbues the material with antibacterial properties.However,due to the small particle size and high activity of AgNCs,ligands are often necessary to prevent spontaneous aggregation [28].Nonetheless, the polyolefin structure of PP, its low surface energy, and absence of active sites render AgNC loading unfeasible [29].Radiation grafting, a material modification technique with wide-ranging applications and straightforward reaction conditions, is frequently used in modifying various polymer materials [30–33].Compared with other modification methods, such as chemical grafting, radiation grafting offers benefits, such as simpler synthesis conditions, universal applicability, and environmental friendliness [34–36].For instance,Li et al.used radiation grafting to modify a polyethylene microporous diaphragm with polyacrylamide to enhance the affinity between the diaphragm and electrolyte in lithium-ion batteries [35].Radiation reduction is another radiation technology, known for its uncomplicated synthesis process, and is extensively used in synthesizing metal nanoparticles [37–39].Lang et al.crafted an efficient catalyst by anchoring bimetallic Ag–Cu nanoparticles onto a polypropylene (PP) fabric via an environmentally friendly grafting reduction strategy [40].In our previous studies, we successfully prepared AgNC aqueous solutions using ligand chain polyacrylic acid (PAA) as a ligand through radiation reduction [41–44].Building upon this, we utilized radiation grafting technology to further modify commercial non-woven PP, thereby providing functional sites for the AgNC ligand to facilitate AgNC loading.In summary,radiation technology is a straightforward, effective, and universal method for endowing nonwoven PP with antibacterial properties.
In this study, we employed radiation technology to load AgNCs into commercial nonwoven polypropylene (PP) to impart antibacterial properties.Initially, we introduced the PAA into PP using radiation grafting technology.This provided ample loading sites for the AgNCs and enhanced the hydrophilicity of the material.The prepared modified PP (PPg-PAA) served as a solid ligand for the in situ synthesis of Ag nanoclusters.Silver nanocluster composites (AgNCs@PP-g-PAA) were directly obtained through radiation reduction.Owing to the coordination between the carboxyl groups in PAA and silver nanoclusters, the prepared AgNCs@PP-g-PAA successfully loaded the silver nanoclusters, displaying unique photoluminescence characteristics.Simultaneously,due to the introduction of PAA, the AgNCs@PP-g-PAA retained the moisture permeability of the original commercial PP non-woven fabric.This was the case even when the voidage decreased, as the material was able to quickly absorb and diffuse water on the surface, preventing the accumulation of water vapor.Most importantly, owing to the incorporation of AgNCs, the AgNCs@PP-g-PAA could rapidly killE.coliandS.aureus, essentially achieving a “touch and kill” effect.Through the use of radiation technology, we synthesized a modified nonwoven fabric with strong antibacterial capabilities via a simple synthetic route, offering better protection against viral infections.
2 Experimental section
2.1 Materials
Acrylic acid (AA), silver nitrate, N, N-methylene diacrylamide (MBA), and isopropyl alcohol were procured from Sinopharm Chemical Reagent Co., Ltd.(Shanghai, China).All chemicals and solvents used in the study were analytical reagents (AR).All chemicals and solvents were used in as-received condition without further purification.Polypropylene (PP) non-woven is a commercial PP material.Water used in all the experiments in the study was purified using a Millipore system.
2.2 Instrumentation
Ultraviolet–visible (UV–vis) spectra were obtained using a HITACHI U-3900 UV–vis spectrophotometer.The fluorescence spectra were recorded using a spectrofluorometer(FS5).FTIR analysis was performed using an FTIR650 spectrophotometer over a range of 600–4000 cm-1at a resolution of 2 cm-1.For the FESEM imaging study, a JSM-6700F instrument was used to capture the SEM images at an accelerating voltage of 10–15 kV.The EDS images were acquired at an accelerating voltage of 15 kV.Photographs of the materials were captured under a microscope.Valence analysis of the elements on the film surface was performed using a SCIENTIFICESCALAB 250Xi (XPS)and fitted using software.Thermogravimetry (TG) 209F4 was used to study the thermal properties of the material.
2.3 Preparation of PP-g-PAA
PP-g-PAA was prepared via impregnation and radiation grafting.The PP nonwoven fabric was cut to an appropriate size and cleaned with deionized water.Specifically,43.23 g of acrylic acid and 0.92 g of N,N′-methylene diacrylamide (MBA) was dissolved into 60 mL of deionized water using ultrasonication to produce the immersion solution.The pretreated PP nonwoven fabric was immersed in an immersion solution for full impregnation.After the fabric was removed, the non-woven PP, imbued with the immersion solution, was placed in a vacuum sealing bag and vacuum-sealed.Subsequently, it was radiated at 10 kGy absorbed doses using60Co.The entirety of the synthesis was conducted at room temperature.Following irradiation, the graft-modified PP (PP-g-PAA) was ultrasonically cleaned with deionized water for 15 min, during which the deionized water was changed three times.The membranes were then dried in an oven at 60 °C for 24 h and weighed again.The degree of grafting (DG) was determined by the percentage increase in the film’s weight,according to Eq.(1):
whereWgandW0denote the weights of the grafted and ungrafted membranes, respectively.
2.4 Preparation of AgNCs@PP-g-PAA
AgNCs@PP-g-PAA were prepared via radiation reduction.First, 0.1 M silver nitrate and 0.5 M isopropyl alcohol supported solutions were prepared via ultrasonic dissolution.PP-g-PAA was immersed in the support solution and incubated for 2 h.Subsequently, nitrogen was injected into the system for 10 min to remove oxygen.Finally, the samples were placed in a Co source and irradiated with60Co.The absorbed dose was 600 Gy.After irradiation, PP-g-PAA loaded with AgNCs (AgNCs@PP-g-PAA) was ultrasonically cleaned with deionized water for 6 min.The washed AgNCs@PP-g-PAA were dried naturally at room temperature.The degree of loading (DL) was determined as percentage increase in film weight based on Eq.(2):
whereWgandW0denote the weights of the loaded and unloaded membranes, respectively.
2.5 Moisture permeability test
The moisture permeability of the materials was assessed by measuring the moisture escape rate.An open test tube was injected with 40 mL of deionized water and sealed with PP, PP-g-PAA, and AgNCs@PP-g-PAA separately.The open samples were used as controls.Subsequently, the test tubes were placed in a constant temperature environment at 40 °C, and the quality of the test tubes was recorded at different times.The escape mass of the deionized water was calculated from the difference in the mass of the test tube over time.The ratio of the obtained mass to the escape mass of the open sample corresponded to the water vapor transmittance.
2.6 Antibacterial test
The antibacterial properties of PP, PP-g-PAA, and AgNCs@PP-g-PAA were evaluated usingE.coliandS.aureus.First, the samples were sterilized using an ultraviolet lamp for 15 min.The bacteria were inoculated onto the sterilized material.Samples were collected before inoculation, after exposure, and 24 h after exposure, and bacterial concentrations were measured using an enzyme marker.The bacterial survival rate was calculated as the ratio of the bacterial concentration in the sample to that of the inoculated bacteria.
3 Results and discussion
3.1 Preparation of AgNCs@PP-g-PAA
In this study, silver nanoclusters (AgNCs) were introduced onto non-woven polyacrylic acid (PAA)-modified polypropylene (PP) using radiation technology, thereby bestowing the material with potent antibacterial properties.The specific synthesis route is illustrated in Fig.1.The materials were prepared employing a two-step method.Initially,PAA was grafted onto nonwoven PP utilizing the impregnation method.Additionally, through the introduction of the crosslinking agent, N, N-methylene diacrylamide (MBA),nonwoven PP could acquire sufficient sites for subsequent AgNC loading.In the second step, silver ions were reduced in situ on PP-g-PAA by radiation to accomplish AgNC loading, thereby obtaining non-woven AgNCs@PP-g-PAA.
The introduction of PAA provides suitable loading sites for AgNCs, which is very important for the preparation of AgNC composites.First, the introduction of PAA was confirmed via infrared (IR) spectroscopy.As shown in Fig.2a,when compared with the original PP spectrum, the spectrum of PP-g-PAA shows a new characteristic peak at 1700 cm-1.The characteristic peak at 1700 cm-1was the absorption peak of the carbonyl group, which originated from the grafted-chain PAA.This indicates the successful introduction of PAA.Owing to the introduction of the crosslinking agent MBA, the spectrum of PP-g-PAA also showed an absorption peak of the amide group at 1250 cm-1.This indicates that MBA was successfully introduced into the graft chain of PAA to form a cross-linked network.The addition of MBA was conducive to the introduction of PAA into the material.Polypropylene (PP) is a radiation-cracking material[45].A higher absorbed dose leads to the destruction of the PP structure, which is unable to maintain its performance.Radiation grafting is a radical polymerization mechanism[46].A higher absorbed dose is more beneficial for grafting PAA.Hence, to preserve the performance of the polypropylene (PP) matrix material while introducing sufficient polyacrylic acid (PAA), a cross-linking agent, N, N-methylene diacrylamide (MBA), was incorporated in this synthesis process.This led to the formation of a PAA cross-linking network within the matrix material, enabling the material to secure ample loading sites even at a low absorbed dose.As illustrated in Fig.S1a, without the addition of MBA, PP-g-PAA demonstrates significant weight loss due to radiationinduced PP cracking.This made it challenging to obtain adequate PAA ligands for ligand materials.After introducing MBA, the mass of the ligand materials increased.Regardless of the inclusion of MBA, the absorption peak of the PAA carboxyl group was observed in the infrared spectra of PPg-PAA, indicating successful introduction of PAA into PP(Fig.S1b).However, due to the integration of MBA, more PAA could be introduced into PP, enabling PP-g-PAA to provide more ligands, which is beneficial for silver nanocluster loading.Additionally, as demonstrated in Fig.S2,the mechanical properties of conventional PP significantly decreased after irradiation due to radiation cracking.This phenomenon was particularly pronounced at higher absorbed doses.After PAA modification, the tensile strength of PPg-PAA was higher than that of unmodified PP at the same absorbed dose, and it was nearly equivalent to that of the original PP due to the grafting and cross-linking network of PAA (Fig.S2).In conclusion, the incorporation of PAA allowed the material to maintain its original mechanical strength, which is beneficial for preserving its performance.

Fig.1 (Color online) Synthetic route of AgNCs@PP-g-PAA

Fig.2 (Color online) a Infrared spectra of PP, PP-g-PAA, AgNCs@PP-g-PAA; b ultraviolet–visible spectra of PP, PP-g-PAA, AgNCs@PP-g-PAA; c the fluorescence spectra (excitation spectra and emission spectra) of AgNCs@PP-g-PAA; d fluorescence spectra of AgNCs@PP-g-PAA at different excitation wavelengths; e Instantaneous fluorescence spectrum of AgNCs@PP-g-PAA; f XPS spectrum of PP, PP-g-PAA, AgNCs@PP-g-PAA; g XPS Ag3d spectrum of AgNCs@PP-g-PAA; h XPS O1s spectrum of PP-g-PAA; i XPS O1s spectrum of AgNCs@PP-g-PAA
Using a solid PP-g-PAA ligand as a basis, AgNCs@PPg-PAA was in-situ prepared via radiation reduction.The loading of AgNCs was further corroborated through ultraviolet–visible and fluorescence spectroscopies.As depicted in Fig.2(b), the spectra of unmodified non-woven PP and PP-g-PAA display a high degree of similarity in the range of 300–800 nm, implying that the introduction of PAA does not alter the UV absorption of the material.However, after incorporating AgNCs, a very faint absorption peak was observed at 500 nm.The extremely low intensity of the absorption peak could be due to the minimal AgNC loading (only 8%).More direct evidence is derived from the fluorescence spectrum shown in Fig.2c.Upon excitation at 540 nm, AgNCs@PP-g-PAA exhibited noticeable fluorescence emission at 640 nm, directly indicating the presence of fluorescent AgNCs.This also validates that the AgNCs were loaded into AgNCs@PP-g-PAA.The fluorescence emission of AgNCs@PP-g-PAA exhibits a distinct excitation wavelength dependence; that is, the fluorescence emission of AgNCs@PP-g-PAA varies slightly under excitation at different wavelengths.As depicted in Fig.2d, AgNCs@PP-g-PAA showed two fluorescence emission peaks at 600 nm and 700 nm when excited at 500 nm.As the excitation wavelength increased, the fluorescence emission of AgNCs@PPg-PAA gradually faded at 700 nm, while the fluorescence emission at 600 nm exhibited a noticeable red shift, coupled with a decrease in fluorescence intensity (Fig.2d).This could be attributed to the fact that the prepared AgNCs comprise a mixture of nanoclusters with varying numbers of Ag atoms.Nanoclusters with different numbers of silver atoms demonstrate different particle sizes, leading to different fluorescence emissions.Upon excitation, the fluorescence emission of these AgNCs formed the fluorescence emission of AgNCs@PP-g-PAA.The quantum yield of AgNCs@PPg-PAA was a mere 1.16%.The transient fluorescence spectra indicated that the fluorescence lifetime of AgNCs@PP-g-PAA was 0.87 ns (Fig.2e).
The interaction between the ligands and AgNCs in AgNCs@PP-g-PAA is critical for realizing a stable loading.The presence of AgNCs was investigated by analyzing the alterations in the chemical states of each element during the material synthesis process.As depicted in Fig.2f, elemental C and trace element O are present in the unmodified PP.C originates from the backbone of the PP molecular chain,while O could come from additives used in the production of commercially available PP.After introducing PAA, PP-g-PAA displayed only C and O peaks (Fig.2f).The XPS C1s spectra of the material were further analyzed.As shown in Fig.S3a, the C in commercial PP exhibits only one chemical state, derived from the PP carbon chain skeleton.After the introduction of PAA, a carbonyl carbon appeared in PP-g-PAA (Fig.S3b), which was attributed to the carboxyl functional group of the grafted PAA chain.Following further loading of AgNCs, a peak corresponding to Ag emerged in the AgNCs@PP-g-PAA spectrum (Fig.2f).Additionally,through analysis of the XPS Ag3d spectrum, it was determined that Ag exhibited zero valence, further confirming the formation of AgNCs (Fig.2g).However, the changes in the XPS O1s spectra during the synthesis of the material were more intriguing.As shown in Fig.2h, before the loading of AgNCs, only two chemical states of oxygen are present in PP-g-PAA: hydroxyl oxygen and carbonyl oxygen.Both originated from the carboxyl group of the grafted PAA chain.However, after the loading of AgNCs, the half-peak width of the original spectral line of O1s in AgNCs@PP-g-PAA became significantly broader, and thereby, a new fitting peak appeared in the spectral diagram (Fig.2i).It is well known that the carboxyl group in PAA can coordinate with metal particles or atoms due to the lone pair of electrons on the oxygen atoms [47, 48].Hence, it is believed that the newly emerged fitting peak resulted from the interaction between the AgNCs and carboxyl group in PAA, leading to a change in the chemical state of O.This also demonstrates that the introduction of the PAA ligand is advantageous for achieving the loading of AgNCs on non-woven PP.
The morphologies of the synthesized materials were investigated using SEM and EDS.Figure 3a shows that a commercial PP nonwoven fabric was constructed by stacking PP fibers.Due to the standardized industrial processing,a rhomboid plane exists on the surface of the non-woven PP.The mutual overlap of the PP fibers results in an interconnected gap in the PP nonwoven fabric, allowing for air flow.Furthermore, based on the stacking density, the PP nonwoven fabric can serve as a barrier against materials of varying particle sizes.Upon closer inspection, the platform and fiber monofilament parts of the PP exhibit a rough surface (Fig.3a).After the integration of PAA, single-chain grafting on PP is achievable, and PAA can also attach to PP in the form of a crosslinking network.Figure 3a reveals the presence of numerous gel-like substances on PP-g-PAA, filling the original voids, which can be attributed to the introduction of the PAA cross-linking network.In PP-g-PAA,the platform and monofilament portions of the structure are enveloped by PAA, unlike in the unmodified state (Fig.3a).Following further loading of AgNCs, the morphology of AgNCs@PP-g-PAA mirrors that of PP-g-PAA.In AgNCs@PP-g-PAA, PAA continues to envelop the monofilament and platform, and a significant amount of PAA gel can be observed in the voids (Fig.3a).The distribution of elements on the material surface was further investigated using EDS.An O peak appeared on the PP surface in the XPS spectrum(Fig.1f).The original commercial nonwoven PP fabric was also tested.As shown in Fig.3b , C and O signals are present in the original PP, with O likely originating from the filler used during the commercial PP preparation process.After introducing PAA, the O signal in PP-g-PAA became more intense and was concentrated in the PAA-filled region(Fig.3b).After the AgNCs loading, AgNCs@PP-g-PAA exhibited not only a uniform distribution of O but also a discrete distribution of Ag (Fig.3b).The discrete distribution of Ag also supports the presence of AgNCs in AgNCs@PPg-PAA.Based on the EDS images (Fig.S4), it is observable that the relative content of AgNCs in the non-woven fibers is higher than that in the plane formed by material processing.This supports the contact between AgNCs and bacteria, thus facilitating the antibacterial function.
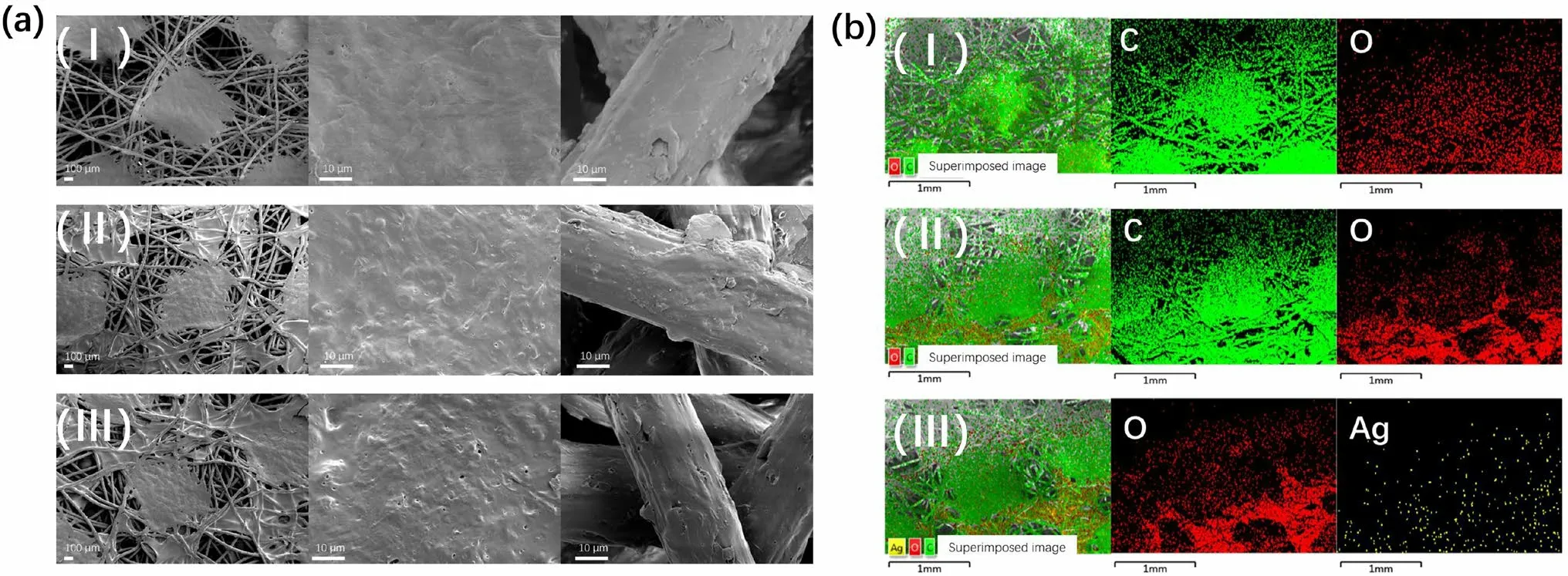
Fig.3 (Color online) a SEM image: (I) PP, (II), PP-g-PAA, (III), AgNCs@PP-g-PAA; b EDS images: (I) PP, (II), PP-g-PAA, (III), AgNCs@PP-g-PAA
The thermal properties of the materials were examined using kernel-derivative thermogravimetric curves.As depicted in Fig.S5, the unmodified non-woven PP exhibits significant thermal decomposition between 400 and 480 °C.This is attributed to the thermal decomposition of the carbon chain at high temperatures.After the incorporation of PAA,PP-g-PAA starts to display noticeable thermogravimetric behavior at 200 °C (Figure S4), resulting from the decarboxylation of the PAA graft chain.Concurrently, at the thermal decomposition temperature of PP, PP-g-PAA continues to show evident thermal decomposition of the carbon chain.Upon further loading of AgNCs, the thermal decomposition behavior of AgNCs@PP-g-PAA aligns with that of PP-g-PAA.
3.2 Moisture permeability of materials
As depicted in Fig.3, the incorporation of PAA results in the occupancy of numerous voids in the material by PAA gel, thereby visibly reducing the number of voids.This had a negative impact on the internal gas flow within the material.As shown in Fig.4a, the material’s porosity decreases substantially after the integration of PAA and loading of AgNCs.Furthermore, traditional PP materials exhibit poor hydrophilicity.When used in masks, water droplets can easily form on the material surface due to the substantial amount of water vapor generated during respiration, leading to significant discomfort during usage.For AgNCs@PP-g-PAA, the passage for water vapor was limited because PAA filled many of the voids.Hence, considering this, it is expected that, following the incorporation of PAA and AgNCs, the material will retain its moisture permeability under operational conditions.According to moisture permeability tests, PP’s water vapor permeability was only a quarter of that of the control sample without the covering material (Fig.4b).However, despite the reduction in voids, the introduction of PAA did not further decrease the material’s moisture permeability, which remained similar to that of commercial non-woven PP (Fig.4b).After the AgNCs loading, which did not significantly impact the material’s morphology (Fig.4b), the moisture permeability of AgNCs@PP-g-PAA remained stable.We hypothesize that the maintenance of material moisture permeability might be due to the improved material hydrophilicity brought about by the introduction of PAA.A surface tensiometer was used to measure the contact angle and its temporal variation.
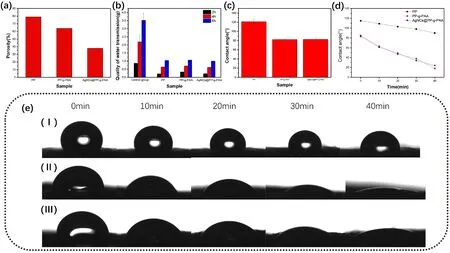
Fig.4 (Color online) a Voidage ratio of PP, PP-g-PAA, AgNCs@PPg-PAA; b moisture permeability of PP, PP-g-PAA, AgNCs@PP-g-PAA; c contact angle of PP, PP-g-PAA, AgNCs@PP-g-PAA; d variation in contact angle with respect to time of PP, PP-g-PAA, AgNCs@PP-g-PAA; e image of contact angle change with respect to time: (I)PP, (II) PP-g-PAA, and (III) AgNCs@PP-g-PAA
As depicted in Fig.4c, the commercial PP nonwoven fabric displays significant hydrophobic properties, with a contact angle of 120°.However, after the introduction of PAA, due to the presence of numerous polar carboxyl groups in PAA, the contact angle of PP-g-PAA decreased to around 80°, indicating pronounced hydrophilicity (Fig.4c).The loading of AgNCs had slight effect on the materials’contact angle, and AgNCs@PP-g-PAA maintained a contact angle similar to that of PP-g-PAA (Fig.4c).Furthermore, as the contact duration between the water droplets and materials increased, the contact angles of AgNCs@PP-g-PAA and PP-g-PAA gradually decreased from an initial 83° to approximately 20° after 40 min (Fig.4d).However, the water droplets on PP only decreased from 120° to around 100° (Fig.4d).A more visual representation of this process is shown in Fig.4e.Over time, the water droplets gradually permeated into AgNCs@PP-g-PAA, whereas the water droplets on the PP surface maintained their original state.In this context, we propose that due to the incorporation of PAA, the material becomes more hydrophilic, enabling water droplets to easily diffuse into the voids of the fiber network and preventing water droplet accumulation on the material surface.Given that during the actual usage of the mask, the water droplets formed by surface-carried water vapor are smaller and diffuse more readily into the material gaps, the diffusion time should be shorter than the test time used in this example.Moreover, water vapor diffused into the material is likely to be transported away from the skin side by airflow, thereby preventing droplet adherence to the material surface.
3.3 Antibacterial properties of materials
AgNCs@PP-g-PAA demonstrated antibacterial activity as a result of AgNCs incorporation (Fig.5a).Escherichia coli(E.coli) andStaphylococcus aureus(S.aureus) were used as test subjects.The survival rates of these different bacteria in PP, PP-g-PAA, and AgNCs@PP-g-PAA were evaluated according to the relevant test standards to assess their antibacterial activities.As depicted in Fig.5b, the conventional commercial PP nonwoven fabric does not exhibit any antibacterial properties.E.coliwas able to maintain its vitality following exposure to non-woven PP and after 24 h, it continued to reproduce, with the bacterial survival rate reaching 152%.This indicates that non-woven PP is ineffective in inhibiting bacterial proliferation.Upon introduction of PAA, PP-g-PAA did not immediately eliminateE.coli, but it realized a near-100% antibacterial rate after 24 h.This might be attributable to the presence of amide groups in the material, resulting from the introduction of the MBA crosslinking agent, a phenomenon discussed in previous reports [49].However, despite its antibacterial activity, PPg-PAA failed to eliminate bacteria promptly and hence did not meet the actual performance requirements.Following the incorporation of AgNCs, AgNCs@PP-g-PAA accomplished nearly 100% bacterial extermination shortly after coming into contact withE.coli, demonstrating potent antibacterial activity.ForS.aureus, PP, PP-g-PAA, and AgNCs@PP-g-PAA showed similar antibacterial characteristics to those observed forE.coli.As indicated in Fig.5c, PP does not eliminateS.aureus.Although PP-g-PAA displayed bactericidal capability, it took a considerable amount of time to take effect.Conversely, AgNCs@PP-g-PAA was able to demonstrate a near-instant “touch and kill” antibacterial capacity.This rapid bactericidal ability could be attributed to the extremely small AgNCs; their high specific surface area allows for a faster Ag+release [9].In summary, the introduction of AgNCs resulted in AgNCs@PP-g-PAA rapidly exterminating bacteria and exhibiting excellent antibacterial performance.
4 Conclusion
Capitalizing on the antibacterial activity of silver nanoclusters (AgNCs), we designed and prepared a modified polypropylene (PP) nonwoven fabric with antibacterial properties.AgNC-modified nonwoven samples, referred to as AgNCs@PP-g-PAA, were successfully produced through radiation grafting and radiation reduction.The employment of crosslinkers in the synthetic route allowed for the introduction of ample loading sites for AgNCs at lower absorbed doses.The interaction between polyacrylic acid (PAA) and AgNCs facilitated the stable loading of AgNCs into AgNCs@PP-g-PAA, endowing the material with an antibacterial function.Simultaneously,the voids within the material were filled with PAA gel.Owing to PAA’s hydrophilicity, the moisture permeability of AgNCs@PP-g-PAA was maintained, enabling surface water vapor to diffuse into the material.This feature contributes to the material’s comfort when utilized as a mask.Moreover, the exceptional antibacterial properties of AgNCs implies that AgNCs@PP-g-PAA can effectively killE.coliandS.aureusafter brief contact, essentially achieving a “touch and kill” effect.In this study, we synthesized a modified nonwoven fabric with robust antibacterial capability through a straightforward synthetic route using radiation technology.This provides a promising candidate material with enhanced protective performance for preventing viral infections.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s41365- 023- 01292-2.
Author contributionsAll authors contributed to the study conception and design.Material preparation, data collection and analysis were performed by Fei Han, Jihao Li and Linfan Li.The first draft of the manuscript was written by Fei Han and all authors commented on previous versions of the manuscript.All authors read and approved the final manuscript.
Data availabilityThe data that support the findings of this study are openly available in Science Data Bank at https:// www.doi.org/ 10.57760/ scien cedb.j00186.00176 and https:// cstr.cn/ 31253.11.scien cedb.j00186.00176.
Declarations
Conflicts of interestThere is no conflict of interest to declare.
杂志排行
Nuclear Science and Techniques的其它文章
- Dynamic scaling characteristics of single-phase natural circulation based on different strain transformations
- Comparison between 4D robust optimization methods for carbon-ion treatment planning
- Aperture shape optimization in intensity-modulated radiation therapy planning
- Calculation of microscopic nuclear level densities based on covariant density functional theory
- Employing adaptive fuzzy computing for RCP intelligent control and fault diagnosis
- EJUSTCO: Monte Carlo radiation transport code hybrid with ANN model for gamma-ray shielding simulation
