Magnetically Separable Straw@Fe3O4/Cu2O Composites for Photocatalytic Degradation of Methyl Orange under Visible Light Irradiation
2023-10-07WANGJingjingZHANGYawen
WANG Jingjing ,ZHANG Yawen
(1.School of Materials Science and Engineering, Yancheng Institute of Technology, Yancheng 224051, China; 2.Key Laboratory for Ecological-Environment Materials of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051, China)
Abstract: Fe3O4 and Cu2O were successively immobilized on alkali-treated straw,and the magnetically separable straw@Fe3O4/Cu2O composite was obtained.The straw@Fe3O4/Cu2O was characterized by Fourier transform infrared spectroscopy,X-ray diffraction,scanning electron microscopy,X-ray photoelectron spectroscopy and vibrating sample magnetometry,respectively.Photocatalytic performance of the straw@Fe3O4/Cu2O was evaluated by measuring the degradation of methyl orange (MO) under irradiation of visible light.The introduction of Fe3O4 not only endowed the straw@Fe3O4/Cu2O with magnetic separation feature but also significantly enhanced photocatalytic activity because Fe3O4 could prevent recombination of hole-electron pairs.The active species capture experiment showed that holes (h+),hydroxyl (·OH) and superoxide (·O2ˉ) radicals all took part in the MO degradation.In addition,the photocatalytic mechanism of straw@Fe3O4/Cu2O was proposed based on the experimental results.After five cycles for the photodegradation of MO,the straw@Fe3O4/Cu2O still displayed good photocatalytic activity,suggesting that the as-prepared composite had great potential for practical use in wastewater treatment.
Key words: straw;Cu2O;Fe3O4;photocatalysis;visible light
1 Introduction
As morden industry develops,huge amount of wastewater containing various organic contaminants has attracted worldwide attention.These organic pollutants are usually toxic and non-biodegradable,which could cause disadvantageous effects on aquatic life and human health[1,2].Therefore,the wastewater must be treated before discharge into water streams.
Photocatalysis has been attracting growing interest because it provides a new and promising way to meet the environmental challenges of energy and sustainability[3,4].As for photocatalytic technology,development of high performance photocatalysts with good photocatalytic efficiency and recyclability is of great importance.In the last decades,photocatalysts based on semiconductor have been widely investigated owing to their applications in degradation of organic pollutants[5,6].
Cuprous oxide (Cu2O),which is an environmentally friendly semiconductor,could be activated by visible light and exhibits photocatalytic activity for degradation of organic pollutants[7,8].However,the application of Cu2O photocatalyst is often restricted because recombination of photogenerated electronhole pairs and instability are always involved in photocatalytic reactions.Nanosized Fe3O4has also been employed for removal of pollutants because of its high adsorption capacity as well as its easiness for separating and recycling[9].Furthermore,nanosized Fe3O4could reduce the recombination of electron-hole pairs in photocatalytic reactions,thus improving quantum efficiency[10].Therefore,the Fe3O4nanomaterial could be considered as a prospective candidate for wastewater purification.
Nevertheless,one major problem associated with nanomaterials lies in their nature of easy aggregation,which could ultimately deteriorate their unique properties and often significantly weaken their activity when they are employed as photocatalysts.Consequently,some materials such as zeolites,clays and polymers have been used as carriers for immobilizing nanosized photocatalysts[11-13].
Rice straw,which is low-cost and abundantly available,could also be used as host for nanomaterials due to its relatively large surface area.For example,Huang used an alkali modified rice straw fiber as a carrier of TiO2photocatalyst,and studied photocatalytic performance of this material[14].
In this study,Fe3O4and Cu2O were immobilized successively on alkali-treated straw,and the obtained magnetically separable straw@Fe3O4/Cu2O composite was used as photocatalyst to degrade methyl orange(MO) under visible light irradiation.The photocatalytic activity of the straw@Fe3O4/Cu2O was systematically studied and the photocatalytic mechanism of straw@Fe3O4/Cu2O was proposed based on the experimental results.
2 Experimental
2.1 Materials
Rice straw was collected from a suburb near Yancheng,China.Urea,FeCl3·6H2O,FeCl2·4H2O,NaOH,NH2OH·HCl,CuCl2were purchased from Aladdin Chemistry Co.Ltd.
2.2 Synthesis of straw@Fe3O4/Cu2O composite
In the present research,alkali-treated straw was used as photocatalyst carrier.For this purpose,rice straw was firstly crushed,soaked in NaOH solution and digested for 2 h at 100 ℃.Then,the alkali-treated straw was washed thoroughly with deionized water and dried under reduced pressure.
Straw@Fe3O4was prepared according to previous report[15].Firstly,constant amount of the alkali-treated straw was dispersed in deionized water,then 2.0 mol/L solution of urea was added into the suspension as stabilizing agent.Subsequently,FeCl3·6H2O and FeCl2·4H2O were added into the above suspension with vigorous stirring under nitrogen atmosphere to prevent oxidation of Fe2+.Finally,NaOH solution (2.0 mol/L)was poured into the mixture under continuous stirring.The final product was centrifuged,washed with deionized water and dried under reduced pressure.
For the preparation of straw@Fe3O4/Cu2O,the as-prepared straw@Fe3O4was dispersed in 100 mL of deionized water.Then,20 mL of 0.1 mol/L CuCl2solution was added into the dispersion with vigorous stirring.The above mixture was sonicated for 30 min,and 25 mL of 1.0 mol/L NaOH solution was introduced dropwise under sonication.Subsequently,30 mL of 0.1 mol/L NH2OH·HCl was quickly injected into the suspension,which was kept in water bath for 1 h.Finally,the suspension was centrifuged,and the obtained precipitate was well washed with distilled water.After being dried under reduced pressure,the straw@Fe3O4/Cu2O was obtained.The whole preparation process of the straw@Fe3O4/Cu2O was shown in Fig.1.For comparison,straw@Cu2O was prepared according to the above procedure without immobilization of Fe3O4.

Fig.1 Illustration for the synthesis procedure of the straw@Fe3O4/Cu2O
2.3 Characterization
Fourier transform infrared (FTIR) spectra were recorded using a Bruker Vector 22 spectrometer.X-ray diffraction (XRD) patterns were obtained using a Bruker KAPPA APEX II X-ray diffractometer with Cu Kα radiation.Scanning electron microscopy (SEM,Hitachi S-4800,Japan) was used to analyze morphology of samples.X-ray photoelectron spectroscopy (XPS)measurements were carried out using a Perkin-Elmer PHI 550 ESCA/SAM spectrometer.Magnetic property was measured on a LakeShore 7404 vibrating sample magnetometer (VSM) at room temperature.
2.4 Photocatalytic degradation tests
In order to study photocatalytic performance,50 mg of catalyst was firstly dispersed in MO aqueous solution in the dark for 30 min to reach adsorptiondesorption equilibrium.Then,the suspension was stirred and irradiated under a 300 W Xenon lamp equipped with an ultraviolet cut off filter (λ> 420 nm)as light source.The characteristic absorption of MO atλ=464 nm was used to monitor the photocatalytic degradation process using a UV-Vis spectrophotometer(Shimadzu UV-2550,Japan).The degradation efficiency was determined based on the value ofC/C0,whereC0was the initial concentration of MO,andCwas the concentration at different time intervals.
2.5 Photoelectrochemical measurements
Photoelectrochemical measurements were performed on an electrochemical workstation (CHI 660D,Chenhua Instrument Co.,China).Fluorinedoped tin oxide (FTO) deposited with samples served as working electrode with active area of 1.0 cm2.Carbon rod and saturated calomel electrode were used as counter and reference electrodes,respectively.The photocurrent and electrochemical impedance spectra were measured in Na2SO4aqueous solution (0.5 mol/L) using a 300 W Xenon lamp equipped with an ultraviolet cut off filter (λ > 420 nm) as light source.
2.6 Reusability study
In the recycling experiments,the straw@Fe3O4/Cu2O was firstly separated from solutions by an external magnetic field.Then,the straw@Fe3O4/Cu2O was washed with distilled water several times before being redispersed in the same MO solution for another cycle.
3 Results and discussion
3.1 Characterization of straw@Fe3O4/Cu2O
Fourier transform infrared (FTIR) spectra of the straw before and after functionalization were shown in Fig.2(a).The pristine straw showed absorption peaks at 1 060,2 920 and 3 420 cm-1,which were assigned to stretching vibrations of C-O-C,C–H and O–H groups,respectively.In the spectrum of straw@Fe3O4,there appeared a new peak at 559 cm-1,which was ascribed to Fe-O group[16].This indicated the existence of Fe3O4particles in the composite.After immobilization of Cu2O,a characteristic peak at 619 cm-1was attributed to Cu-O group[17].

Fig.2 FTIR spectra (a) and XRD patterns (b) of pristine straw,straw@Fe3O4 and straw@Fe3O4/Cu2O
Fig.2(b) showed XRD patterns of pristine straw,straw@Fe3O4and straw@Fe3O4/Cu2O.In the XRD pattern of pristine straw,diffraction peaks at 17.1° and 24.7° were attributed to crystalline form of cellulose in the straw[18].After immobilization of Fe3O4particles,the characteristic diffraction peaks at 30.1°,35.5°,43.1°,53.5°,57.0° and 62.6° were assignable to (220),(311),(400),(422),(551) and (440) planes of Fe3O4lattice,respectively[19].The diffraction peaks at 29.6°,36.5°,42.4°,61.4°,73.7°,and 77.5° in the XRD pattern of straw@Fe3O4/Cu2O were indexed to (110),(111),(200),(220),(311) and (222) crystal planes of Cu2O,respectively[20].No peaks of metallic copper(Cu) or oxidized copper (CuO) could be found in the XRD patterns,indicating the exact reduction of Cu(II)precursors to Cu2O by NH2OH·HCl.
Scanning electron microscopy (SEM) was employed to study morphology of the samples.Fig.3 showed images of the pristine straw,straw@Fe3O4and straw@Fe3O4/Cu2O with low magnification.The pristine straw presented compact and smooth surfaces before functionalization (Fig.3(a)).After subsequent immobilization with Fe3O4and Cu2O,the straw was well decorated by many particles (Figs.3(b) and 3(c)).Image of the straw@Fe3O4/Cu2O with high magnification (Fig.3(d)) showed that spherical Fe3O4and cubical Cu2O particles without agglomeration were immobilized successfully on the straw.

Fig.3 SEM images of pristine straw (a),straw@Fe3O4 (b),and straw@Fe3O4/Cu2O (c,d)
Chemical composition of the straw@Fe3O4/Cu2O was analyzed by XPS spectra.Fig.4(a) showed that C 1s,O 1s,Fe 2p and Cu 2p signals appeared in the wide scan spectrum,which suggested that carbon,oxygen,iron and copper existed on the straw@Fe3O4/Cu2O.The Fe 2p spectrum of the straw@Fe3O4/Cu2O was presented in Fig.4b.The binding energies at 712.3 and 725.8 eV were associated with Fe(III) (Fe 2p3/2) and Fe(II) (Fe 2p1/2) respectively[21],which suggested that Fe3O4was successfully immobilized on the straw.In the Cu 2p spectrum (Fig.4(c)),two peaks at 932.7 and 952.5 eV were attributed to the Cu 2p3/2and Cu 2p1/2[22],confirming that copper existed in the form of Cu2O.The above results indicated successful synthesis of the straw@Fe3O4/Cu2O composite.

Fig.4 XPS wide scan (a),Fe 2p (b) and Cu 2p (c) spectra of the straw@Fe3O4/Cu2O
The magnetic property of the as-prepared straw@Fe3O4/Cu2O was evaluated at room temperature.Fig.5 showed magnetic hysteresis curve of the straw@Fe3O4/Cu2O.The straw@Fe3O4/Cu2O displayed superparamagnetism with high saturation magnetization(Ms) of 14.8 emu/g.It was suggested that the straw@Fe3O4/Cu2O could be conveniently separated from solutions by external magnetic field,which was advantageous in practical wastewater treatment.

Fig.5 Magnetization curve of the straw@Fe3O4/Cu2O
3.2 Photocatalytic performance
Photocatalytic activity of the catalysts was studied by evaluating degradation of MO under visible light.Fig.6 showed the effect of initial MO concentration on photocatalytic performance of straw@Fe3O4/Cu2O and straw@Cu2O.As shown in Fig.6,reduced concentration of MO was observed with extension of the irradiation time.The degradation rates of MO were 91.9% and 71.6% in the presence of straw@Fe3O4/Cu2O after irradiation time of 90 min at the initial MO concentration of 20 and 40 mg/L,respectively(Fig.6(a)).The higher the initial concentration,the longer the time required for complete degradation.When initial MO concentration was relatively high,the limited reactive sites on the photocatalyst surface were covered by dye molecules,and photons were difficult to reach the surface of catalyst.Moreover,a certain amount of photocatalysts could only generate a certain amount of reactive species at certain time,and relatively high dye concentration would deplete much reactive species.So longer time was needed as the initial concentration of MO increased.

Fig.6 Photocatalytic degradation rate of MO over the straw@Fe3O4/Cu2O (a) and straw@Cu2O (b) at the initial MO concentration of 20 and 40 mg/L
Fig.6 also showed that degradation performance of the straw@Cu2O was inferior to that of the straw@Fe3O4/Cu2O.The poorer catalytic performance of the straw@Cu2O could be ascribed to inherent defects of Cu2O.On one hand,the hole-electron pair in the Cu2O was easy to recombine,leading to lower quantum efficiency.On the other hand,thermodynamically unsuitable band energy structure resulted in low productivity of active oxygen species generated from redox reaction[23,24].After immobilization of Fe3O4,the catalytic activity of the straw@Fe3O4/Cu2O was significantly improved,which indicated that Fe3O4could enhance photocatalytic activity by preventing recombination of hole-electron pairs[10].
3.3 Photoelectrochemical analysis
Photocurrent provides an effective method to study the generation,separation and migration efficiency of the photogenerated carriers.Fig.7(a)showed that the straw@Fe3O4/Cu2O exhibited higher photocurrent intensity than the straw@Cu2O.The rise of photocurrent suggested that separation efficiency of the photoinduced electrons and holes was greatly enhanced,which indicated the specific interactions between Fe3O4and Cu2O phases.

Fig.7 (a) Transient photocurrent responses and (b) electrochemical impedance spectra of the straw@Cu2O and straw@Fe3O4/Cu2O
In order to further confirm this result,electrochemical impedance spectroscopy (EIS)measurement was conducted,which was widely employed to detect transfer resistance across the electrode–electrolyte interfaces and separation rate of charge carriers.Generally,smaller radius of the semicircle indicated faster separation and transfer of photogenerated carriers.As shown in Fig.7(b),the straw@Fe3O4/Cu2O showed smaller radius than the straw@Cu2O,indicating the efficient separation and fast transportation of photogenerated charge carriers for straw@Fe3O4/Cu2O.Based on the above results,it was found that introduction of Fe3O4could greatly promote separation efficiency of electron-hole pairs,suppress recombination of charge carriers and thus improve photocatalytic activity[10].
3.4 Photocatalytic mechanism
In order to clarify photocatalytic mechanism of the straw@Fe3O4/Cu2O,active species trapping experiments were performed.Three typical scavenges,ethylenediaminetetraacetic acid disodium (EDTA-2Na),isopropanol (IPA) and 1,4-benzoquinone (BQ)were used as quenchers of hole (h+),hydroxyl radical(·OH) and superoxide radical (·O2ˉ),respectively.As shown in Fig.8,when IPA was introduced,the degradation of MO was signicantly decreased,suggesting that ·OH was predominant active species during the photodegradation reaction.With the addition of EDTA-2Na,the degradation of MO was also obviously suppressed,indicating that h+exerted obvious influence on the photocatalytic degradation of MO.In addition,it was found that BQ suppressed the degradation of MO to some degree.Therefore,it could be concluded that ·O2ˉ was also active species during the photodegradation reaction.Overall,·OH,h+and·O2ˉ all took part in the MO degradation[25].According to the above results,generation of active species could be summarized as follows:
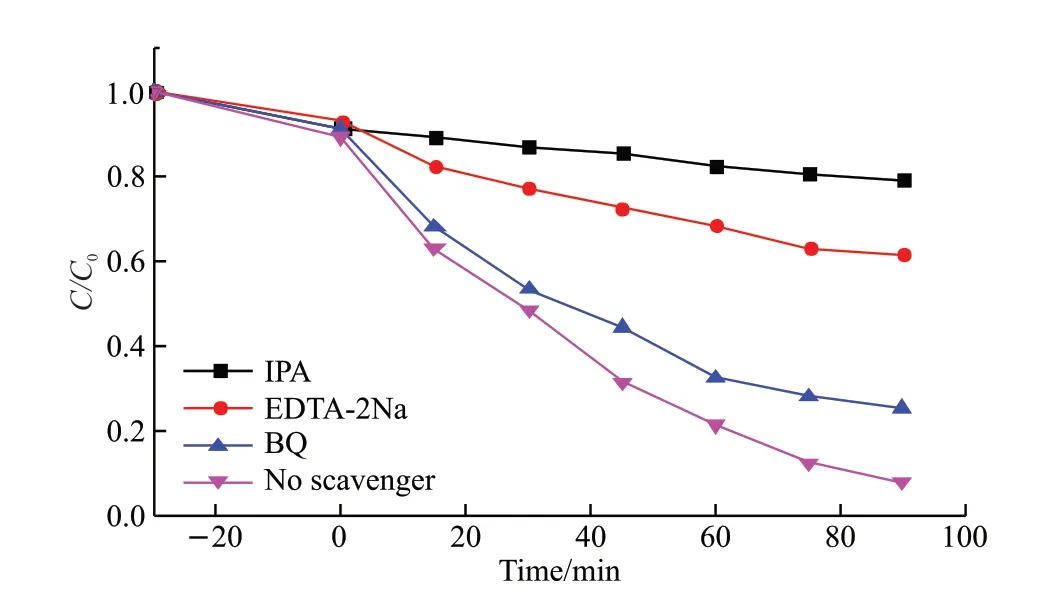
Fig.8 Effect of different scavengers on the MO degradation efficiency over straw@Fe3O4/Cu2O
3.5 Reusability of the photocatalyst
Evaluating reusability and stability of the magnetically separable straw@Fe3O4/Cu2O photocatalyst is indispensable for practical applications.The recyclability experiments were carried out at initial concentration of 20 mg/L MO.After each run,the photocatalyst was collected by external magnet force and then washed several times with distilled water.Fig.9 showed that the degradation percentage declined from 91.9% to 88.5% after five cycles for the photodegradation of MO.It was suggested that the straw@Fe3O4/Cu2O was stable during the photocatalytic degradation of MO,which was of critical importance for practical applications.Overall,the asprepared straw@Fe3O4/Cu2O photocatalyst displayed excellent reusability and promising future for practical wastewater treatment.

Fig.9 Reusability of the straw@Fe3O4/Cu2O for photocatalytic degradation of MO under visible light
4 Conclusions
Fe3O4and Cu2O were immobilized successively on alkali-treated straw,and the obtained magnetically separable straw@Fe3O4/Cu2O composite was used for degradation of MO under visible light irradiation.The higher the initial concentration of MO,the longer the time required for complete degradation.The existence of Fe3O4enhanced the catalytic performance by inhibiting the recombination of electron-hole pair during the catalytic process.The active species capture experiment showed that h+,·OH and ·O2ˉ radicals all took part in the MO degradation.After being reused for five cycles,catalytic efficiency of the straw@Fe3O4/Cu2O declined from 91.9% to 88.5%,which suggested that the as-prepared straw@Fe3O4/Cu2O displayed excellent potential applications in wastewater treatment.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Core-shell-embedded Mesoporous Silica Capsules for Atmospheric Water Harvesting
- Preparation of B2O3-ZnO-SiO2 Glass and Sintering Densification of Copper Terminal Electrode Applied in Multilayer Ceramic Capacitors
- Structural Characterization of Carbon-implanted GaSb
- Development of Eggshell Waste Incorporated with a Porous Host as a Humidity Adsorption Material
- Low Temperature Heat Capacity of Zn Substituted Cobalt Ferrite Nanosphere: The Relation between Magnetic Properties and Microstructure
- The Enhanced Electrons and Holes Separation for Bi2MoO6/TiO2 Z-scheme Heterojunction by Ag Loading
