Differential seed removal,germination and seedling growth as determinants of species suitability for forest restoration by direct seeding – A case study from northern Thailand
2023-10-07KhuanphiromNaruangsriPimonratTiansawatStephenElliott
Khuanphirom Naruangsri, Pimonrat Tiansawat, Stephen Elliott
a Forest Restoration Research Unit (FORRU), Department of Biology, Faculty of Science, Chiang Mai University, 239 Huaykeaw Road, Mueang District, Chiang Mai,50200, Thailand
Keywords:
ABSTRACT Background: Direct seeding is potentially a more cost-effective alternative to conventional tree planting for restoring tropical forest ecosystems.However,seed loss,due to removal and damage by animals,can substantially reduce seedling establishment.Therefore, this study examined the impact of seed predation on seedling establishment of five tree species, native to upland evergreen forests of northern Thailand: Hovenia dulcis, Alangium kurzii, Prunus cerasoides, Choerospondias axillaris and Horsfieldia amygdalina.We tested the hypothesis that excluding animals would significantly reduce seed removal,and increase both germination and seedling survival.The objective was to calculate a composite index of the relative suitability of the species studied for direct seeding.Methods:Seeds were placed on the ground in a deforested site and subjected to five predator-exclusion treatments:wire cage, insecticide, cage + insecticide, open cage and no exclusion (control).
1.Introduction
Despite recent international commitments to “halt and reverse”deforestation by 2030(UK Government,2021),losses of primary tropical forest increased by 10%in 2022 to 4.12 million hectares(compared with 3.75 million the previous year), releasing 2.7 gigatonnes (Gt)of carbon dioxide into the atmosphere (Weisse et al., 2023).Such rapid deforestation also results in substantial biodiversity loss (Thomas et al., 2004;Giam, 2017; Oakley and Bicknell, 2022) and exacerbates rural poverty(Chomitz, 2007).Agriculture remains by far the most significant deforestation driver, accounting for more than 90% of forest loss globally(Seydewitz et al.,2023).To counteract such deforestation,restoration of diverse forest ecosystems on deforested/degraded areas is being implemented on vast scales in many countries,under ambitious schemes such as the UN's“Decade on Ecosystem Restoration”(2021–2030)(UNEP and FAO,2020)and the Bonn Challenge,which calls for reforestation of 350 million hectares by 2030(Wentink, 2015).
Most usually, conventional forest restoration involves growing tree saplings in nurseries, transporting them to restoration plots, planting them and maintaining them thereafter(Lamb and Gilmour,2003;Elliott et al., 2013; Verdone, 2015), although so-called “passive” restoration(which relies on natural regeneration)is now becoming popular,despite doubts about its effectiveness (Reid et al., 2018).Even though tree-planting involves several arduous, time-consuming and expensive tasks (Elliott et al., 2013), it is still widely practiced and is often successful (Ruiz-Jaen and Aide, 2005; Elliott et al., 2013; Ceccon et al.,2016;de Souza and Engel,2018).However,to meet the ambitious global restoration targets mentioned above, increases in the cost-effectiveness of forest restoration methods are needed, particularly where motor-vehicle access is limited.
Instead of planting trees, direct seeding (sowing tree seeds directly into the soil)can be an effective restoration technique(Lamb et al.,2005;Doust et al., 2008; Grossnickle and Iveti′c, 2017; Freitas et al., 2019; de Souza,2022).Several authors have reported higher field performance of saplings established from direct seeding, compared with conventional tree-planting(Tunjai,2005;Doust et al.,2008;Tunjai and Elliott,2012),due to better root-system development(Doust et al.,2008).In addition,direct seeding can be used to achieve the high stand density, needed to shade out weeds, particularly on poor sites, provided high-quality,affordably-priced, seeds are available (Schmidt, 2008).Direct seeding has been successful in several places (e.g., Lamb and Gilmour, 2003;Douglas et al.,2007;Doust et al.,2008;St-Denis et al.,2013;Freitas et al.,2019), but it has not been widely adopted in tropical Asia (Lamb et al.,2005;Ruiz-Jaen and Aide,2005).
In the seasonal tropics,direct seeding can be challenging(Holl,2012)due to seasonal drought(Schmidt,2008;Oliet et al.,2015),competition with herbaceous weeds(Douglas et al.,2007;Doust et al.,2008;Schmidt,2008; Piiroinen et al., 2017), as well as seed and seedling removal/predation (Hau, 1997; Orrock et al., 2006; Fricke et al., 2014; Piiroinen et al., 2017; Palma et al., 2020).Low seed germination and seedling establishment are critical barriers (Palma and Laurance, 2015;Ceccon et al.,2016;Grossnickle and Iveti′c,2017;Palma et al.,2020).In the tropics, seed germination, following direct seeding, averages about 38%, with establishment rates of around 17% (Grossnickle and Iveti′c,2017).Furthermore,the costs of site preparation and weed control can be high for direct seeding(Schmidt,2008).Therefore,selecting tree species with high rates of seedling establishment from direct seeding could make the technique more cost-effective and practicable on large scales.
Predation of seeds and seedlings is probably the most serious limitation of direct-seeding success (Woods and Elliott, 2004; Orrock et al.,2006; Fricke et al., 2014; Naruangsri, 2017; Piiroinen et al., 2017).In degraded sites on ex-forest land in northern Thailand and on shrublands in Hong Kong, China, seed predations rates of up to 100% are common(Hau, 1997; Naruangsri, 2017).Rodents are the most common seed predators of large seeded species (Hau, 1997; Woods and Elliott, 2004;Fricke et al.,2014;Naruangsri,2017;Piiroinen et al.,2017).Therefore,it is necessary to quantify seed loss due to animals,and to examine whether such losses differ among different tree species.
The study, presented here, focused on the effects of natural enemies on seeds and emergent seedlings during direct seeding.Both vertebrates and invertebrates are seed predators.Vertebrates, particularly rodents,are the most widely reported(Sharp,1995;Fricke et al.,2014).Birds are also seed predators, but seed loss due to birds is lower than that by rodents (Villalobos et al., 2020).In addition, invertebrates such as ants consume seeds (Woods and Elliott, 2004; Arnan et al., 2012; Pearson et al., 2014).Animals kill seeds by completely consuming them or by damaging their embryos (Han et al., 2018).Vertebrate predators attack large seeds more frequently than small ones (Vongkamjan, 2003),because the former are easier to find and offer a greater nutritional reward per unit effort expended(Wang and Ives,2017).
At the seedling stage, predators, particularly insects, kill or inhibit seedlings(Doust et al.,2008;Fricke et al.,2014).In addition,rodents and ungulates also attack germinating tree seedlings (e.g., Wahungu et al.,2002; Bricker et al., 2010; Piiroinen et al., 2017; Zhang et al., 2017;Villalobos et al., 2020).These animals either kill seedlings outright or seriously reduce their growth and competitive ability (Barton and Hanley, 2013).In tropical forests, most herbivore damage occurs on young leaves (Kursar and Coley, 2003).They are particularly attractive to herbivores, because they have not had enough time to accumulate structural carbohydrates(which toughen the leaves)and defensive,toxic and distasteful secondary plant compounds(Wahungu et al.,2002).
Species selection for forest restoration by direct seeding is more complex and challenging (Meli et al., 2014) than it is for conventional tree planting.Previous species-selection studies were based on seed characteristics, such as seed-coat thickness and seed size (Tunjai and Elliott,2012).Since attacks by seed and seedling predators often lead to failure of direct-seeding trials (Farlee, 2013), we propose that species selection should take into account the likelihood of escaping seed predation, followed by germination success, as well as seedling predation resistance or resilience.
This study addressed two main questions: How much do animal predators affect seed removal and germination of different species?Subsequently, how much do invertebrate and vertebrate seedling predators affect seedling survival? We used seed removal as an index of the intensity of seed predation.We hypothesized that if animals remove seeds and/or reduce germination and seedling survival, then excluding them would increase seedling establishment—the ultimate goal of direct seeding.Consequently,seed removal,germination,seedling survival and growth-rate data were used to calculate a composite index of the relative suitability of those species studied for direct seeding.
2.Materials and methods
2.1.Study site
Experiments were conducted in an upland degraded area of formerly forested land,in Chiang Mai Province,northern Thailand(18°56′19′′N,98°49′15′′E;at 1,296 m above sea level)(Fig.1).The area was managed by the Nong Hoi Royal Project Foundation (Mon-Cham).The site formerly supported upland evergreen forest (sensu Maxwell and Elliott,2001), remnants of which remained nearby (nearest was 70 m away).Average annual climate variables were 1,419 mm precipitation per year and 22°C and 75.4%, mean temperature and humidity respectively(Meteorological Department of Thailand,2016)(Fig.S1).Since 2012,the site had been designated for forest restoration as part of a national flood-prevention program.Previously, it had been used for intensive strawberry cultivation, with heavy use of pesticides.The ground flora was dominated by bracken fern (Pteridium aquilinum (L.) Kuhn), cogon grass (Imperata cylindrica (L.) Raeusch.) and green panic grass (Panicum maximum Jacq.).
In addition to the field experiments, seed germination tests were conducted at the research tree nursery of Chiang Mai University's Forest Restoration Research Unit (FORRU-CMU), in the former headquarters compound of Doi Suthep-Pui National Park (18°48′3.7′′N, 98°54′59.6′′E, at about 1,000 m above sea level).
2.2.Tree species studied
Seeds were collected of five indigenous tree species,characteristic of upland, evergreen forest (>1,000 m elevation) (Forest Restoration Research Unit,2005).Although all selected species had previously been proven useful for forest-ecosystem restoration in the region (Forest Restoration Research Unit,2000),selection depended largely on locating seed trees in remnant natural forest at the start of the study: Hovenia dulcis Thunb.(Rhamnaceae), Alangium kurzii Craib (Cornaceae), Prunus cerasoides D.Don (Rosaceae), Choerospondias axillaris (Roxb.) (Anacardiaceae), and Horsfieldia amygdalina (Wall.) Warb.(Myristicaceae)(Table 1, Fig.S2).Propagules (seeds or pyrenes—one or more seeds contained within fruit endocarp (for P.cerasoides and C.axillaris)),ranging in dry mass from 0.03 to 4.25 g were collected early in the rainy season, from May to July 2015 (Fig.S2).At least 600 propagules were collected from at least five different trees of each species, in evergreen forest remnants in Doi Suthep-Pui National Park.Seeds from each tree were mixed,cleaned,dried and stored at room temperature,until used in experiments.The species were identified in the field using the Field Guide to Forest Trees of Northern Thailand (Gardner et al., 2007) and checked with FORRU-CMU's tree-species database.Species names were confirmed using herbarium specimens and checked for acceptability,using Plants of the World Online (POWO, 2019).Voucher specimens of the individual mother trees were collected and stored at Chiang Mai University's Herbarium in the Biology Department (CMU-B).
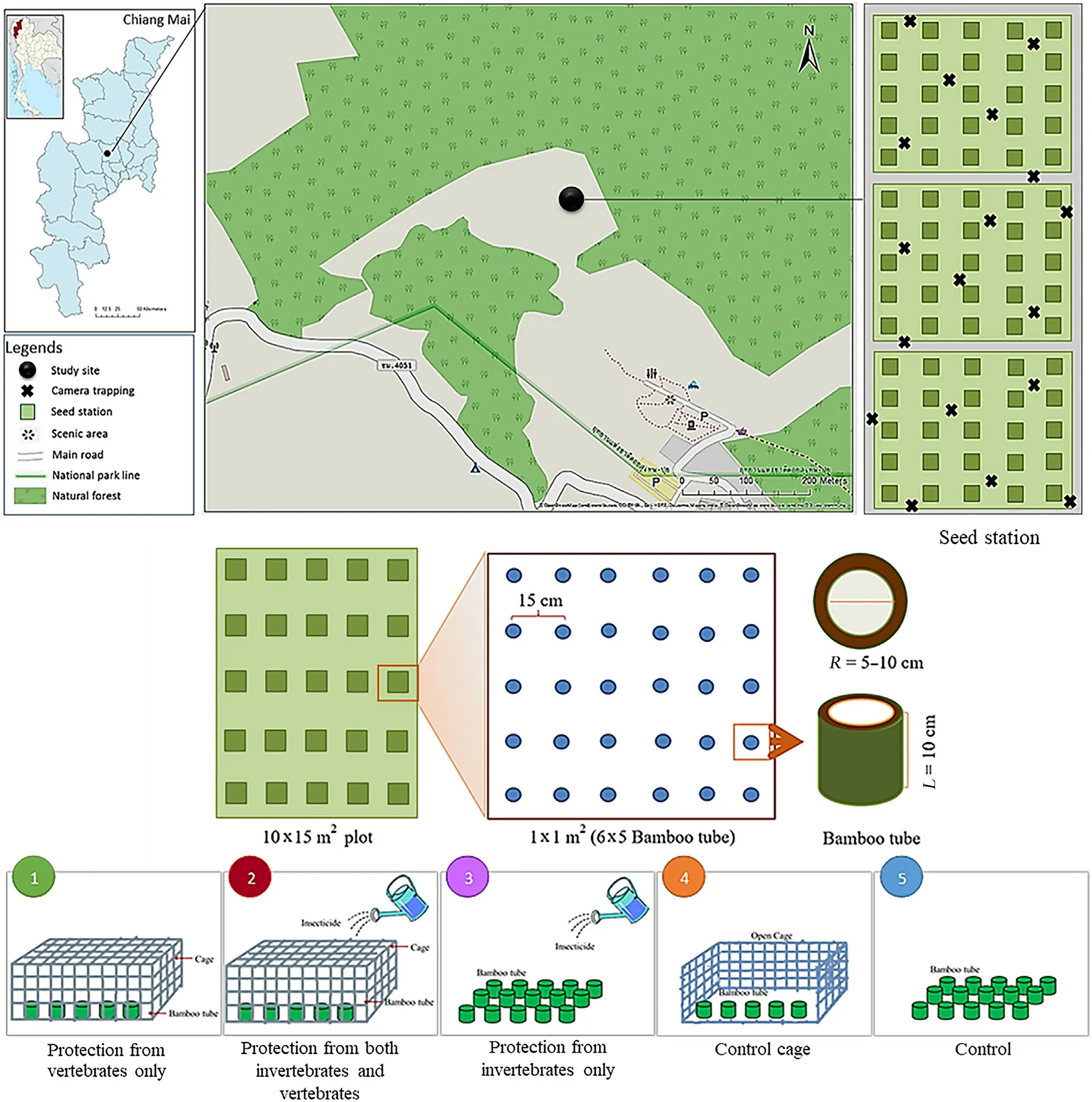
Fig.1.Experiment location at Mon Cham (a degraded area near Ban Nong Hoi, Mae Rim, Chiang Mai, Thailand), and layout of the experimental plots (one plot for each replicate).The plot(light grey rectangle)consisted of five rows(for five species)and five columns(for five treatments)of sub-plots.Each sub-plot contained 30 bamboo tubes(10 cm long and 5–10 cm diameter),within which seeds were pressed into the soil.The bamboo tubes were 15 cm apart and used to prevent seeds from rolling out from the five experiments.
2.3.Experimental design
Predator-exclusion experiments were conducted from July to December 2015, to determine the effects of seed predators on seed removal and germination.The five treatments were, 1) wire cage, to protect seeds and seedlings from vertebrates, 2) insecticide only, to protect seeds and seedlings from invertebrates, 3) wire cage + insecticide, to protect seeds and seedlings from both vertebrates and invertebrates,4)open cage to control for the presence of the cage,and 5)control with no cage or insecticide, exposed to both invertebrates and vertebrates.
Cages were constructed from bamboo canes and steel wire(1 m×1 m×0.6 m)(Fig.S3).Open cages were made in the same fashion as the wire cages, except that the top and one lateral side of the cage was removed.The insecticide Chlorpyrifos (Trade name: Kino505) was sprayed weekly,starting from seed sowing in July until December 2015,after all seedlings had emerged.The insecticide was mixed with water in the ratio of 2.5 mL of insecticide per 1 L of water in a pressure sprayer.For each species, 200 mL of the insecticide mixture was sprayed homogenously onto each replicate of the insecticide and the cage+insecticide treatments.The insecticide had no effect on seed germination, when tested during germination trails under controlled conditions in a nursery(Fig.S4).
At the Mon Cham site, three 10 m × 15 m experimental plots (5 m apart) were established in July 2015 in the middle of the rainy season.Each plot was divided into five rows,spaced 2 m apart,to accommodate five treatments.In each row,five 1 m×1 m sub-plots were established,to accommodate each of the five species.To secure the seeds in place for monitoring purpose,in each sub-plot,30 bamboo tubes(10 cm long and 5–10 cm diameter, Fig.1) were inserted into the soil at 5-cm depth.Ineach tube, one seed was pressed into the soil, so that the surface of the seed was level with the soil surface.The bamboo tubes prevented seeds from rolling out from the experiments and made it possible to follow the fate of the seeds.
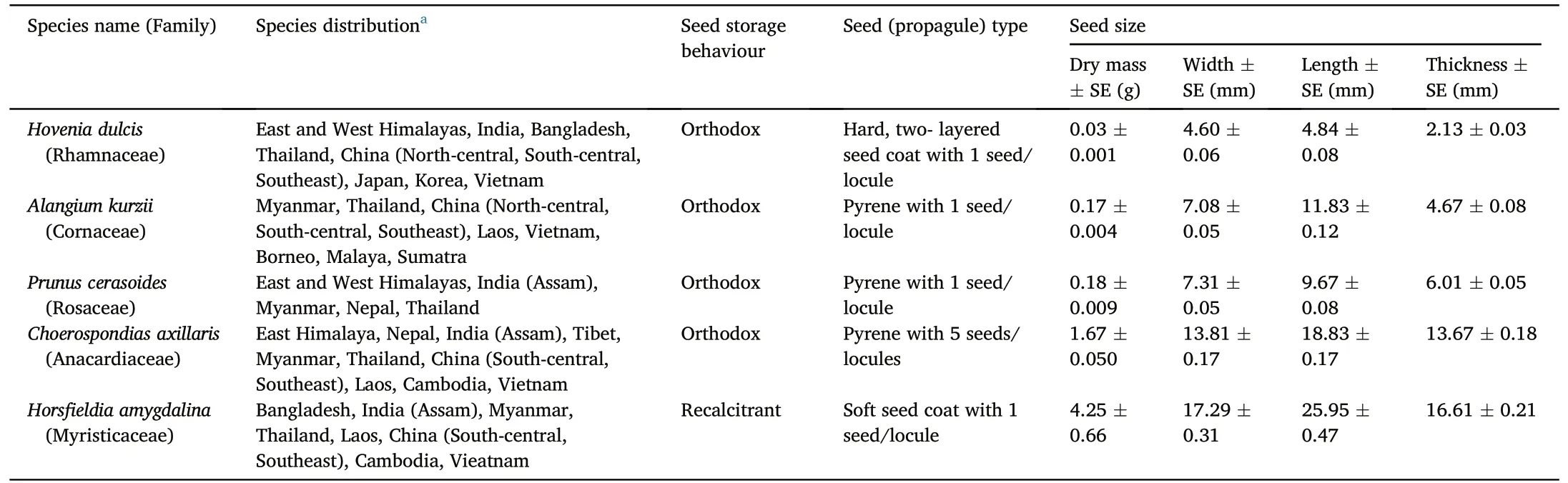
Table 1 Taxonomy, distribution and seed details of the five native tree species selected for this study.
2.4.Data collection and analysis
All statistical analyses,described below,were performed using the R Programming language, version 4.1.0 (R Core Team, 2021).P values of<0.05 were used to determine significant differences unless otherwise stated in the results.
2.4.1.Seed removal and germination
The numbers of seeds that had been removed from the bamboo tubes were recorded weekly.At the same time,visible emergence of the radicle and/or hypocotyl was recorded as germination.For each species, seed germination percentage was calculated as the number of germinated seeds,divided by the number of seeds that remained after seed removal.In addition, median length of dormancy (MLD) was calculated as the number of days from sowing to germination of half of the total number of seeds that eventually germinated (Elliott et al., 2013).A generalized linear model(GLM)with a logit link function was used,to determine the effects of treatments on seed removal and germination.Species and treatments were used as independent variables.The dependent variable was the proportion of seeds removed or germinated.We used the dispmod package version 1.2 (Scrucca, 2018) to handle over-dispersion in the logistic regression models.
2.4.2.Seedling mortality and the exclusion treatments
Germinants were classified as seedlings, if they possessed expanded cotyledons and/or true leaves had fully expanded.The numbers of seedlings that had died were recorded weekly until the end of the experiment.The effects of the treatments on seedling mortality were analyzed by a GLM with a logit link function.Species and treatments were used as independent variables.The dependent variables were the proportion of dead and surviving seedlings.We used the dispmod package version 1.2 (Scrucca, 2018) to handle over-dispersion in the logistic regression models.
2.4.3.Seedling yield and relative growth rate after treatments were terminated
To obtain longer term survival data, post-treatment seedlings were maintained on-site after the exclusion experiments were terminated in December 2015.The numbers of seedlings that survived after the first hot, dry season (February to April 2016) were recorded in July and percent survival of those that had germinated was calculated.A GLM with a logit link function was carried out,to compare differences among species.The independent variable was tree species.The dependent variable was the proportion of surviving seedlings.
Seedling height, crown width (CW) and root-collar diameter (RCD)were monitored three times,in October 2015,April 2016,and after the dry season in July 2016.For each species, mean relative growth rates(RGR) (averaged across all seedlings which survived until July 2016)were calculated from changes in height (RGR-H), root-collar diameter(RGR-RCD) and crown width (RGR-CW).RGR's for each individual seedling were calculated, using the following formula for each growth variable.
Daily proportional growth,relative to the average plant size over the measurement interval, was multiplied by 100 (to convert to a per cent)and by 365 to derive an annual value (modified from Hoffmann and Poorter,2002).
Analysis of variance(ANOVA)was used to test whether differences in mean height, CW, RCD and their RGRs among species were significant.The species were used as independent variables.The depended variables were height,CW,RCD and their RGRs.The Shapiro-Wilk normality test was conducted,to evaluate the normality of the data,while Levene's test was performed,to assess equality of variances.When the assumptions of ANOVA were met and significant effects were detected,the significance of differences between means was determined by Tukey's multiple comparison test.When ANOVA assumptions were not met, the Kruskal-Wallis test was performed, for height and RGR-RCD, followed by the Nemenyi post hoc test.
2.4.4.Species performance index
For each species, a performance score was calculated, based on seedling establishment(the number of surviving seedlings divided by the total number of seeds sown (450 per species)) and mean root-collar diameter (RCD) across all surviving seedlings 11 months after sowing.For each species,seedling establishment was averaged across three replicates.We used mean seedling RCD as a measurement of seedling size,because it is a more stable measurement than height (the latter being affected by broken stems, wind damage, trampling etc.) and is closely and positively correlated with plant biomass(Tian et al., 2017).
Our proposed score combined both the yield and size of the plants resulting from direct seeding, since a few large plants may contribute more to ecosystem restoration (in terms of shading out weeds and recovery of structural complexity) than several smaller ones.The equation implies equal weighting of stocking density and seedling size.
A higher score represented higher species performance for direct seeding.Differences in performance score among species were evaluated in the same way as for seedling growth.The independent variable was species, with performance score as the dependent variable.Due to nonnormal distribution of the data and unequal variance, the Kruskal-Wallis test was performed (instead of ANOVA), with Nemenyi post hoc test conducted after significant Kruskal-Wallis tests.
3.Results
3.1.Seed removal
The GLM indicated no interaction effect between species and treatments.However, the main effects of species and treatments, separately,on seed removal were significant.H.amygdalina exhibited very high seed removal(mean=85.8%(±11.4%[SE])across treatments),whereas the other 4 species displayed low and very similar seed removal rates:0.7%(±0.7% [SE]) for P.cerasoides, 1.1% (±0.9% [SE]) for H.dulcis, 1.1%(±0.6%[SE])for A.kurzii and 2.4%(±0.9%[SE])for C.axillaris,with no significant differences among them(P>0.05).
The GLM showed that the probability of H.amygdalina seeds being removed in the control treatment was about 206 times greater than that of the other species(Coefficient estimate±SE=10.56±1.78,ɀ=5.92,P < 0.001).For this species, seed removal from the cage treatment was significantly lower than that from the other treatments including the cage +insecticide treatment(P<0.001) (Fig.2).
In general, the cage treatment significantly and substantially decreased seed removal, compared with the control, by 12.4% (Coefficient estimate±SE=-5.58±1.62,ɀ=-3.45,P<0.001).The cage+insecticide treatment was marginally effective at protecting seeds from being removed compared with the control.The open cage and the insecticide treatments did not significantly prevent seed removal(Fig.2).
3.2.Seed germination
No H.amygdalina seeds germinated in the field.Therefore,H.amygdalina was not included in the analysis of seed germination.For the other four species, the treatments had no significant effect on germination percent, compared with the controls.Averaging across species,percent germination of seeds remaining after removal by animals was 46%(±11.2%[SE])in the control,55%(±19.0%[SE])in the cage treatment,44%(±8.4%[SE])in the insecticide treatment,54%(±14.8%[SE]) with insecticide plus cages, and 48% (±17.5% [SE]) in the open cage treatment.
However, germination differed significantly among the four tree species (Fig.3).A.kurzii (74% ± 5.6% [SE]) and P.cerasoides (73% ±5.2% [SE]) germinated the most (Coefficient estimate ± SE = 1.01 ±0.21, ɀ = 4.76, P < 0.001).C.axillaris (33% ± 2.8% [SE]) germinated moderately,whilst H.dulcis germinated the least(17%± 2.1%[SE]).
3.3.Seedling mortality and the exclusion treatments
For the four species, which germinated, both species and treatment affected seedling mortality (expressed as a percent of the seeds that germinated).Seedling mortality of H.dulcis was highest (42% ± 22.5%[SE]), significantly higher than that of C.axillaris (15% ± 7.9% [SE])(Fig.4).The insecticide treatment significantly increased seedling mortality (35% ± 10.3% [SE]) compared with the cage treatment (13% ±5.0%[SE]),whereas mortality did not differ among the other treatments(Fig.4).
3.4.Seedling yield after exclusion treatments were terminated
Seedling yield (as a percent of seeds sown) differed significantly among species, ranging from 4% (±3.0% [SE]) for H.dulcis to a maximum of 42% (±0.6% [SE]) for P.cerasoides.Seedling yield of the latter exceeded that of A.kurzii, C.axillaris and H.dulcis significantly(Table 2).
3.5.Relative growth rates and species performance index
Among the species tested,P.cerasoides seedlings grew the tallest,had the broadest crown width (CW), and the largest root-collar diameter(RCD)by the end of the study period.Relative growth rates(RGR)varied among species.P.cerasoides grew fastest with mean RGR-H>200%per year (Table 2).The species also achieved highest RGR-CW.In contrast,H.dulcis had the highest RGR-RCD among the four species(Table 2).
P.cerasoides had the highest performance score 1.98.This species also had shortest MLD (18 days), highest percent seedling establishment(42%) and exhibited rapid growth.A.kurzii and C.axillaris were intermediate among the four species, with performance scores of 0.53 and 0.45, respectively.H.dulcis had the lowest performance score (0.21).It also had low germination and small seedling size.No score could be calculated for H.amygdalina, since no seeds germinated.Differences in performance scores among plots were statistically insignificant (F(2,45)=0.055,P=0.95).
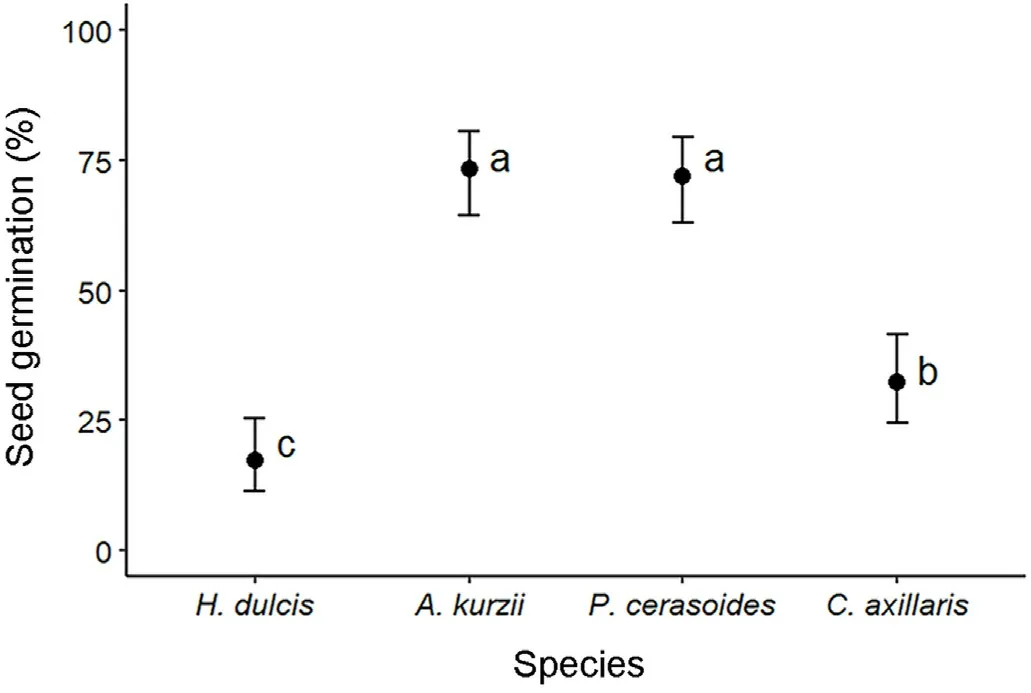
Fig.3.Percent seed germination(±1 SE),averaged across all treatments of the four species that germinated (GLM model with logit link function).Points not sharing the same letters are significantly different (P < 0.05).
4.Discussion
We explored the impact of seed and seedling predation on the efficacy of direct seeding for restoring a seasonally-dry,upland,evergreen,forest ecosystem in northern Thailand.In particular, we investigated whether animals affect seed removal of five native forest tree species,previously proven of value for restoring upland evergreen forest(Forest Restoration Research Unit,2000).We then examined germination of remaining seeds and seedling survival.Finally,we compared performance among species by combining seedling establishment with growth.
Protecting seeds in wire cages reduced seed removal, but had no effect on seed germination.The intensity of seed removal, seed germination,and seedling survival differed among species,indicating variation in the suitability of different species for direct seeding.
4.1.Seed removal
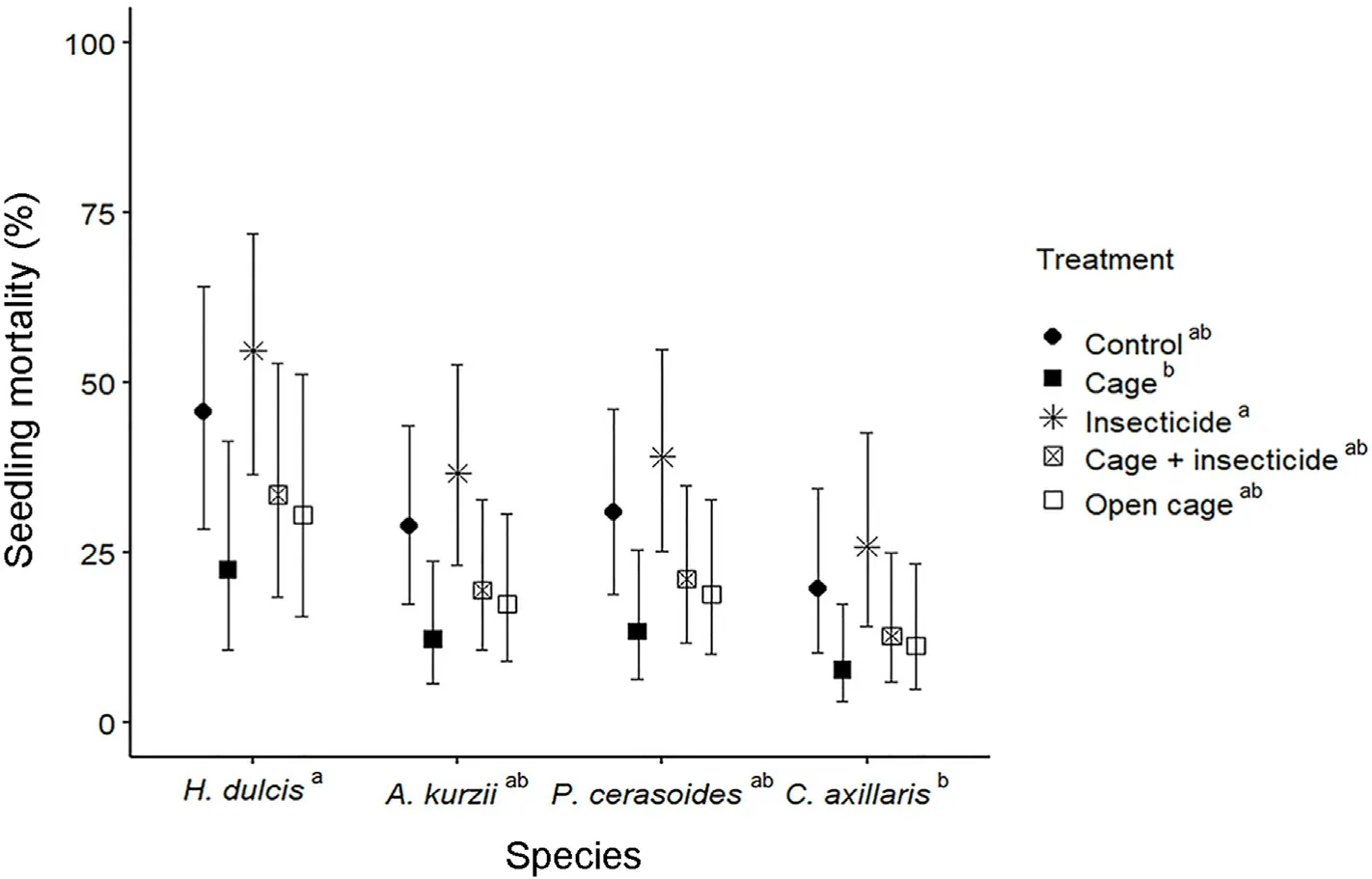
Fig.4.Seedling mortality(±1 SE)of the four species that germinated,with different predator-exclusion treatments(GLM model with logit link function).Species and treatments not sharing the same letters are significantly different (P < 0.05).
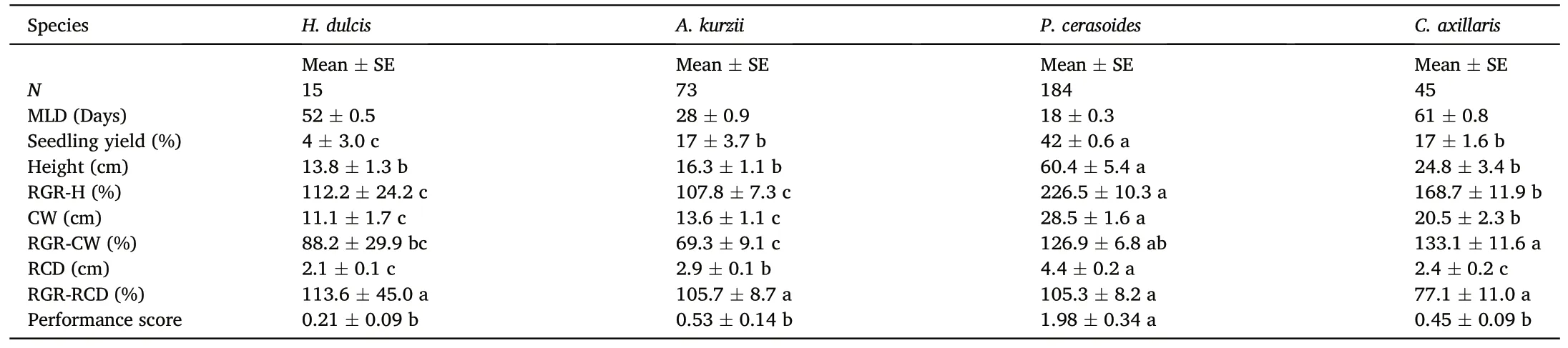
Table 2 Summary performance by species,including MLD(median length of dormancy),seedling yield(number of surviving seedling/number of seeds sown×100),mean size variables - height, crown width (CW) and root-collar diameter (RCD) –and their relative growth rates (RGR: percent per year), and performance scores ((number of surviving seedlings/number of seeds sown)×RCD).The seedling yield and growth of H.amygdalina were not available,because no seeds germinated in the field.In each row, species not sharing the same letters are significantly different (P <0.05).
Vertebrates were the major seed removers, since their exclusion by cages reduced seed removal.Several Rattus species were common in the study sites as evidenced by camera traps placed in the study site(Fig.S5)(Naruangsri and Tiansawat,2016;Piiroinen et al.,2017;Villalobos et al.,2020).The cages did not completely prevent seed removal of H.amygdalina, which had the largest seed amongst the species tested.Rats dug their way into and removed most of the seeds(>80%)from four out of the six cages(with and without insecticide).This accounted for the unusually high seed removal of this species from the cage treatment and cage + insecticide treatment (Fig.2).Sharp (1995) also presented evidence of rodent predation of tree seeds nearby the location of the study presented here (with similar elevation and vegetation type) but concluded that environmental factors limited seed germination more than seed predation did.Hau (1997) reported relatively low seed-removal rates of C.axillaris in a study of rodent predation in Hong Kong grasslands and shrublands, which he ascribed to the protective nature of the species’ tough seed coverings (which includes the woody fruit endocarp).
Although ants (Pheidole spp.) were abundant at the study site (Naruangsri, 2017), insects were not major seed removers.The insecticide treatment did not reduce seed removal, suggesting that the seeds were too large for insects to remove, or that the seeds were not attractive to insects.In contrast, in a nearby study site, of similar elevation and vegetation type,Woods and Elliott(2004)reported that ants,not rodents,were the main seed predators, attacking seeds,without removing them,in cages designed to exclude rodents.One of the species in the Woods-and-Elliott study, Spondias axillaris (synonym of C.axillaris) was the same as in the present study.The authors reported that ant attacks on the scarified pyrenes, exposed on the soil surface, reduced mean germination from 21% to 9%, whereas burial of the pyrenes reduced ant attacks,significantly increasing germination to 57%(P<0.05).Lack of ant attack in the present study might be explained by the fact that the pyrenes were not scarified and were pressed into the soil.These contrasting results show that micro-habitat differences may dramatically alter the relative impact of different kinds of seed predators.
4.2.Seed germination
The exclusion treatments had no significant effects on seed germination, but differences in seed dormancy types, median length of dormancy(MLD)and germination did occur among species.P.cerasoides seeds have mechanical dormancy.Hard seed coverings(testa and endocarp) restrict embryo expansion until they break down, whereas dormancy of A.kurzii seeds is probably physiological (due to chemical inhibitors) (Baskin and Baskin,2014).However, these dormancy mechanisms were short-lived, since seeds both species germinated relatively rapidly (MLDs of 18 days for P.cerasoides and 28 days for A.kurzii).In contrast,H.dulcis and C.axillaris exhibited longer dormancy in the field(MLDs of 52 days for H.dulcis and 61 days for C.axillaris).H.dulcis seeds probably have both physical and physiological dormancy (restriction of water and oxygen transport to the embryo by the testa),while C.axillaris seeds probably have physiological dormancy(Baskin and Baskin,2014).
In this study, H.amygdalina seeds did not germinate, both in the nursery and in the field.Waiboonya(2017)conducted a study at around the same time as this study and reported 55% germination of H.amygdalina seeds, immediately after collection in May, which fell to zero germination after 24 days storage in a refrigerator,thus determining that seeds of this species are recalcitrant.Our seeds were collected in May and by the time of experimental set-up in July, seeds had probably lost viability.Knowledge of seed storage behaviour and seed dormancy mechanisms is therefore clearly instructive when planning direct seeding strategies.
4.3.Seedling mortality and seedling yield
Seedling mortality varied among the tree species selected and increased with decreasing seed size.Seedling mortality was relatively high,resulting in relatively low seedling yield for H.dulcis,which had the smallest seeds amongst the four species that germinated.Highest yield was achieved by P.cerasoides, which had medium-sized seeds.This finding was consistent with that of Tunjai and Elliott (2012), who demonstrated that seedlings of small-seeded species(<0.01 g)had lower survival, compared with those with medium-sized seeds (0.10–4.99 g)and large seeds (>5.0 g).Previous studies indicated that small seeded species have low tolerance of harsh environmental conditions and lower competitive ability than do species with larger seeds (Pizo et al., 2006;Doust et al., 2008; St-Denis et al., 2013).Larger seeds provision their seedlings better and hold resources in reserve,to cope with stressors,as seedlings grow.This increases survival probability until such time as expanding leaves render seedlings independent of seed reserves.This is the larger-seed-later-commitment mechanism, validated by Kidson and Westoby (2000).Therefore, without some treatments to promote faster seedling growth, conventional tree planting is probably a more suitable technique for small seeded species than direct seeding is.
4.4.Species performance and species selection for direct seeding
Our study demonstrated differential suitability among the species for direct seeding.P.cerasoides was the most highly suitable for direct seeding, due to low seed predation and high percent germination,seedling survival and RGR(Fig.2,Table 2).Studies in the same area by Tunjai(2005)and Waiboonya(2017)support this result,increasing our confidence that this species is indeed an excellent candidate for inclusion in forest-restoration projects by direct seeding.Furthermore,Elliott et al.(2003) also reported P.cerasoides as an excellent species, for inclusion forest ecosystem restoration by conventional tree planting, not only for its high survival and growth,but also because it flowers and fruits within 2.5 years,attracting seed-dispersing and nesting birds.
A.kurzii and C.axillaris were both ranked as acceptable for direct seeding, due to low seed removal (see also Hau, 1997), but they may require pretreatments to increase germination.H.amygdalina and H.dulcis were considered the least favorable for direct seeding.For H.dulcis, germination and seedling establishment were low, despite it having the highest RGR-RCD.When planted as saplings,however,Elliott et al.(2003)considered it an“excellent”tree species for restoration.The recalcitrant nature of H.amygdalina seeds mean that they could only be included in direct seeding projects if they were sown immediately after seed collection(in May)and even then,seed predation would most likely severely limit seedling establishment.Furthermore H.amygdalina was rejected as a suitable species for restoration,when planted saplings were tested in field trials(=H.thorelii in Elliott et al.,2003).
4.5.Further research needed
Whilst the levels of seed removal were not high enough to reject direct seeding as a restoration tool,further research is needed to increase seedling establishment and ensure that the method contributes substantially towards global forest-restoration targets.
Coating seeds before direct seeding can provide both physical and chemical deterrents to predation.Candidate materials include clay and biochar (Williams et al., 2016).Seed containers (seed shelters) made from polypropylene are also being investigated as physical barriers to predation (Castro et al., 2015).Chemical deterrents may include the urine of carnivores that prey on rodents and capsaicin (from chili peppers),which is a powerful irritant(Villalobos et al.,2019).
Techniques to accelerate germination and seedling growth may also increase the effectiveness of direct seeding.Pre-sowing treatments, to shorten dormancy,include scarification or soaking seeds in acid or water(Hossain et al., 2014; FAO, 2017).Seeds may also be coated with fertilizer before sowing (Scott, 1998; Sousa et al., 2017).Pre-treatment effectiveness depends on species and seed characteristics (Mng'omba et al.,2007).Further research on developing appropriate species-specific seed pretreatments may therefore increase the effectiveness of direct seeding for forest ecosystem restoration.Above of all, however, the technique must be tested for many more forest tree species.A standardized process to test direct-seeding trials is urgently needed, to support the expansive restoration projects that are already underway.
Funding
This study was funded by the Thailand Research Fund (TRF Grant Number:MRG5980177),the Development and Promotion of Science and Technology Talents Project (Royal Thai Government Scholarship).Chiang Mai University partially supported this research, including the inputs of Stephen Elliott.
Authors’ contributions
KN, PT conceived and designed the research; KN performed the experiments;PT,SE advised on the experiments;KN,PT analyzed the data;KN,PT, SE wrote and edited the manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgements
The authors thank the staff of Chiang Mai University's Forest Restoration Research Unit(FORRU-CMU)for helping with seed collection and seedling care, both in the field and nursery.We are also grateful to Dia Panitnard Shannon and Wirong Chanthorn for helpful comments on the study and to the Nong Hoi Royal King Project (Mon Cham), Ban Nong Hoi, Mae Rim District, Chiang Mai Province, for permission to conduct the field experiments.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2023.100133.
杂志排行
Forest Ecosystems的其它文章
- Climate and fire drivers of forest composition and openness in the Changbai Mountains since the Late Glacial
- Complexity responses of Rhododendron species to climate change in China reveal their urgent need for protection
- Response of fungal communities to afforestation and its indication for forest restoration
- Words apart: Standardizing forestry terms and definitions across European biodiversity studies
- Regeneration of Nothofagus dombeyi (Mirb.) Ørst.in little to moderately disturbed southern beech forests in the Andes of Patagonia (Argentina)
- Spatial niche segregation between bird species in the Białowie˙za primeval forest (NE Poland)
