中药渣制备超高比表面积活性炭及其甲苯吸附性能研究*
2023-08-31廖达秀阳济章李德念袁浩然
谈 强,廖达秀,阳济章,李德念,袁浩然,
中药渣制备超高比表面积活性炭及其甲苯吸附性能研究*
谈 强1,廖达秀1,阳济章2,3,4,李德念2,3,4,袁浩然2,3,4,†
(1. 广州环投永兴集团股份有限公司,广州 510015;2. 中国科学院广州能源研究所,广州 510640;3. 中国科学院可再生能源重点实验室,广州 510640;4. 广东省新能源与可再生能源研究开发与应用重点实验室,广州 510640)
以中药渣为碳源,采用KOH辅助活化制备了具有超高比表面积的中药渣活性炭吸附剂。探索了碱炭质量比(KOH/C)、活化温度对吸附剂孔隙结构及其对甲苯吸附行为的影响。在KOH/C为5、温度为800 ℃的热解条件下,活性炭的比表面积和总孔容分别达到了3 549 m2/g和2.12 cm3/g,微孔比表面积和微孔孔容分别为2 529 m2/g和1.33 cm3/g,微孔占比达到了62.7%。在25 ℃、相对压力/0为0.9 ~ 1时的甲苯吸附量更是高达2 612 mg/g。该中药渣活性炭吸附剂在挥发性有机物去除方面具有广阔的应用前景。
中药渣;KOH;活性炭;甲苯吸附;挥发性有机物
0 引 言
在中国,中药被广泛用于预防和治疗疾病,大多数中药是草本植物。中药在生产与使用的过程中,会产生大量的中药残渣[1],其年产量高达6 000万t ~ 7 000万t,但这些中药残渣并没有得到有效利用。如果将这些中药残渣直接扔掉或进行焚烧处理,将会带来一定的环境问题[2-4]。因此,如何实现中药渣的资源化利用,被认为是一个亟待解决的重大问题[5]。
在众多的处理技术中,热解技术为中药渣的工业化应用提供了一条高效可靠的途径[6]。生物炭是热解过程中产生的固相产物,在土壤改良、碳质吸附剂、功能复合材料的制备等方面具有广阔的应用前景[7-9]。例如,连翘、耳丁、金银花等中草药残渣被直接用作碳源,通过控制氧的浓度和温度,制备出一种用于去除水中四环素的碳质吸附剂[10]。通过尿素和KOH辅助炭化刺五加残渣,可得到富微孔、氮掺杂的多孔炭材料,并用于锂硫电池载体[11]。多孔炭在对挥发性有机物(volatile organic compounds, VOCs)的吸附和环境修复具有重要意义[12-16]。
甲苯是一种典型的VOCs[12,17],严重威胁人体健康[18]。目前,已发展出多种甲苯去除技术,如吸收、吸附、膜分离、等离子体降解等[19-22]。其中,吸附法操作简单、效率高、能耗低,性价比最高。用于去除甲苯的吸附剂种类很多,包括活性炭[22]、碳纳米管[23]、沸石[24]、金属有机骨架(metal-organic framework, MOF)[25]等。比表面积和孔容是影响吸附剂吸附能力的关键因素。活性炭具有超过1 000 m2/g的比表面积和发达的孔隙结构,因此活性炭成为最受欢迎的VOCs吸附剂[12-13]。
本文以KOH辅助活化热解中药渣,制备出具有较大比表面积和丰富表面官能团的分层多孔活性炭。通过设置正交试验,详细研究活化温度和活化剂用量对活性炭孔隙结构和表面官能团的影响,以及对甲苯的吸附性能的影响,旨在为中药渣的高值化利用提供一种新的路径。
1 实 验
1.1 试剂与材料
中药渣来自广东揭阳某制药厂,KOH(分析纯)购自上海麦克林生化有限公司,去离子水为实验室自行制备。
1.2 中药渣活性炭的制备
中药渣活性炭的制备过程可分为三步。①将新鲜中药渣放入温度为105 ℃的烘箱中进行烘干处理,烘干后用粉碎机将中药渣粉碎成粉末,取60 g粉末放置于石英舟并转入管式炉中进行预炭化,以5 ℃/min的升温速率升至450 ℃,并保持恒温0.5 h,得到预炭化的中药渣炭,记为PHRC-450;②取10 g PHRC-450,并与一定质量的KOH混合均匀,转入管式炉中进行活化,以5 ℃/min 的升温速率升至700 ~ 900 ℃,并保持恒温1 h;③降至室温后,先用稀盐酸浸泡24 h,再用去离子水清洗至中性,最后于105 ℃下烘干,得到活化后的中药渣炭,记为HRC-K-(其中为KOH与PHRC-450的质量比,= 2,3,4,5,6;代表不同的活化温度)。
1.3 结构、形貌及吸附性能表征
扫描电子显微镜(scanning electron microscope, SEM)(日本,Hitachi,S-4800)和透射电子显微镜(transmission electron microscopy, TEM)(日本,JEOL JEM-2100F)用于表征材料的形貌结构。X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)(美国,Thermo Fisher Scientific,ESCALAB250xi)用于分析材料表面元素。X射线衍射(X-ray diffraction, XRD)图谱通过X射线衍射仪(荷兰,PANalytical,X’Pert Pro MPD)获得。傅里叶变换红外光谱仪(Fourier transform infrared spectrometer, FT-IR)(美国,Thermo Fisher Scientific,Nicolet iS50/Nicolet iN10)用于分析材料表面官能团。用气体吸附仪(美国,Quantachrome,Quadrasorb)分析氮气吸脱附曲线,获取甲苯吸附曲线,并用吸附仪自带软件进行数据分析。
2 结果与讨论
2.1 微观形貌
通过扫描电子显微镜和透射电子显微镜对材料的微观形貌结构进行研究。图1是HRC-5K-800的SEM和TEM图,图中可见HRC-5K-800含有大量的孔洞。
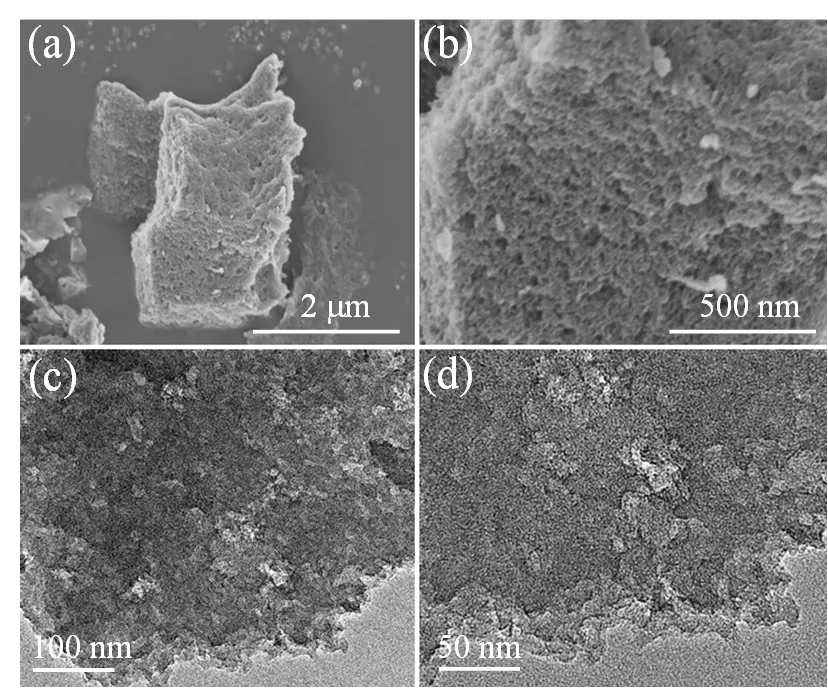
图1 HRC-5K-800的SEM(a、b)图和TEM(c、d)图
此外,HRC-K-的微观形貌也因的不同,发生了明显的变化,由原来致密的块状固体,逐渐转变为具有多孔结构的固体,如图2和图3所示。结果表明,随着的增大与的升高,中药渣炭与KOH之间的反应也随之加剧[26],但整体结构并没有发生变化。
2.2 结构特性
通过氮气等温吸脱附实验,进一步研究HRC-K-的孔隙分布情况。如图4(a)所示,PHRC-450的吸脱附曲线几乎是一条直线,表明在整个压力范围内,PHRC-450的氮气吸附量可以忽略不计,说明PHRC-450的孔隙结构不发达此外,随着和的不同,HRC-K-的孔隙度发生了明显的变化,如图4(b)和4(d)所示。HRC-K-的吸脱附曲线为典型的I型等温曲线,在相对压力/0小于0.05时,氮气吸附量急剧增加,且很快达到饱和。而在较高的相对压力下,吸附能力几乎没有明显的增加,说明HRC-K-的结构以微孔为主。有趣的是,对于HRC-6K-800而言,在相对压力大于0.9时,吸附能力开始增强,说明HRC-6K-800的孔隙变大,这可能是由于过量的氢氧化钾和较高的活化温度,使活化过程过于剧烈,进而引起微孔坍塌成大孔,导致孔容降低[13,25,27],这与HRC的孔结构特性参数和孔径分布结果是一致的,详见表1和图4(b)。
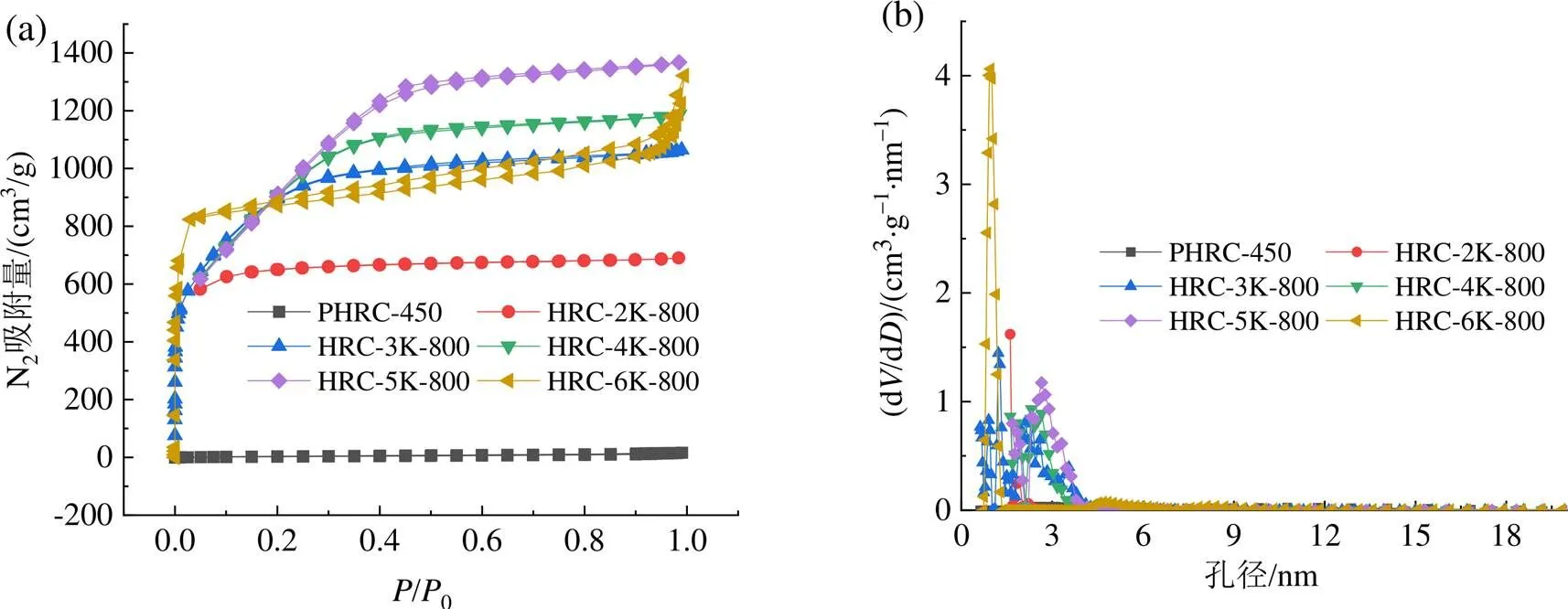

表1 HRC的孔结构特性参数
用X射线衍射仪分析活化温度和活化剂量对HRC结晶度的影响,图5展示了HRC-K-的XRD图谱。由图可见,在26°和44°附近有两个宽峰,对应的是石墨微晶(002)和(100)晶面的特征峰。其中,(002)表示石墨微晶层片的空间排列规则程度,(100)表示石墨微晶的晶面直径大小[28]。结果表明,热解温度低,石墨化程度不明显,得到的活性炭是无定形结构[29]。然而,(100)的峰强度在不断下降,说明金属钾嵌入碳晶格中,不断发生反应,从而导致晶面直径减小[26]。对于HRC-K-800而言,保持温度不变,增大活化剂量,活化程度加剧,(002)面的空间排列趋于无序,因此峰强度下降。对于HRC-5K-而言,保持活化剂量不变,升高温度会促使孔结构坍塌,空间排列规则程度降低,因此峰强度降低[28,30]。

图5 HRC-nK-T的XRD衍射图
X射线光电子能谱用于表征材料表面的化学结构,图6和表2显示HRC-5K-800含有C、N、O三种元素。由图6(b)~ 6(d)和表3可知,C1s谱分裂成6个峰,表明C存在6种成键形式,分别为C=C(283.8 eV)、C—C(284.6 eV)、C—O(285.1 eV)、C—N(286.2 eV)、C=O(287.5 eV)、COOH(289.4 eV);O1s分裂成4个峰,分别为O=C(531.5 eV)、O—C(533.1 eV)、HO—C(534.4 eV)和COOH(537.1 eV);N1s则分裂成4个峰,分别为吡啶N(398.1 eV)、吡咯N(400.2 eV)、石墨化N(401.1 ~ 401.5 eV)和氧化型N(403.0 ~ 406.0 eV)[31-34]。
值得注意的是,随着值增大到4,C=C和C—C含量逐渐降低,可能是C=C和C—C优先参与活化过程,且在HRC-5K-800中的相对含量略微减少,而在HRC-5K-900中的含量却有所增加。这是由于当温度高于700 ℃时,金属钾嵌入碳晶格中并发生反应,导致含量降低。当温度为900 ℃时,有利于提高石墨化进程,进而提高其含量[26-27]。当= 4和5时,C—O和C=O的相对含量因O的掺入而明显增加[35]。
与HRC-5K-700相比,HRC-5K-800中的石墨化N和吡啶N的相对含量略有减少,这是由于800 ℃时,N的类型发生了转变。随着温度继续升高,吡咯N和氧化型N因不稳定而分解,导致其相对含量降低[15,36-38]。
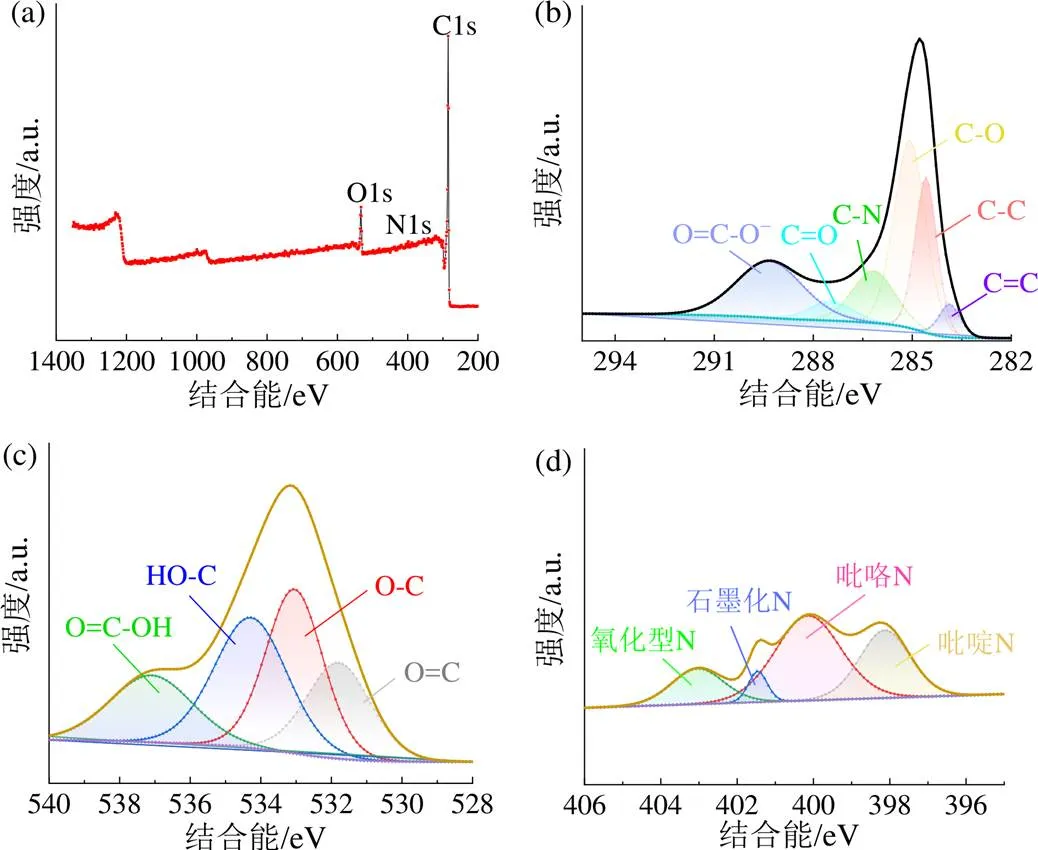
图6 HRC-5K-800的XPS全谱(a)和C1s(b)、O1s(c)和N1s(d)的拟合图

表2 HRC-nK-T中C、N和O的相对含量

表3 HRC-nK-T中C1s、N1s和O1s的各种成键形式的相对含量
图7是HRC-K-的傅里叶变换红外光谱。如图7(a)所示,所有的样品在3 450 cm−1附近均存在一个明显的峰,这是由羟基(—OH)的拉伸振动引起的。C=O从1 610 cm−1移动至1 590 cm−1,出现了明显的红移现象,这种现象说明因强烈的π-π叠加作用,引起了C=O偶极矩的变化[39-40]。
图7(b)展示了在相同的活化剂量下,温度对羟基的影响。与HRC-5K-700相比,在800 ℃的温度下,氧更容易掺杂到碳骨架当中,因此羟基的振动信号由弱增强。当温度升高至900 ℃时,羟基的振动信号减弱,这可能是由于温度升高,导致C—OH断裂。

2.3 甲苯吸附
图8(a)是HRC-K-的甲苯等温吸附曲线。如图8(a)和表4所示,当≤ 5时,HRC-K-800的吸附量呈现逐渐增加的趋势,而HRC-6K-800的吸附量却急剧下降,这可能是由于HRC-6K-800的孔结构坍塌,导致孔径增大,微孔占比降低,不利于其对甲苯的吸附行为。对于HRC-5K-(= 700、800和900 ℃)来说,随着温度的升高,吸附量先升高后降低。这可能是由于温度低于800 ℃时,微孔结构发育不完善,而高于800 ℃时,微孔不稳定,容易坍塌形成介孔。结合氮气等温吸附结果,表明= 5、温度为800 ℃是制备具有高比表面积、高甲苯吸附性能活性炭的最优条件,且其甲苯吸附性能优于大部分已报道的生物质活性炭,如表5所示。

图8 HRC-5K-800的甲苯等温吸附曲线(a)和甲苯等温吸附拟合结果(b)

表4 HRC-nK-T的甲苯吸附容量(P/P0 = 0 ~ 1,T = 25 ℃)

表5 HRC-5K-800与已报道的活性炭的甲苯吸附对比结果
为了理解HRC-5K-800的甲苯吸附行为,采用Freundlich模型和Langmuir模型对HRC-5K-800的吸附曲线进行拟合,模型方程分别如式(1)和式(2)所示[27,45]:

式中:e为平衡吸附量,mg/g;F为Freundlich方程的吸附容量常数,mg1−1/n∙L1/n∙g−1;为吸附强度系数;e为平衡浓度,mg/L。

式中:m为饱和吸附量,mg/g;为Langmuir常数, L/mg。
由图8(b)和表6中的等温吸附曲线拟合结果可知,两种模型都能较好地反映HRC-5K-800的吸附行为。

表6 HRC-5K-800的等温吸附曲线拟合结果(P/P0 = 0.9 ~ 1,T = 25 ℃)
3 结 论
以中药渣为原料,采用KOH为活化剂,通过辅助活化热解法制备超高孔隙率的活性炭,探讨了活化剂用量和活化温度对活性炭孔隙结构的影响,以及活性炭的结构特性和表面性质对其吸附甲苯性能的影响及其构效关系。
研究表明,KOH辅助活化热解法可制备孔隙发达且具有较高微孔占比、表面氮氧杂原子含量高的活性炭,不同活化剂用量与温度对活性炭的孔结构和吸附性能有显著影响。当碱炭质量比为5∶1、温度为800 ℃时,活性炭比表面积和总孔容分别达到了3 549 m2/g和2.12 cm3/g,微孔比表面积和孔容分别为2 529 m2/g和1.33 cm3/g,微孔占比达到了62.7%。发达的孔隙度、较高的微孔占比与氮氧含量,能为活性炭提供更多的有效吸附位点,有利于提高其甲苯吸附性能。本研究为中药渣的高值化利用提供了一种新的策略。
[1] LU Q, LI C L. Comprehensive utilization of Chinese medicine residues for industry and environment protection: turning waste into treasure[J]. Journal of cleaner production, 2021, 279: 123856. DOI: 10.1016/j. jclepro.2020.123856.
[2] WANG M H, LIU Y, WANG S Q, et al. Development of a compound microbial agent beneficial to the composting of Chinese medicinal herbal residues[J]. Bioresource technology, 2021, 330: 124948. DOI: 10.1016/j.biortech. 2021.124948.
[3] FERRONATO N, TORRETTA V. Waste mismanagement in developing countries: a review of global issues[J]. International journal of environmental research and public health, 2019, 16(6): 1060. DOI: 10.3390/ijerph16061060.
[4] 李俊, 陈夏, 李蕴钰, 等. 典型中药渣的热解特性研究[J]. 环境污染与防治, 2022, 44(12): 1601-1606. DOI: 10.15985/j.cnki.1001-3865.2022.12.009.
[5] GUO X Y, WANG S M, LI N, et al. Preparation of SnS nanosheet-loaded traditional Chinese medicine slag-derived carbon composite (SnS/NC) by one-pot hydrothermal method used as anodes for lithium-ion batteries[J]. Ionics, 2021, 27(11): 4721-4729. DOI: 10.1007/s11581-021-04230-7.
[6] 陈梅倩, 胡德豪, 黄友旺. 基于热重分析法的生物质变温热解特性实验研究[J]. 华北电力大学学报(自然科学版), 2019, 46(6): 99-104. DOI: 10.3969/j.ISSN. 1007-2691.2019.06.13.
[7] GAO J, CHU X J, LU H B, et al. Efficient carbon-based electrocatalyst derived from biomass for hydrogen peroxide generation[J]. Materials today communications, 2021, 26: 102051. DOI: 10.1016/j.mtcomm.2021.102051.
[8] SHEN Q B, WANG Z Y, YU Q, et al. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues[J]. Environmental research, 2020, 183: 109195. DOI: 10.1016/j.envres.2020.109195.
[9] LIAN F, SUN B B, SONG Z G, et al. Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole[J]. Chemical engineering journal, 2014, 248: 128-134. DOI: 10.1016/j. cej.2014.03.021.
[10] ZHANG S N, WANG J H. Removal of chlortetracycline from water byimmobilized on Chinese medicine residues biochar[J]. Environmental technology & innovation, 2021, 24: 101930. DOI: 10.1016/j.eti. 2021.101930.
[11] LIANG J F, XU Y Q, LI C, et al. Traditional Chinese medicine residue-derived micropore-rich porous carbon frameworks as efficient sulfur hosts for high-performance lithium-sulfur batteries[J]. Dalton transactions, 2022, 51(1): 129-135. DOI: 10.1039/D1DT02595C.
[12] SHI R, LIU K K, LIU B G, et al. New insight into toluene adsorption mechanism of melamine urea-formaldehyde resin based porous carbon: experiment and theory calculation[J]. Colloids and surfaces A: physicochemical and engineering aspects, 2022, 632: 127600. DOI: 10.1016/j.colsurfa.2021.127600.
[13] YAN M, RONG Y, WU F, et al. Micro-mesoporous graphitized carbon fiber as hydrophobic adsorbent that removes volatile organic compounds from air[J]. Chemical engineering journal, 2023, 452: 139184. DOI: 10.1016/j.cej.2022.139184.
[14] HE S, SHI G B, XIAO H, et al. Self S-doping activated carbon derived from lignin -based pitch for removal of gaseous benzene[J]. Chemical engineering journal, 2021, 410: 128286. DOI: 10.1016/j.cej.2020.128286.
[15] XU X, GUO Y, SHI R, et al. Natural honeycomb-like structure cork carbon with hierarchical micro-mesopores and N-containing functional groups for VOCs adsorption[J]. Applied surface science, 2021, 565: 150550. DOI: 10.1016/j.apsusc.2021.150550.
[16] JALILOV A S, LI Y L, TIAN J, et al. Ultra-high surface area activated porous asphalt for CO2capture through competitive adsorption at high pressures[J]. Advanced energy materials, 2017, 7(1): 1600693. DOI: 10.1002/ aenm.201600693.
[17] HE C, CHENG J, ZHANG X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources[J]. Chemical reviews, 2019, 119(7): 4471-4568. DOI: 10.1021/acs. chemrev.8b00408.
[18] HIROTA K, SAKAI H, WASHIO M, et al. Application of electron beams for the treatment of VOC streams[J]. Industrial & engineering chemistry research, 2004, 43(5): 1185-1191. DOI: 10.1021/ie0340746.
[19] ZOU W X, GAO B, OK Y S, et al. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: a critical review[J]. Chemosphere, 2019, 218: 845-859. DOI: 10.1016/j.chemosphere.2018.11.175.
[20] LI X Q, ZHANG L, YANG Z Q, et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review[J]. Separation and purification technology, 2020, 235: 116213. DOI: 10.1016/j.seppur.2019.116213.
[21] YAN X R, ANGUILLE S, BENDAHAN M, et al. Ionic liquids combined with membrane separation processes: a review[J]. Separation and purification technology, 2019, 222: 230-253. DOI: 10.1016/j.seppur.2019.03.103.
[22] CHANG T, WANG Y, WANG Y Q, et al. A critical review on plasma-catalytic removal of VOCs: catalyst development, process parameters and synergetic reaction mechanism[J]. Science of the total environment, 2022, 828: 154290. DOI: 10.1016/j.scitotenv.2022.154290.
[23] SHI W B, PLATA D L. Vertically aligned carbon nanotubes: production and applications for environmental sustainability[J]. Green chemistry, 2018, 20(23): 5245-5260. DOI: 10.1039/C8GC02195C.
[24] JAFARI S, GHORBANI-SHAHNA F, BAHRAMI A, et al. Adsorptive removal of toluene and carbon tetrachloride from gas phase using zeolitic imidazolate Framework-8: effects of synthesis method, particle size, and pretreatment of the adsorbent[J]. Microporous and mesoporous materials, 2018, 268: 58-68. DOI: 10.1016/j.micromeso. 2018.04.013.
[25] MENG X M, YANG L, JIANG W J, et al. Adsorption of acetone and toluene by N-functionalized porous carbon derived from ZIF-8[J]. Journal of industrial and engineering chemistry, 2022, 111: 137-146. DOI: 10.1016/j.jiec.2022.03.046.
[26] OTOWA T, TANIBATA, R, ITOH M. Production and adsorption characteristics of MAXSORB: high-surface-area active carbon[J]. Gas separation & purification, 1993, 7(4): 241-245. DOI: 10.1016/0950-4214(93)80024-Q.
[27] LI D N, YANG J Z, ZHAO Y, et al. Ultra-highly porous carbon from wasted soybean residue with tailored porosity and doped structure as renewable multi-purpose absorbent for efficient CO2, toluene and water vapor capture[J]. Journal of cleaner production, 2022, 337: 130283. DOI: 10.1016/j.jclepro.2021.130283.
[28] 汪树军. X射线衍射法对树脂炭微观结构测试分析[J]. 炭素技术, 2000(6): 8-12. DOI: 10.14078/j.cnki.1001-3741.2000.06.003.
[29] LIU S N, ZHAO T Q, TAN X H, et al. 3D pomegranate-like structures of porous carbon microspheres self-assembled by hollow thin-walled highly-graphitized nanoballs as sulfur immobilizers for Li-S batteries[J]. Nano energy, 2019, 63: 103894. DOI: 10.1016/j.nanoen. 2019.103894.
[30] 陈轶贤. 炭黑基多孔碳材料制备及甲苯吸附性能研究[D]. 重庆:重庆工商大学, 2022.
[31] GUO D, WEI H, CHEN X, et al. 3D hierarchical nitrogen-doped carbon nanoflower derived from chitosan for efficient electrocatalytic oxygen reduction and high performance lithium-sulfur batteries[J]. Journal of materials chemistry A, 2017, 5(34): 18193-18206. DOI: 10.1039/C7TA04728B.
[32] LIU J H, LI W F, DUAN L M, et al. A graphene-like oxygenated carbon nitride material for improved cycle-life lithium/sulfur batteries[J]. Nano letters, 2015, 15(8): 5137-5142. DOI: 10.1021/acs.nanolett.5b01919.
[33] PANG Q, TANG J T, HUANG H, et al. A nitrogen and sulfur dual-doped carbon derived from polyrhodanine@ cellulose for advanced lithium-sulfur batteries[J]. Advanced materials, 2015, 27(39): 6021-6028. DOI: 10.1002/adma.201502467.
[34] HOU T Z, XU W T, CHEN X, et al. Lithium bond chemistry in lithium-sulfur batteries[J]. Angewandte chemie international edition, 2017, 56(28): 8178-8182. DOI: 10.1002/anie.201704324.
[35] WANG J C, KASKEL S. KOH activation of carbon-based materials for energy storage[J]. Journal of materials chemistry, 2012, 22(45): 23710-23725. DOI: 10.1039/ c2jm34066f.
[36] PELS J R, KAPTEIJN F, MOULIJN J A, et al. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis[J]. Carbon, 1995, 33(11): 1641-1653. DOI: 10.1016/0008-6223(95)00154-6.
[37] ZHOU X S, QIU L L, FAN R Q, et al. Metal-organic framework-derived n-rich porous carbon as an auxiliary additive of hole transport layers for highly efficient and long-term stable perovskite solar cells[J]. Solar RRL, 2020, 4(3): 1900380. DOI: 10.1002/solr.201900380.
[38] JI Y X, WANG S S, DONG Y H, et al. Tuning nitrogen species on natural biomass derived porous carbon for efficient acetone adsorption[J]. Materials chemistry and physics, 2020, 253: 123338. DOI: 10.1016/j. matchemphys.2020.123338.
[39] MEHTA A, RAO J R, FATHIMA N N. Electrostatic forces mediated by choline dihydrogen phosphate stabilize collagen[J]. The journal of physical chemistry B, 2015, 119(40): 12816-12827. DOI: 10.1021/acs.jpcb. 5b07055.
[40] 王林萍. 基于(4-二茂铁乙炔基)苯胺/石墨烯复合物的电化学传感器研究[D]. 长沙:湖南师范大学, 2013. DOI: 10.7666/d.Y2325606.
[41] DU Y K, CHEN H Y, XU X, et al. Surface modification of biomass derived toluene adsorbent: hierarchically porous characterization and heteroatom doped effect[J]. Microporous and mesoporous materials, 2020, 293: 109831. DOI: 10.1016/j.micromeso.2019.109831.
[42] CHENG H R, SUN Y H, WANG X H, et al. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: ultrahigh surface area and impressive improvement in toluene adsorption[J]. Journal of hazardous materials, 2020, 392: 122298. DOI: 10.1016/j.jhazmat.2020.122298.
[43] JIN Z H, WANG B D, MA L, et al. Air pre-oxidation induced high yield N-doped porous biochar for improving toluene adsorption[J]. Chemical engineering journal, 2020, 385: 123843. DOI: 10.1016/j.cej.2019.123843.
[44] 刘培慧, 刘宇喆, 李琳, 等. 具有多级孔道结构的高比表面多孔炭活化策略及VOCs吸附性能[J]. 化工进展, 2022, 41(S1): 613-621. DOI: 10.16085/j.issn.1000-6613.2022-0647.
[45] SAHA D, MIRANDO N, LEVCHENKO A. Liquid and vapor phase adsorption of BTX in lignin derived activated carbon: equilibrium and kinetics study[J]. Journal of cleaner production, 2018, 182: 372-378. DOI: 10.1016/j.jclepro.2018.02.076.
Ultra-High-Specific-Area Activated Carbon from Herb Residue as Excellent Absorbent for Toluene Adsorption
TAN Qiang1, LIAO Daxiu1, YANG Jizhang2,3,4, LI Denian2,3,4, YUAN Haoran2,3,4,†
(1. Grandtop Yongxing Group Co., Ltd., Guangzhou 510015, China;2. Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China;3. CAS Key Laboratory of Renewable Energy, Guangzhou 510640, China;4. Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou 510640, China)
Potassium hydroxide (KOH) was used as a coactivator to produce activated carbon with an ultra-high specific area, and herb residue was used as a carbon source. The effects of alkali carbon mass ratio (KOH/C) and activation temperature on the pore structure of activated carbon were explored, while the adsorption behavior of toluene was also investigated. The total specific surface area and total pore volume of activated carbon increased up to 3 549 m2/g and 2.12 cm3/g when theKOH/Cand the temperature were 5 and 800 °C, respectively. The proportion of micropores reached 62.7%, while the specific surface area and pore volume of the micropores were 2 529 m2/g and 1.33 cm3/g, respectively. Moreover, the toluene adsorption capacity was reached at 2 612 mg/g when the temperature and relative pressure/0were 25 °C and around 0.9 to 1, respectively. It should be noted that activated carbon produced from herb residue can be used to effectively remove volatile organic compounds in the future.
herb residue; KOH; activated carbon; toluene adsorption; volatile organic compounds
2095-560X(2023)04-0365-09
TK6
A
10.3969/j.issn.2095-560X.2023.04.009
2023-03-10
2023-04-11
广州市科技计划项目(202201010687);广东省基础与应用基础研究基金资助项目(2022A1515011653);中国科学院青年创新促进会项目
袁浩然,E-mail:yuanhr@ms.giec.ac.cn
谈强, 廖达秀, 阳济章, 等. 中药渣制备超高比表面积活性炭及其甲苯吸附性能研究[J]. 新能源进展, 2023, 11(4): 365-373.
: TAN Qiang, LIAO Daxiu, YANG Jizhang, et al. Ultra-high-specific-area activated carbon from herb residue as excellent absorbent for toluene adsorption[J]. Advances in new and renewable energy, 2023, 11(4): 365-373.
谈 强(1972-),男,硕士,高级工程师,主要从事固体废弃物能源化与资源化利用研究。
袁浩然(1981-),男,博士,研究员,主要从事固体废弃物能源化与资源化利用研究。
