肿瘤相关缺血性脑卒中
2023-08-26崔雪晨马爱军
崔雪晨 马爱军
[摘要]脑卒中和肿瘤是常见的疾病,亦是导致死亡和残疾的两种常见病因。肿瘤引起缺血性脑卒中的发病机制多种多样,包括肿瘤的高凝状态及其相关的放、化疗等。肿瘤相关缺血性脑卒中的治疗包括抗凝、溶栓等。本文对肿瘤相关缺血性脑卒中发病机制及防治策略进行综述,以期为临床实践提供参考。
[关键词]缺血性卒中;肿瘤;血液凝固障碍;化放疗;综述
[中图分类号]R743.3;R739.9[文献标志码]A[文章编号]2096-5532(2023)03-0454-04
doi:10.11712/jms.2096-5532.2023.59.105[开放科学(资源服务)标识码(OSID)]
[网络出版]https://link.cnki.net/urlid/37.1517.R.20230807.1616.008;2023-08-0809:10:47
TUMOR-ASSOCIATED ISCHEMIC STROKE CUI Xuechen, MA Aijun (Departments of Neurology, The Affiliated Hospital of Qingdao University, Qingdao 266100, China)
[ABSTRACT]Stroke and tumors are common conditions, which frequently lead to death and disability. Various mechanisms are involved in the pathogenesis of tumor-induced ischemic stroke, including tumor-related hypercoagulability and radiotherapy and chemotherapy. The treatment of tumor-related ischemic stroke includes anticoagulation, thrombolysis, and other approaches. This article reviews the pathogenesis and prevention and treatment strategies of tumor-associated ischemic stroke, aiming to provide a reference for clinical practice.
[KEY WORDS]ischemic stroke; neoplasms; blood coagulation disorders; chemoradiotherapy; review
脑卒中和肿瘤是老年人群的常见疾病,亦是导致死亡和残疾的两种常见病因。由于肿瘤与脑卒中有共同的危险因素如吸烟、肥胖、高龄等[1-2],且肿瘤的治疗可增加脑卒中风险,如放射治疗加速动脉粥样硬化,放疗、化疗、手术和肿瘤本身也会导致机体凝血功能障碍[1-3]。因此,肿瘤对脑卒中的发病因素如动脉粥样硬化、心血管疾病和心脏栓塞等具有促进作用[2,4],而脑卒中亦会导致肿瘤病人严重残疾[3]。瑞典一项针对820 491例肿瘤病人的调查研究显示,其发生缺血性脑卒中的比例超过50%[5]。因此,对肿瘤相关的缺血性脑卒中进行全面的了解将有助于提高病人的生存率。本文就肿瘤相关缺血性脑卒中的发生机制及防治策略进行综述,以期为临床实践提供参考。
1发病机制
1.1凝血功能障碍
系统性凝血障碍是血栓形成的基础,并被认为与肿瘤之间存在复杂的相互作用。研究表明,人体内可促进血栓形成的代谢产物如具有类似血小板生成素活性的肿瘤来源因子、生长因子、血小板来源的微粒、骨髓内皮细胞释放的因子,以及巨核细胞分泌的生长因子、组织因子、凝血酶等在肿瘤病人体内呈高表达状态[6]。这些代谢产物可以使机体处于高凝状态(HCS),导致系统性血栓形成以及脑梗死。其中由恶性肿瘤诱导的HCS [7]所引起的脑梗死统称为特鲁索综合征(Trousseau综合征)相关脑梗死,常见的恶性肿瘤类型包括肺癌、胰腺癌、结直肠癌、乳癌、泌尿生殖道癌和前列腺癌等[8-9]。最新发表的神经学研究指出,Trousseau综合征的高度特异性标志为“三区征”,即双侧前后循环急性缺血性弥散加权成像病变[10-11]。有研究结果表明,在弥散加权成像上,梗死涉及3个特定的血管区域,在没有可识别的栓塞源或与此类病变相关的其他疾病的情况下,则高度提示肿瘤相关的高凝性脑卒中[12]。此外,多个血管区域多处分散的微栓子是Trousseau综合征相关脑梗死的另一个常见特征[11]。有研究结果表明,Trousseau综合征病人NIHSS评分和mRS评分通常表现为更严重的神经功能缺损及更差的预后[11]。因此,对没有已知恶性肿瘤的病人,当出现来源不明的栓塞性脑卒中时,鉴别是否存在未知的恶性肿瘤显得尤为重要。D-二聚体、纤维蛋白原水平、肿瘤标志物、深静脉血栓筛查及胸、腹、盆腔CT检查等均可以作为鉴别诊断的辅助检查方法[11-12]。
1.2非細菌性心内膜炎
非细菌性血栓性心内膜炎(NBTE)最常见于肿瘤,发生率为40%~85%[13]。肿瘤介导的HCS,导致心脏瓣膜上形成无菌性赘生物。该赘生物主要由纤维蛋白组成,包括少量白细胞和红细胞,类似于微血管内的血栓。该血栓结构松散,极易脱落形成栓子。NBTE形成的赘生物可以出现在心脏的任何一个瓣膜上,但二尖瓣和主动脉瓣最常受累[13]。NBTE的赘生物通常表现为圆形、无柄、形状不均匀,超声心动图测量长度>3 mm[14]。NBTE是肿瘤病人出现缺血性脑卒中的主要原因之一。自2005年至2009年一项关于263名被诊断为患有急性缺血性脑卒中的肿瘤病人的随访调查报告显示,NBTE在出现缺血性脑卒中的肿瘤病人中所占比例为7%~27%[15]。NBTE诊断方法包括经胸超声心动图和经食管超声心动图,后者在检测瓣膜赘生物方面比前者更敏感,被认为是该疾病的首选诊断工具[16]。
1.3肿瘤栓塞
肿瘤栓塞是一种罕见的缺血性脑卒中病因。良性肿瘤和恶性肿瘤均可引起肿瘤栓塞。心房黏液瘤由一种高度表达基质金属蛋白酶的间叶细胞组成,是心脏最常见的原发肿瘤,约占心脏肿瘤的50%[17]。该肿瘤结构松散,易脱落形成栓子,导致缺血性脑卒中及短暂性脑缺血发作(TIA)[18]。恶性肿瘤易引起肺栓塞和门静脉栓塞,缺血性脑卒中的发生率较低,恶性肿瘤晚期,肿瘤细胞易广泛转移至软脑膜[19],引起血管痉挛可表现为TIA,或侵及动脉壁造成缺血性脑卒中。系统性肿瘤栓塞最常被描述的主要是肺肿瘤和心脏左心房黏液瘤等肿瘤。大多数肺部肿瘤病例中,肿瘤栓塞主要发生在手术过程中或术后立即发生栓塞[20],自发的肿瘤栓塞很少见。
1.4肿瘤治疗相关并发症
1.4.1化疗目前已有的化疗并发症的报道中,脑血管事件缺乏统一的报道,因此很难确定化疗是否是单独的血栓形成的原因。某些化疗药物具有较高的诱发缺血性脑卒中的风险。一项关于21 853例口腔癌病人的调查显示,接受卡铂治疗的病人发生缺血性脑卒中的风险概率高于对照组[21]。可能是由于铂类化学药物具有损伤血管内皮的作用,血管内皮细胞損伤后诱发血小板黏附等凝血反应,从而导致血管狭窄甚至闭塞。
1.4.2放疗目前放疗所致血管损伤的病理机制尚未完全阐明,大多数研究认为放疗引起的动脉损伤是包括内皮细胞损伤及功能障碍、氧化应激反应、慢性炎症反应、外膜滋养血管狭窄和闭塞在内的多种机制共同作用的结果[22-23]。射线可以直接损伤动脉管壁,导致内膜增生、中膜坏死及外膜周围纤维化,并加速动脉粥样硬化[22-24]。此外,射线诱导的滋养脉管闭塞可引起管壁缺血,在放疗导致的血管狭窄中也起到了一定作用[25]。血管狭窄可导致TIA或梗死。包括口咽鳞状细胞癌[26]、垂体腺瘤[27-28]和动静脉畸形[29]等头颈部疾病的研究显示,放疗与脑血管疾病具有相关性。
2肿瘤相关缺血性脑卒中的治疗
2.1肿瘤病人原发病的治疗
对于肿瘤相关缺血性脑卒中的治疗,首先应积极治疗其原发肿瘤。当病人的影像学检查符合肿瘤相关缺血性脑卒中的特征但没有已知肿瘤的情况下,需要进行广泛的检查如肿瘤标志物、胸部CT、腹部CT、PET/CT及全身骨扫描等以明确是否存在原发肿瘤,并与心血管疾病及风湿免疫系统疾病进行鉴别,从而制定相关治疗方案。对于已知实体肿瘤,与有关科室进行沟通并进行积极的治疗,可显著降低血栓栓塞事件的发生。此外,对可影响血栓栓塞事件发生率的原发疾病如动脉粥样硬化、高血压、糖尿病、高脂血症等,也要通过改变生活方式和药物治疗,如阿司匹林、氯吡格雷等抗血小板聚集治疗,他汀类药物调脂治疗,降压、降糖治疗等来进行积极的控制。
2.2肿瘤病人急性脑梗死期的治疗
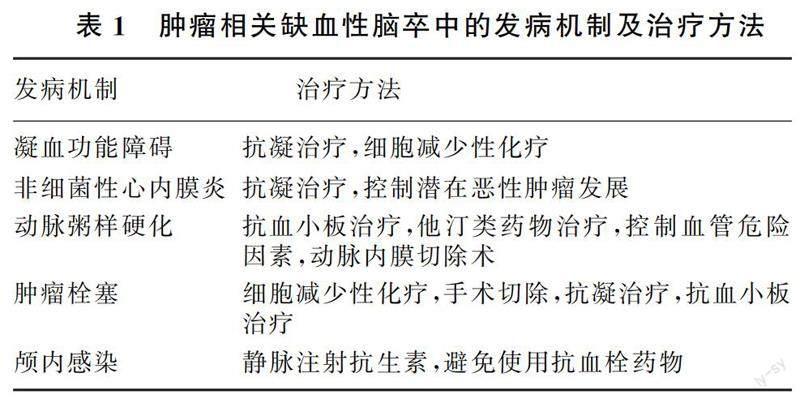
肿瘤病人在脑梗死急性期治疗遵循《中国脑卒中防治指导规范(2021年版)》,针对病人病情制定个性化治疗方案,包括改善脑血液循环(静脉溶栓、血管内治疗、抗血小板、抗凝、降纤、扩容等方法)、他汀类药物及神经保护等治疗。见表1。
2.2.1抗凝治疗肝素是癌症相关静脉血栓栓塞(VTE)治疗的金标准,尤其是低分子肝素被主要共识指南推荐用于肿瘤相关VTE的初始和长期治疗[30]。然而,由于低分子肝素不便于长期使用,近年来越来越多的肿瘤病人选择口服抗凝药如华法林、Ⅹ因子抑制剂、凝血酶抑制剂等进行长期抗凝治疗。一些随机试验证明,新型口服抗凝药Ⅹ因子抑制剂和凝血酶抑制剂在预防癌症病人复发VTE和大出血方面的安全性相当,且更方便临床使用[31]。然而,使用抗凝药物治疗必然要考虑到出血风险,因此,对使用抗凝药物的病人进行密切观察,及时调整药物用量显得尤为重要。
2.2.2溶栓治疗溶栓治疗是目前最重要的恢复急性缺血性脑卒中病人脑血流的措施之一,我国目前常用的药物为重组组织型纤溶酶原激活剂和尿激酶。然而,尽管多篇文章报道缺血性脑卒中病人是否合并肿瘤与接受溶栓治疗后的住院病死率无关,但肿瘤合并缺血性脑卒中病人溶栓治疗后出现脑出血的概率明显增加[32-34]。因此,肿瘤合并缺血性脑卒中病人在进行溶栓治疗时需进行密切观察预防出血的发生。
3肿瘤相关缺血性脑卒中的预后
肿瘤相关缺血性脑卒中的预后不佳,病死率和复发率明显升高。肿瘤病人在缺血性脑卒中发生后的1个月和1年后病死率分别为18.3%和71.6%,中位生存期仅为84 d。而在一般人群中,缺血性卒中后1个月和1年后病死率则为15.0%和25.0%,5年的病死率为50.0%[35-36]。肿瘤相关缺血性脑卒中病人预后不良的预测因素包括肿瘤转移、隐基因机制、D-二聚体和C反应蛋白水平升高等[37]。评估和识别与肿瘤病人卒中预后相关的因素对于确定最佳治疗策略非常重要。关于缺血性脑卒中病人的研究表明,缺血性脑卒中病人患有肿瘤的概率与D-二聚体、高敏C反应蛋白、血红蛋白和血小板计数水平等具有相关性[38-43]。此外,一项针对480例缺血性脑卒中病人为期15个月的调查显示,D-二聚体水平升高的病人通过CT成像发现隐匿性肿瘤的概率增高了约10倍[44]。但将D-二聚体作为标志物用于预测肿瘤病人急性缺血性脑卒中的诊断方案尚未得到正式评估。如果这些变量确实影响了预后,对肿瘤病人缺血性脑卒中不良预后的预测和制定治疗计划会有所帮助。
4小结
肿瘤通常会增加缺血性脑卒中风险。对于肿瘤病人来说,死于缺血性脑卒中的可能性仅次于死于肿瘤本身。近年来,随着分子靶向药物和免疫检查点抑制剂的应用,肿瘤病人的生存率得到了提高,因此预防这类病人的缺血性脑卒中对提高生活质量和生存预后极为重要。临床医生通过了解肿瘤病人缺血性脑卒中的病因,观察病人临床表现及检查检验报告,对疾病进行早期诊断,尽早采取预防措施,有助于明显改善病人的预后。
[参考文献]
[1]MARTINEZ-MAJANDER N, TATLISUMAK T. Cancer-associated ischemic stroke[J]. Acta Neurologica Scandinavica, 2020,141(3):202-203.
[2]BANG O Y, CHUNG J W, LEE M J, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms[J]. Journal of Stroke, 2020,22(1):1-10.
[3]NAVI B B, IADECOLA C. Ischemic stroke in cancer patients: a review of an underappreciated pathology[J]. Annals of Neurology, 2018,83(5):873-883.
[4]TSUCHIHASHI Y, SHIMIZU T, AKIYAMA H, et al. The risk factors for death within 6 months after ischemic stroke in patients with cancer[J]. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 2020,29(12):105365.
[5]Z?LLER B, JI J G, SUNDQUIST J, et al. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden[J]. European Journal of Cancer (Oxford, England:1990), 2012,48(12):1875-1883.
[6]FRANCHINI M, MONTAGNANA M, FAVALORO E J, et al. The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread[J]. Seminars in Thrombosis and Hemostasis, 2009,35(7):644-653.
[7]GRAZIOLI S, PACIARONI M, AGNELLI G, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study[J]. Thrombosis Research, 2018,165:33-37.
[8]SALAZAR-CAMELO R A, MORENO-VARGAS E A, CARDONA A F, et al. Ischemic stroke: a paradoxical manifestation of cancer[J]. Critical Reviews in Oncology/Hematology, 2021,157:103181.
[9]DAVIES A, VAN LEER L, CHAN J, et al. Stroke in patients with cancer in the era of hyperacute stroke intervention[J]. Internal Medicine Journal, 2022,52(9):1513-1518.
[10]NOUH A, STAFF I, FINELLI P. Three Territory Sign: an MRI marker of malignancy-related ischemic stroke (Trousseau syndrome)[J]. Neurology: Clinical Practice, 2019,9:124-128.
[11]BAO L, ZHANG S Y, GONG X Y, et al. Trousseau syndrome related cerebral infarction: clinical manifestations, laboratory findings and radiological features[J]. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 2020,29(9):104891.
[12]FINELLI P F, NOUH A. Three-territory DWI acute infarcts: diagnostic value in cancer-associated hypercoagulation stroke (trousseau syndrome)[J]. American Journal of Neuroradiology, 2016,37(11):2033-2036.
[13]IKUSHIMA S, ONO R, FUKUDA K, et al. Trousseaus syndrome: cancer-associated thrombosis[J]. Japanese Journal of Clinical Oncology, 2016,46(3):204-208.
[14]LIU J, FRISHMAN W H. Nonbacterial thrombotic endocarditis[J]. Cardiology in Review, 2016,24(5):244-247.
[15]PACKER R J, RORKE L B, LANGE B J, et al. Cerebrovascular accidents in children with cancer[J]. Pediatrics, 1985,76(2):194-201.
[16]BENEDETTI M, MORRONI S, FIASCHINI P, et al. Nonbacterial thrombotic endocarditis with multiple systemic emboli in a patient with primary lung cancer[J]. Journal of Cardiovascular Echography, 2022,32(2):129-131.
[17]REYNEN K. Cardiac myxomas[J]. The New England Journal of Medicine, 1995,333(24):1610-1617.
[18]P?REZ ANDREU J, PARRILLA G, ARRIBAS J M, et al. Neurological manifestations of cardiac myxoma: experience in a referral hospital[J]. Neurologia (Barcelona, Spain), 2013,28(9):529-534.
[10]WHEEN L C, ANDERSON N E, BAKER P C, et al. Leptomeningeal infiltration as the presenting manifestation of a malignant glioma[J]. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 2006,13(2):298-301.
[20]WHYTE R I, STARKEY T D, ORRINGER M B. Tumor emboli from lung neoplasms involving the pulmonary vein[J]. The Journal of Thoracic and Cardiovascular Surgery, 1992,104(2):421-425.
[21]WU Y T, CHEN C Y, LAI W T, et al. Increasing risks of ischemic stroke in oral cancer patients treated with radiotherapy or chemotherapy: a nationwide cohort study[J]. The International Journal of Neuroscience, 2015,125(11):808-816.
[22]PLUMMER C, HENDERSON R D, O'SULLIVAN J D, et al. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review[J]. Stroke, 2011,42(9):2410-2418.
[23]GUJRAL D M, SHAH B N, CHAHAL N S, et al. Clinical features of radiation-induced carotid atherosclerosis[J]. Clinical Oncology (Royal College of Radiologists (Great Britain)), 2014,26(2):94-102.
[24]SO N M, LAM W W, CHOOK P, et al. Carotid intima-media thickness in patients with head and neck irradiation for the treatment of nasopharyngeal carcinoma[J]. Clinical Radiology, 2002,57(7):600-603.
[25]LI C S, SCHMINKE U, TAN T Y. Extracranial carotid artery disease in nasopharyngeal carcinoma patients with post-irradiation ischemic stroke[J]. Clinical Neurology and Neurosurgery, 2010,112(8):682-686.
[26]AIZER A A, DU R, WEN P Y, et al. Radiotherapy and death from cerebrovascular disease in patients with primary brain tumors[J]. Journal of Neuro-Oncology, 2015,124(2):291-297.
[27]ERFURTH E M, B?LOW B, SVAHN-TAPPER G, et al. Risk factors for cerebrovascular deaths in patients operated and irradiated for pituitary tumors[J]. The Journal of Clinical Endocrinology and Metabolism, 2002,87(11):4892-4899.
[28]SATTLER M G, VROOMEN P C, SLUITER W J, et al. Incidence, causative mechanisms, and anatomic localization of stroke in pituitary adenoma patients treated with postoperative radiation therapy versus surgery alone[J]. International Journal of Radiation Oncology, Biology, Physics, 2013,87(1):53-59.
[29]GROSS B A, ROPPER A E, DU R. Vascular complications of stereotactic radiosurgery for arteriovenous malformations[J]. Clinical Neurology and Neurosurgery, 2013,115(6):713-717.
[30]STREIFF M B, HOLMSTROM B, ANGELINI D, et al. Cancer-associated venous thromboembolic disease, version 2.2021, NCCN clinical practice guidelines in oncology[J]. Journal of the National Comprehensive Cancer Network, 2021,19(10):1181-1201.
[31]SUN Y C, LIU X H, XU Y Z. Meta-analysis of efficacy and safety of new oral anticoagulants compared with warfarin in Japanese patients undergoing catheter ablation for atrial fibrillation[J]. Journal of Interventional Cardiac Electrophysiology, 2020,58(3):381-399.
[32]WEEDA E R, BOHM N. Association between comorbid can-cer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis[J]. International Journal of Stroke: Official Journal of the International Stroke Society, 2019,14(1):48-52.
[33]LADAK A A, SANDHU S, ITRAT A. Use of intravenous thrombolysis in acute ischemic stroke management in patients with active malignancies: a topical review[J]. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 2021,30(6):105728.
[34]NAM K W, KIM C K, KIM T J, et al. Intravenous thrombo-lysis in acute ischemic stroke with active cancer[J]. BioMed Research International, 2017,2017:4635829.
[35]LEE M J, CHUNG J W, AHN M J, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-CANCER study[J]. Journal of Stroke, 2017,19(1):77-87.
[36]NAVI B B, SINGER S, MERKLER A E, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer[J]. Neurology, 2014,83(1):26-33.
[37]SHIN Y W, LEE S T, JUNG K H, et al. Predictors of survi-val for patients with cancer after cryptogenic stroke[J]. Journal of Neuro-Oncology, 2016,128(2):277-284.
[38]GON Y, OKAZAKI S, TERASAKI Y, et al. Characteristics of cryptogenic stroke in cancer patients[J]. Annals of Clinical and Translational Neurology, 2016,3(4):280-287.
[39]SCHWARZBACH C J, SCHAEFER A, EBERT A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology[J]. Stroke, 2012,43(11):3029-3034.
[40]KIM S G, HONG J M, KIM H Y, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea[J]. Stroke, 2010,41(4):798-801.
[41]GON Y, SAKAGUCHI M, TAKASUGI J, et al. Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke[J]. European Journal of Neurology, 2017,24(3):503-508.
[42]SORGUN M H, KUZU, OZER I S, et al. Risk factors, biomarkers, etiology, outcome and prognosis of ischemic stroke in cancer patients[J]. Asian Pacific Journal of Cancer Prevention, 2018,19(3):649-653.
[43]KARLI?SKA A G, GROMADZKA G, KARLI?SKI M A, et al. The activity of malignancy may determine stroke pattern in cancer patients[J]. Journal of Stroke and Cerebrovascular Di-seases: the Official Journal of National Stroke Association, 2015,24(4):778-783.
[44]BAEK S H, PARK S, LEE N J, et al. Effective mechanical thrombectomy in a patient with hyperacute ischemic stroke associated with cardiac myxoma[J]. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 2014,23(9): e417-e419.
(本文編辑刘宁)