长链非编码RNA H19在肝脏疾病中作用的研究进展*
2023-08-07侯梦贞余芸王建青
侯梦贞, 余芸, 王建青△
· 综述 ·
长链非编码RNA H19在肝脏疾病中作用的研究进展*
侯梦贞1,2, 余芸1, 王建青1,2△
(1安徽医科大学第一附属医院,安徽省公共卫生临床中心,安徽 合肥 230012;2安徽医科大学药学院,安徽 合肥 230032)
长链非编码RNA H19;胆汁淤积;肝细胞癌;非酒精性脂肪性肝病
人类基因组计划发现,仅有不到2%的基因组编码蛋白质,而近70%的基因组转录成非编码RNA[1]。非编码RNA是指不具备蛋白质编码能力的RNA,包括转运RNA、核糖体RNA、小核仁RNA和长链非编码RNA(long noncoding RNA, lncRNA)。其中,lncRNA是一类长度大于200个核苷酸、没有开放阅读框而不具备蛋白编码能力的特殊RNA[2]。lncRNA过去被认为是RNA聚合酶II转录产生的副产物,因不具备生物学功能而仅被当作是转录“噪音”[3]。但越来越多的研究显示,lncRNA可通过表观遗传学、转录和转录后调控等多个层面调控基因表达水平,在多种生命过程中发挥重要作用[4]。H19是lncRNA中第一个被鉴定的印迹基因,在出生后表达显著减少。研究发现,H19在胆汁淤积[5]、肝细胞癌(hepatocellular carcinoma, HCC)[6]和非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)[7]等肝脏疾病中异常表达,参与了疾病的发生发展。但是H19在不同肝脏疾病中的作用不同,具体的作用机制也有差异,其自身表达也受多种因素的调控。因此,本文就H19在胆汁淤积、HCC和NAFLD等肝脏疾病中的作用和分子机制进行综述,为肝脏疾病的诊断以及开发新的治疗方法提供理论依据。
1 H19概述
基因位于人染色体11p15.5和鼠7号染色体,含5个外显子和4个内含子,剪接加工后的转录本H19长度为2.3 kb。H19具有mRNA的经典特征,如由RNA聚合酶II转录,由RNA剪接加工,并被多聚腺苷酸化,但不能编码蛋白质,而是作为一种特殊的RNA分子发挥作用[8]。的第一个外显子可编码一个微小RNA,miR-675[9]。基因位点还可转录出两个反义RNA分子即91H[10]和HOTS (H19 opposite tumor suppressor)[11]。作为母源性印迹基因,与相邻的父源性印迹基因胰岛素样生长因子2(insulin-like growth factor 2,)均受其启动子上游4 kb处差异甲基化区或印记调控区调控[12]。在胚胎发育过程中H19高表达,但出生后在大多数组织中表达迅速降低[13]。作为肝脏发育的重要调节因子,H19在肝脏病理状态下显著上调,并参与肝脏疾病中肝细胞和胆管细胞的增殖和凋亡、巨噬细胞和肝星状细胞的激活、炎症、纤维化、物质能量代谢等多种病理生理过程[14]。
2 H19与胆汁淤积
胆汁淤积主要是由多种因素导致胆汁生成、分泌和排泄障碍的一种疾病。长时间的胆汁淤积可导致严重的肝胆损伤、炎症、纤维化甚至肝硬化,最终发展为恶性肿瘤[15]。胆道闭锁患者肝和血清外泌体H19水平与胆汁淤积性肝损伤和肝纤维化严重程度呈正相关[16]。而敲除可显著缓解胆管结扎和(multidrug resistance 2)-/-模型小鼠肝纤维化症状[17]。这些研究表明,H19在胆汁淤积性肝损伤中发挥了重要作用(图1)。
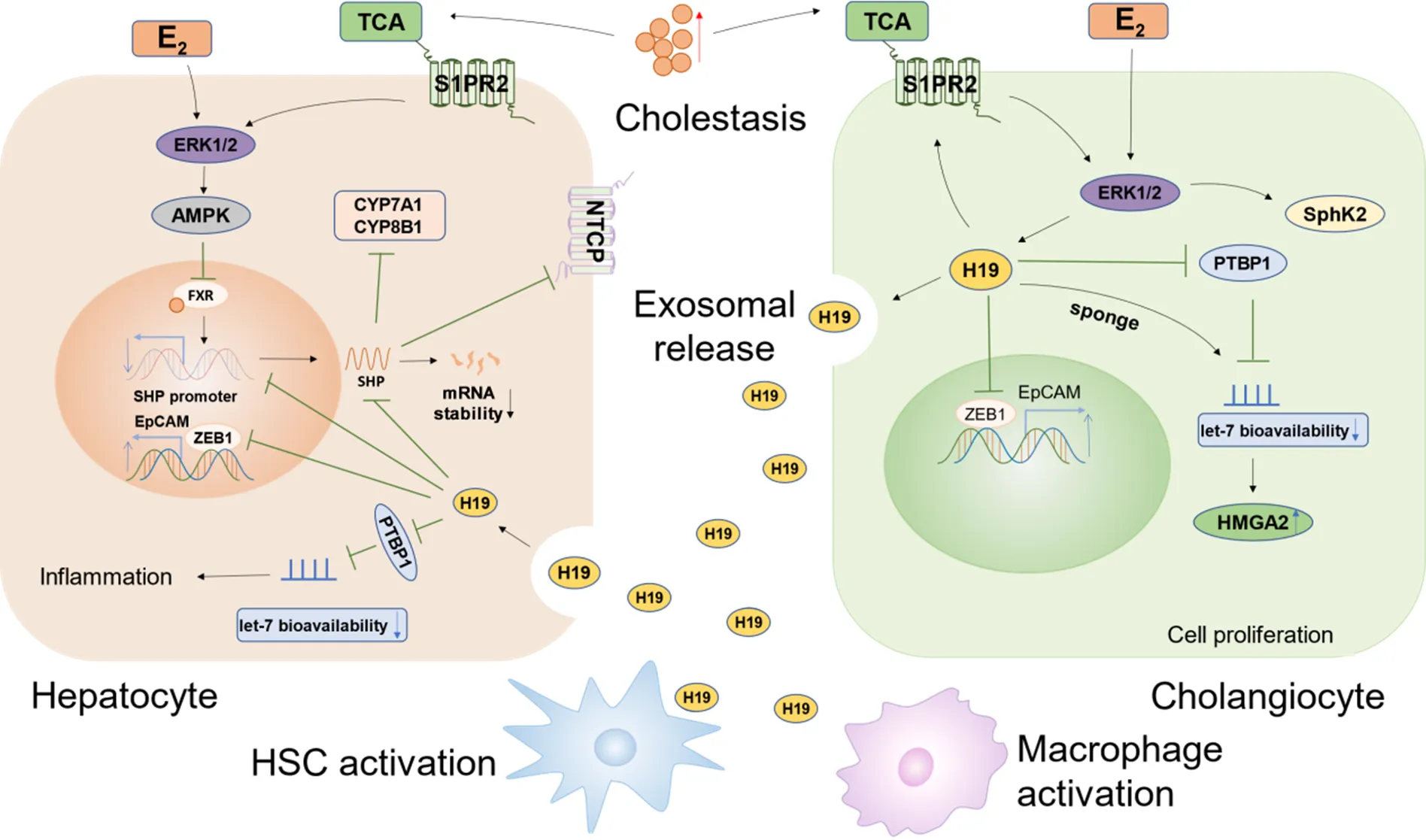
Figure 1. Regulatory mechanisms of H19 in cholestasis. E2: 17β-estradiol; TCA: taurocholate; ERK1/2: extracellular signal-regulated kinase 1/2; S1PR2: sphingosine-1-phosphate receptor 2; SphK2: sphingosine kinase 2; AMPK: AMP-activated protein kinase; FXR: farnesoid X receptor; SHP: small heterodimer partner; CYP7A1: cytochrome P450 family 7 subfamily A member 1/cholesterol 7α-hydroxylase; CYP8B1: cytochrome P450 family 8 subfamily B member 1/sterol 12α-hydroxylase; NTCP: sodium-taurocholate cotransporting polypeptide; PTBP1: polypyrimidine tract-binding protein 1; ZEB1: zinc finger E-box-binding protein 1; EpCAM: epithelial cell adhesion molecule; HMGA2: high mobility group A2.
2.1相关分子机制目前,胆汁酸合成与转运障碍被认为是导致胆汁淤积的重要因素之一。当肝细胞内胆汁酸水平升高时,法尼醇X受体被激活,继而诱导肝脏小异源二聚体伴侣(small heterodimer partner, SHP)表达上调,SHP一方面通过抑制两种关键酶即胆固醇7α-羟化酶和固醇12α-羟化酶的表达,从而抑制胆汁酸合成;另一方面通过下调Na+-牛磺胆酸共转运多肽,进而抑制肝细胞对胆汁酸的摄取[18-19]。有趣的是,在腺病毒介导的Bcl-2过表达小鼠模型中,肝细胞SHP蛋白水平与胆管来源的H19表达水平呈负相关[20]。进一步的深入研究发现,胆管细胞来源的外泌体可介导H19转移到肝细胞,通过降低启动子活性和SHP mRNA稳定性而抑制SHP表达[21-22]。
除分泌胆汁外,胆管细胞还可对肝细胞生成的胆汁进行加工修饰,并输送至胆囊和肠道。胆汁淤积诱发胆管细胞增殖活化,不仅会影响胆汁的分泌与排泄,还可通过各种调节因子激活肌成纤维细胞使细胞外基质沉积,从而导致肝纤维化[23]。Xiao等[16]发现,H19一方面上调胆汁酸诱导的1-磷酸-鞘氨醇受体2/鞘氨醇激酶2(sphingosine kinase 2, SphK2)轴的表达;另一方面,作为分子海绵作用于miRNA let-7家族使高迁移率族蛋白A2上调,进而促进胆道闭锁患者的胆管细胞增殖和肝纤维化。此外,H19还可通过降低胆汁淤积小鼠肝脏中多聚嘧啶区结合蛋白1(polypyrimidine tract binding protein 1, PTBP1)的表达水平,促进miRNA let-7的成熟,并且降低miRNA let-7对其靶点的生物利用度,提示H19可增加miRNA let-7的炎症相关靶基因表达进而加剧肝脏炎症反应[24]。据报道,E盒结合锌指蛋白1(zinc finger E-box-binding protein 1, ZEB1)作为调控上皮-间充质转化(epithelial-mesenchymal transition, EMT)的重要蛋白,可与H19相互作用,进而抑制ZEB1与上皮细胞黏附分子(epithelial cell adhesion molecule,)启动子结合,减弱ZEB1对EpCAM的抑制作用,导致胆管增生和肝纤维化[5]。其次,有研究发现,H19可通过单核细胞趋化蛋白1[25]和Rho家族GTP酶[26]促进巨噬细胞募集、激活和分化,而选择性清除巨噬细胞可抑制胆管增殖和肝纤维化。
研究发现,-/-模型小鼠的胆汁淤积性肝损伤和纤维化发生程度存在性别差异,这可能与雌激素和牛磺胆酸的增加有关[27]。进一步研究表明,雌激素和牛磺胆酸可分别激活雌激素受体和SIPR2并进一步诱导细胞外信号调节激酶1/2(extracellular signal-regulated kinases 1/2, ERK1/2)信号通路的激活,上调胆管细胞H19的表达,而高表达的H19可能是促进雌性-/-小鼠发生胆汁淤积性肝损伤的因素之一[22]。以上研究表明,H19不仅在胆汁淤积的发生发展中发挥重要作用,并且可能是导致胆汁淤积性肝损伤性别差异的关键因素。
3 H19与HCC
HCC是原发性肝癌的主要类型,每年因HCC死亡的人数高居世界癌症死亡人数的第4位[28]。由于早期无明显症状,发现时多为晚期,故早期诊断和早期治疗是提高患者生存率的关键。甲胎蛋白(alpha-fetoprotein, AFP)是HCC最主要的血清学诊断指标,但易误诊或漏诊,缺乏足够的灵敏度与特异性。Hernandez等[29]发现,HCC患者中H19表达水平升高,且与AFP有显著相关性,提示与AFP联合检测可提高早期诊断的准确率。近年来多项研究表明,H19与HCC的发生、发展及预后密切相关,有望成为HCC新的诊断标志物和治疗靶点[30]。见图2。

Figure 2. Regulatory mechanisms of H19 in hepatocellular carcinoma (HCC). AFB1: aflatoxin B1; E2F1: E2F transcription factor 1; NSUN2: NOP2/Sun domain family, member 2; G3BP1: Ras-GTPase-activating protein SH3 domain binding protein 1; CDC42: cell division cycle 42; PAK1: p21-activated kinase 1; LIMK1: LIM kinase 1; PTEN: phosphatase and tensin homolog; EGFR: epidermal growth factor receptor; IGF1R: insulin-like growth factor 1 receptor; MAPK: mitogen-activated protein kinase; Rb: retinoblastoma protein; HP1α: heterochromatin protein 1α; ZEB1/2: zinc finger E-box-binding protein 1/2; hnRNP U: heterogeneous nuclear ribonucleoprotein U; PCAF: p300/CBP associated factor; Pol II: RNA polymerase II; ERK: extracellular signal-regulated kinase; MDR1: multidrug resistance 1; GST-π: glutathione S-transferase-π; EMT: epithelial-mesenchymal transition; TAM: tumor-associated macrophages.
3.1相关分子机制研究发现,化学致癌物黄曲霉素B1可增加细胞周期相关转录因子E2F1 (E2F transcription factor 1)和H19表达水平进而促进肝癌HepG2细胞增殖和侵袭,其中E2F1可与的启动子结合并激活H19[31]。此外,NSUN2 (NOP2/Sun RNA methyltransferase 2)对H19进行m5C修饰后可增加H19稳定性进而提高H19表达水平;而m5C修饰的H19可通过招募G3BP1 (Ras-GTPase-activating protein SH3 domain binding protein 1)导致MYC积累,促进HCC的发生发展[32]。
H19可作为“miRNA海绵”,解除miRNA对下游靶基因的抑制作用,从而促进HCC细胞生长、侵袭和转移。例如,H19可竞争性结合miR-15b、miR-326和miR‐520a‐3p,分别激活细胞分裂周期蛋白42/p21活化激酶1、Twist1转录因子及LIM激酶1[30, 33-34]。此外,作为miR-675的前体,H19还可通过其转录产物miR-675发挥作用且二者多协同过表达。miR-675下调Rb (retinoblastoma protein),可减轻对E2F1的抑制作用并上调H19表达[29]。研究发现,miR-675还可通过调控异染色质蛋白1α和早期生长反应蛋白1,上调H19表达水平,进而激活丙酮酸激酶M2的表达,促进HCC发生发展[35]。此外,miR-675还能通过增强EMT促进HCC对索拉非尼的耐药性[36]。
作为肿瘤微环境的重要组成部分,肿瘤相关巨噬细胞(tumor-associated macrophages, TAMs)在促进肿瘤干细胞(cancer stem cell, CSC;或称肿瘤起始细胞,tumor initiating cell, TIC)的维持和自我更新上发挥重要作用。研究表明,TAMs可诱导肝癌细胞HepG2和Hep3B中H19表达并与miR-193b竞争性结合,减弱其对下游多种癌症驱动基因(磷酸酶及张力蛋白同源物、表皮生长因子受体和胰岛素样生长因子1受体)和丝裂酶原活化蛋白激酶1(mitogen-activated protein kinase 1, MAPK1)的抑制作用,进而促进EMT和干细胞转化,最终导致HCC侵袭和转移[37]。CSC或TIC在HCC发生、转移、复发及耐药中起着关键作用。研究发现,转化生长因子β受体2失活的TIC中,H19是表达上调最多的lncRNA之一,在TIC中敲低会减弱转化生长因子β受体2失活诱导的成瘤性,提示H19可参与调控TIC[38]。Conigliaro等[39]发现,CD90+CSC可释放含H19的外泌体,调节血管内皮细胞表型,促进HCC血管生成,从而促进转移。Ding等[40]报道,下调H19可通过阻断MAPK/ERK信号通路下调MDR1和谷胱甘肽-转移酶π的表达,从而逆转CD133+CSC的耐药性。上述研究表明,H19与HCC增殖、转移、耐药以及预后密切相关,主要在HCC中起到促癌作用。
然而,Zhang等[41]发现,H19可与蛋白复合物核异质核糖核蛋白U-p300/CBP相关因子-RNA聚合酶II相结合,通过增加组蛋白乙酰化激活miR-200家族,继而抑制其靶点ZEB1/2,逆转EMT进程,最终抑制HCC转移。Schultheiss等[42]也报道,H19可抑制HCC生长与化疗耐药。出现这种不一致的结果,可能与实验条件不同(细胞系的选择、体内动物模型的多样性和人类HCC样本含量不同)以及H19的特点(基因多态性和细胞特异性表达)有关,可加大样本含量尤其是体内实验,进一步明确H19在HCC中发挥的作用。
4 H19与NAFLD
NAFLD已成为全球最常见肝病之一,是一类包含单纯性脂肪肝、非酒精性脂肪性肝炎(nonalcoholic steatohepatitis, NASH)等的临床病理综合征,并可能进一步发展为肝硬化、肝衰竭和肝癌[43]。研究发现,与无脂肪变性和脂肪性肝的其他肝病患者相比,H19在NAFLD患者血清中表达水平显著升高,且随肝脏脂肪变性程度的加重而增加;相关性分析显示,NAFLD患者血清H19与甘油三酯、低密度脂蛋白胆固醇和胰岛素抵抗指数呈正相关,提示H19在调节肝脏脂质代谢中发挥重要作用,有望成为预测NAFLD的潜在标志物和治疗靶点[44]。见图3。
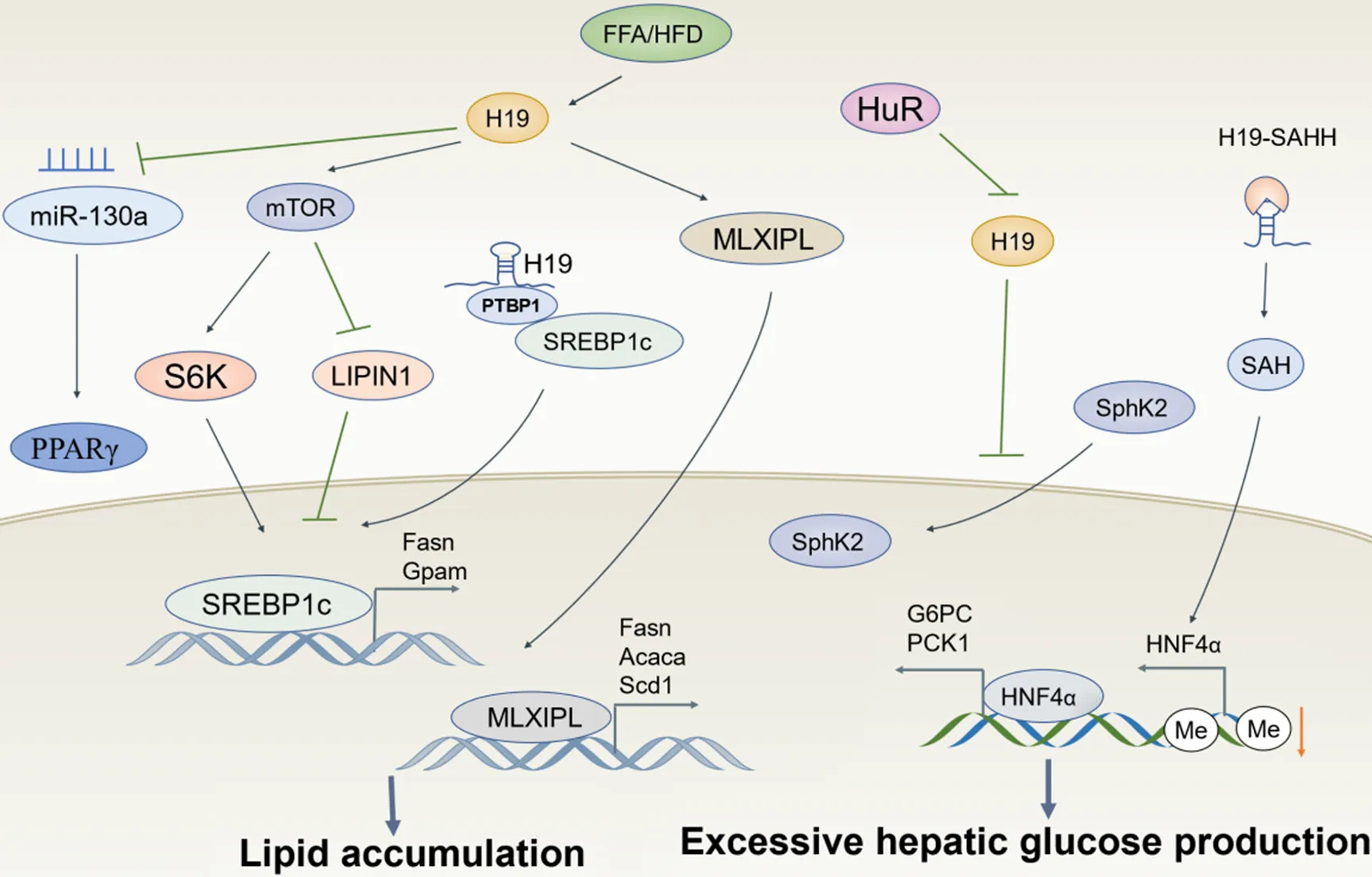
Figure 3. Regulatory mechanisms of H19 in non-alcoholic fatty liver disease. FFA: free fatty acids; HFD: high-fat diet; PPARγ: peroxisome proliferator-activated receptor γ; mTOR: mammalian target of rapamycin; S6K: S6 kinase; PTBP1: polypyrimidine tract-binding protein 1; SREBP1c: sterol regulatory element-binding protein 1c; MLXIPL: MLX interacting protein-like; Fasn: fatty acid synthase; Gpam: glycerol-3-phosphate acyltransferase; Acaca: acetyl-CoA carboxylases alpha; Scd1: stearoyl-CoA desaturase 1; HuR: human antigen R; SAHH: S-adenosylhomocysteine hydrolase; SAH: S-adenosylhomocysteine; HNF4α: hepatocyte nuclear factor 4 α; G6PC: glucose-6-phosphatase; PCK1: phosphoenolpyruvate carboxykinase 1.
4.1相关分子机制NAFLD的主要特征是脂肪变性,即肝脏中甘油三酯的异常积累[45]。研究发现,在油酸诱导的脂肪变性和高脂饮食诱导的NAFLD小鼠中H19上调并通过激活肝细胞中哺乳动物雷帕霉素靶蛋白复合体1信号轴和MLX相互作用蛋白样蛋白转录网络来上调多个脂质代谢相关基因,进而诱导肝脏脂肪变性[7]。有研究报道,H19还能直接下调miR-130a的表达,进而激活肝脏过氧化物酶体增殖物激活受体γ,促进肝脏脂肪生成[46]。固醇调节元件结合蛋白1c(sterol regulatory element-binding protein 1c, SREBP1c)作为一种转录因子,通过调节胆固醇和脂肪酸代谢在调节脂质稳态中发挥重要作用[47]。研究发现,H19可与PTBP1相互作用,增加其下游靶点SREBP1c的稳定性和核转录活性,诱导脂质累积[48]。最近的一项研究表明,肝细胞特异性人类抗原R缺失可显著上调NASH患者和西方饮食加糖水诱导的NASH小鼠肝脏中H19的表达,而高表达的H19通过调控SphK2从细胞质转移至细胞核,降低SphK2的核蛋白水平而促进肝脏脂质累积[49]。
糖代谢紊乱和炎症反应参与了NAFLD发生发展的全过程,是加速NAFLD恶性进展的重要机制。据报道,高脂饮食小鼠肝脏中H19表达增加,而肝脏特异性过表达H19可促进高血糖和胰岛素抵抗,敲除则可以增加胰岛素对肝葡萄糖产生的抑制作用[50]。进一步机制研究表明,H19可与⁃腺苷同型半胱氨酸水解酶结合并抑制其活性,造成⁃腺苷同型半胱氨酸累积,使肝细胞核因子4α(hepatocyte nuclear factor 4α,)启动子甲基化程度降低而促进HNF4α表达,从而使磷酸烯醇式丙酮酸羧激酶1和葡萄糖-6-磷酸酶催化亚基这两种糖异生反应的关键酶表达增加。此外,Cheng等[51]发现,抑制H19可通过上调miR-29b抑制血管内皮生长因子A的表达,最终激活蛋白激酶B/内皮型一氧化氮合酶信号通路而使内皮细胞免受高糖诱导的氧化应激和炎症,减少NAFLD并发症的发生。
5 小结和展望
综上所述,lncRNA H19作为最早被鉴定的印迹基因之一,在肝脏病理状态下被激活,参与多种肝脏细胞的增殖、凋亡、炎症、纤维化、物质和能量代谢等病理生理过程,通过多种调控机制参与胆汁淤积、HCC和NAFLD等肝脏疾病的发生发展。但是肝脏疾病的发生机制是多因素的且基因本身的作用机制较为复杂,目前仍有很多问题亟待解决。近年来/基因组印迹在人类肝脏疾病中的作用受到广泛关注。/基因印迹异常可引起一系列病理生理学的改变,甚至导致肿瘤的发生。人体微环境、基因上游印迹调控区的甲基化状态和印记丢失等均会影响H19的表达水平;的单核苷酸多态性变异还可能改变H19的二级结构,进而影响H19与miRNA的相互作用等多种生物学功能。这方面的研究提示未来H19不仅有望作为一种新的诊断标志物和治疗靶点,还将有助于肝脏疾病的个体化治疗。此外,与相邻的印迹基因也可能参与了肝脏疾病进展,基因位点还能编码miR-675,并产生两种反义RNA 91H和HOTS,使得H19在肝脏疾病中的作用机制变得更复杂。深入探讨单核苷酸多态性变异对H19功能与人群易感性的影响,甲基化水平对H19致病机制的影响,H19与IGF2、miR-675、91H和HOTS之间的调控关系,以及H19的这三种转录产物在肝脏疾病的不同阶段发挥的作用,对肝脏疾病的诊断和治疗具有重要意义。
[1] ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome[J]. Nature, 2012, 489(7414):57-74.
[2]张毅, 冯康倪, 陈鉴涛, 等. 非编码RNA在脑缺血再灌注损伤中的作用[J]. 中国病理生理杂志, 2021, 37(2):347-355.
Zhang Y, Feng KN, Chen JT, et al. Role of noncoding RNA in cerebral ischemia-reperfusion injury[J]. Chin J Pathophysiol, 2021, 37(2):347-355.
[3] Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II[J]. Nat Struct Mol Biol, 2007, 14(2):103-105.
[4] Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions[J]. Nat Rev Genet, 2009, 10(3):155-159.
[5] Song Y, Liu C, Liu X, et al. H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of epithelial cell adhesion molecule[J]. Hepatology, 2017, 66(4):1183-1196.
[6] Yang J, Qi M, Fei X, et al. LncRNA H19: a novel oncogene in multiple cancers[J]. Int J Biol Sci, 2021, 17(12):3188-3208.
[7] Wang H, Cao Y, Shu L, et al. Long non-coding RNA (lncRNA) H19 induces hepatic steatosis through activating MLXIPL and mTORC1 networks in hepatocytes[J]. J Cell Mol Med, 2020, 24(2):1399-1412.
[8] Brannan CI, Dees EC, Ingram RS, et al. The product of the H19 gene may function as an RNA[J]. Mol Cell Biol, 1990, 10(1):28-36.
[9] Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor[J]. RNA, 2007, 13(3):313-316.
[10] Berteaux N, Aptel N, Cathala G, et al. A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression[J]. Mol Cell Biol, 2008, 28(22):6731-6745.
[11] Onyango P, Feinberg AP. A nucleolar protein, H19 opposite tumor suppressor (HOTS), is a tumor growth inhibitor encoded by a human imprinted H19 antisense transcript[J]. Proc Natl Acad Sci U S A, 2011, 108(40):16759-16764.
[12] Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of thedifferentially methylated domain results in loss of imprinted expression ofand[J]. Genes Dev, 1998, 12(23):3693-3702.
[13] Li X, Liu R. Long non-coding RNA H19 in the liver-gut axis: a diagnostic marker and therapeutic target for liver diseases[J]. Exp Mol Pathol, 2020, 115:104472.
[14] Xu J, Cao X. Long noncoding RNAs in the metabolic control of inflammation and immune disorders[J]. Cell Mol Immunol, 2019, 16(1):1-5.
[15] Onofrio FQ, Hirschfield GM. The pathophysiology of cholestasis and its relevance to clinical practice[J]. Clin Liver Dis, 2020, 15(3):110-114.
[16] Xiao Y, Liu R, Li X, et al. Long noncoding RNA H19 contributes to cholangiocyte proliferation and cholestatic liver fibrosis in biliary atresia[J]. Hepatology, 2019, 70(5):1658-1673.
[17] Liu R, Li X, Zhu W, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis[J]. Hepatology, 2019, 70(4):1317-1335.
[18] Yang Z, Zhang T, Han S, et al. Long noncoding RNA H19: a new player in the pathogenesis of liver diseases[J]. Transl Res, 2021, 230:139-150.
[19] 邹步, 唐莹, 杨文玲, 等. 肠道菌群-FXR轴在代谢性疾病中的作用[J]. 中国病理生理杂志, 2019, 35(9):1716-1720.
Zou B, Tang Y, Yang WL, et al. Role of intestinal microbiota-farnesoid X receptor axis in metabolic diseases[J]. Chin J Pathophysiol, 2019, 35(9):1716-1720.
[20] Zhang Y, Liu C, Barbier O, et al. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function[J]. Sci Rep, 2016, 6(1):20559.
[21] Li X, Liu R, Huang Z, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans[J]. Hepatology, 2018, 68(2):599-615.
[22] Li X, Liu R, Yang J, et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice[J]. Hepatology, 2017, 66(3):869-884.
[23] 陈瑞玲, 马雄. 胆汁淤积导致肝纤维化的机制及其阻断策略[J]. 临床肝胆病杂志, 2019, 35(2):247-251.
Chen RL, Ma X. Pathogenesis of cholestasi-induced liver fibrosis and thoughts for blockade[J]. J Clin Hepatol, 2019, 35(2):247-251.
[24] Zhang L, Yang Z, Huang W, et al. H19 potentiates let-7 family expression through reducing PTBP1 binding to their precursors in cholestasis[J]. Cell Death Dis, 2019, 10(3):168.
[25] Li X, Liu R, Wang Y, et al. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions[J]. Cells, 2020, 9(1):190.
[26] Tian X, Wang Y, Lu Y, et al. Conditional depletion of macrophages ameliorates cholestatic liver injury and fibrosis via lncRNA-H19[J]. Cell Death Dis, 2021, 12(7):646.
[27] van Nieuwerk CM, Groen AK, Ottenhoff R, et al. The role of bile salt composition in liver pathology of-/-mice: differences between males and females[J]. J Hepatol, 1997, 26(1):138-145.
[28] Tietze L, Kessler SM. The good, the bad, the question: H19 in hepatocellular carcinoma[J]. Cancers, 12(5):E1261.
[29] Hernandez JM, Elahi A, Clark CW, et al. miR-675 mediates downregulation of Twist1 and Rb in AFP-secreting hepatocellular carcinoma[J]. Ann Surg Oncol, 2013, 20(Suppl 3):S625-S635.
[30] Zhou Y, Fan RG, Qin CL, et al. LncRNA-H19 activates CDC42/PAK1 pathway to promote cell proliferation, migration and invasion by targeting miR-15b in hepatocellular carcinoma[J]. Genomics, 2019, 111(6):1862-1872.
[31] Lv J, Yu YQ, Li SQ, et al. Aflatoxin B1 promotes cell growth and invasion in hepatocellular carcinoma HepG2 cells through H19 and E2F1[J]. Asian Pac J Cancer Prev, 2014, 15(6):2565-2570.
[32] Sun Z, Xue S, Zhang M, et al. Aberrant NSUN2-mediated m5C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma[J]. Oncogene, 2020, 39(45):6906-6919.
[33] Wei LQ, Li L, Lu C, et al. Involvement of H19/miR-326 axis in hepatocellular carcinoma development through modulating TWIST1[J]. J Cell Physiol, 2019, 234(4):5153-5162.
[34] Wang D, Xing N, Yang T, et al. Exosomal lncRNA H19 promotes the progression of hepatocellular carcinoma treated with Propofol via miR-520a-3p/LIMK1 axis[J]. Cancer Med, 2020, 9(19):7218-7230.
[35] Li H, Li J, Jia S, et al. miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancer[J]. Oncotarget, 2015, 6(31):31958-31984.
[36] Xu Y, Liu Y, Li Z, et al. Long non‑coding RNA H19 is involved in sorafenib resistance in hepatocellular carcinoma by upregulating miR‑675[J]. Oncol Rep, 2020, 44(1):165-173.
[37] Ye Y, Guo J, Xiao P, et al. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma[J]. Cancer Lett, 2020, 469:310-322.
[38] Zhang J, Han C, Ungerleider N, et al. A transforming growth factor-β and H19 signaling axis in tumor-initiating hepatocytes that regulates hepatic carcinogenesis[J]. Hepatology, 2019, 69(4):1549-1563.
[39] Conigliaro A, Costa V, Lo Dico A, et al. CD90+liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA[J]. Mol Cancer, 2015, 14:155.
[40] Ding K, Liao Y, Gong D, et al. Effect of long non-coding RNA H19 on oxidative stress and chemotherapy resistance of CD133+cancer stem cells via the MAPK/ERK signaling pathway in hepatocellular carcinoma[J]. Biochem Biophys Res Commun, 2018, 502(2):194-201.
[41] Zhang L, Yang F, Yuan JH, et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma[J]. Carcinogenesis, 2013, 34(3):577-586.
[42] Schultheiss CS, Laggai S, Czepukojc B, et al. The long non-coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma[J]. Cell Stress, 2017, 1(1):37-54.
[43] Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact[J]. J Hepatol, 2018, 68(2):268-279.
[44] 阎春英, 张蓉, 王雪, 等. 非酒精性脂肪性肝病患者血清LncRNA H19表达变化及临床意义[J]. 中国中西医结合消化杂志, 2021, 29(8):545-549.
Yan CY, Zhang R, Wang X, et al. Change of serum LncRNA H19 expression in patients with non-alcoholic fatty liver disease and its clinical significance[J]. Chin J Integr Tradit West Med Dig, 2021, 29(8):545-549.
[45] Chen Q, Wang T, Li J, et al. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD)[J]. Nutrients, 2017, 9(2):E96.
[46] Liu J, Tang T, Wang GD, et al. LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARγ axis in non-alcoholic fatty liver disease[J]. Biosci Rep, 2019, 39(7):BSR20181722.
[47] Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c[J]. J Clin Invest, 2004, 113(10):1408-1418.
[48] Liu C, Yang Z, Wu J, et al. Long noncoding RNA H19 interacts with polypyrimidine tract‐binding protein 1 to reprogram hepatic lipid homeostasis[J]. Hepatology, 2018, 67(5):1768-1783.
[49] Wang Y, Tai YL, Way G, et al. RNA binding protein HuR protects against NAFLD by suppressing long noncoding RNA H19 expression[J]. Cell Biosci, 2022, 12(1):172.
[50] Zhang N, Geng T, Wang Z, et al. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia[J]. JCI Insight, 2018, 3(10):e120304.
[51] Cheng XW, Chen ZF, Wan YF, et al. Long non-coding RNA H19 suppression protects the endothelium against hyperglycemic-induced inflammation via inhibiting expression of miR-29b target gene vascular endothelial growth factor a through activation of the protein kinase B/endothelial nitric oxide synthase pathway[J]. Front Cell Dev Biol, 2019, 7:263.
Progress in role of lncRNA H19 in liver diseases
HOU Mengzhen1,2, YU Yun1, WANG Jianqing1,2△
(1,,230012,;2,,230032,)
Long noncoding RNAs (lncRNAs) refer to a class of special RNAs having more than 200 nucleotides in their transcripts but featuring no protein-coding function. They play significant roles in epigenetic, transcriptional, and post-transcriptional regulation. H19 is the first identified imprinted lncRNA. It participates in the biological process of hepatocytes, cholangiocytes, immune cells and other cells, and has gradually become a new hotspot in the study of liver diseases. In this review, we introduce the characteristics of H19 and summarize its effect and molecular mechanism in 3 common liver diseases including cholestasis, hepatocellular carcinoma, and non-alcoholic fatty liver disease. We hope this review can provide new insights for related research and lead to potential therapeutic targets for liver disease treatment.
long noncoding RNA H19; cholestasis; hepatocellular carcinoma; non-alcoholic fatty liver disease
1000-4718(2023)07-1289-07
2022-11-04
2023-02-28
0551-66330208; E-mail: Jianqingwang81@126.com
R575; R363
A
10.3969/j.issn.1000-4718.2023.07.016
[基金项目]国家自然科学基金资助项目(No. 82073566);安徽省高校优秀青年人才支持计划项目重点项目(No. gxyq2019014);安徽省高等学校省级质量工程项目(No. 2020xsxxkc246);临床药学与药理学共建项目
(责任编辑:宋延君,罗森)
