CO2 valorization through methyl N-phenylcarbamate synthesis
2023-08-01XUNJiayaoSONGQingwenZHANGQianxiaHANLihuaZHANGKanZHANGJianliLIUPing
XUN Jia-yao,SONG Qing-wen,*,ZHANG Qian-xia,HAN Li-hua,ZHANG Kan,ZHANG Jian-li,LIU Ping,*
(1.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences,Taiyuan 030001, China;2.State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering,Ningxia University, Yinchuan 750021, China)
Abstract: Methyl N-phenylcarbamate (MPC) is an important intermediate for the synthesis of diphenylmethane diisocyanate(MDI),and its preparation using CO2 or its equivalents/derivatives as carbon source represents a green and sustainable manner for fine chemicals synthesis.This review will highlight the development of MPC synthetic methods from the viewpoint of chemical fixation of CO2.The contents mainly include the introduction of MPC synthesis through CO2 equivalents (urea or phenyl urea) alcoholysis,dimethyl carbonate (DMC) aminolysis,and the coupling of DMC and diphenyl urea.Furthermore,one-pot synthesis of carbamates/MPC from aliphatic amines/aniline,CO2 and alcohols is highlighted which represents one of the most promising schemes in direct CO2 utilization.What is more,the reaction mechanisms and selection of catalysts are also discussed in detail.The advances will provide important theories on further improving the efficiency of green catalysis and sustainable chemical processes.
Key words: carbon dioxide;CO2 equivalent;methyl N-phenylcarbamate;catalysis;synthetic method
For a long time,a large amount of CO2from the consumption of fossil fuels is emitted which continuously increases the greenhouse effect.Meanwhile,CO2is also a safe,abundant and inexpensive carbon resource.Considering environmental protection and resource utilization,it is of great significance to launch the study on the synthesis of highly value-added chemicals using CO2as the raw material[1,2].As a green building block,CO2has been widely used in the synthesis of urea,carbonates,carboxylic acids,formamides,hydrocarbons and so on[3-5].Among them,the synthesis of methylNphenylcarbamate (MPC) through the coupling of aniline with CO2or its equivalents and derivatives has attracted great attention.
MPC is one kind of methyl carbamate being widely used in synthesis of diphenylmethane diisocyanate (MDI) which is the main raw material for polyurethane (PU) production[6].In 2019,the total production capacity of isocyanates in the world was about 12.7 million t/a,and that of MDI was about 9.2 million t/a.China is the largest MDI producer in the world at present,and the production capacity reaches 3.7 million t/a accounting for around 68.9% of the world's total production capacity[7].In recent years,with the increasing global demand of PU and the awareness of energy conservation,the demand for MDI is also increasing.Besides,MPC is also used as an important organic intermediate in the synthesis of non-toxic polyurethane,isocyanate,polyvinyl amine,etc.[8]
At present,industrial production of MDI is from the phosgene method,namely the two-step reaction from aniline,formaldehyde and phosgene.In the process,the phosgene is highly toxic,and the byproduct hydrogen chloride is corrosive which can cause many potential environmental problems[9].In recent years,much attention is paid to the non-phosgene synthesis technology of MDI.As shown in Figure 1,the traditional non-phosgene route to MDI contains aniline oxidation carbonyl method[10],Hofmann rearrangement method[11]and nitrobenzene carbonyl reduction carbonyl method,etc.[12].In these methods,the use of precious metals as catalyst,harsh reaction conditions and the generated by-products are inevitable.Therefore,these non-phosgene protocols for MDI synthesis only stay in the theoretical stage.In the further,the green,economic and efficient routes will be indispensable and urgently needed.
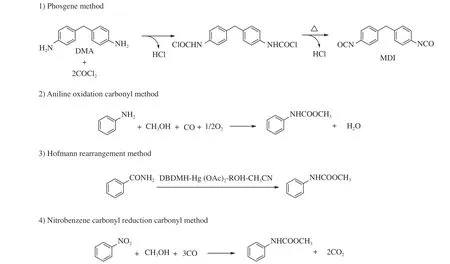
Figure 1 Traditional MDI synthetic methods
The synthesis of MDI using CO2or its equivalents and derivatives as carbon source represents the green and sustainable method with high atom economy under mild reaction conditions.Herein,the advances on the synthesis of methylN-phenylcarbamate,the key intermediate for the preparation of MDI,through CO2or its equivalents and derivatives as C1source are summarized.The highlights of synthetic methods,catalytic processes and reaction mechanism are introduced,and the results under different reaction conditions and catalysts are compared and discussed,which will provide the guidance for fundamental research and application exploration.
1 Synthesis of MPC through CO2 equivalents and derivatives
1.1 Phenyl urea alcoholysis
Industrial process for the synthesis of urea is via the reaction of CO2with ammonia.Moreover,phenyl urea and diphenyl urea can be easily synthesized through the reaction of urea and aniline in equal or excessive amount.As the equivalents of CO2,phenyl urea and diphenyl urea also play an important role in the synthesis of MPC[13].MPC can be formed via an exchange reaction between phenyl urea and methanol along with the generation of NH3(eq.(1)).Simultaneously,a competitive side reaction of phenyl urea and methanol occurs to give the aniline and methyl carbamate (eq.(2)).Besides,N-methylaniline can also be generated by the subsequent methylation reaction of aniline and methanol (eq.(3)).
In the previous work,Pb(OCH3)2was demonstrated to be the active species and showed good catalytic activity among the catalysts tested in the reaction of phenyl urea and methanol.In addition,the reaction conditions (including reaction temperature,time and methanol/phenyl urea mole ratio) also strongly affected the synthetic efficiency of MPC.Specially,the influence of methanol-pretreated PbO catalyst in the reaction of phenyl urea and methanol was studied[14].Comparing with the system without any catalyst,the conversion of phenyl urea and the selectivity of MPC increased after introducing Pbbased catalysts such as PbO,Pb3O4or methanolpretreated PbO,and meanwhile the selectivity of the byproduct aniline reduced.Using methanol-pretreated PbO catalyst under the same conditions,the selectivities of aniline,methyl carbamate andNmethylaniline were 14.51%,11.32% and 0.64%,respectively.After the pretreatment via methanol,PbO was transferred into the Pb(OCH3)2bearing better catalytic activity,and therefore the reactivity of phenyl urea was enhanced.Finally,a new addition-elimination reaction mechanism was proposed on the basis of the experimental results.In the presence of methanolpretreated PbO catalyst (Pb(OCH3)2species),the nucleophilic species CH3O-attacks the carbonyl carbon and meanwhile (Pb-OCH3)+combines with the nitrogen atom in -NH2.As a result,a tetrahedral transition state forms through the reaction of phenyl urea with Pb(OCH3)2),and then (NH2-PbOCH3)+detaches from the process with the generation of MPC.Meanwhile,the reaction of methanol with the intermediate NH2-PbOCH3regenerates Pb(OCH3)2and releases NH3.Due to the large diameter of Pb(OCH3)2and the geometrical barrier of benzene ring unit,the combination of (Pb-OCH3)+with nitrogen atom in -NH is suppressed.Therefore,MPC and ammonia are mainly produced.In addition,methanol can be activated via Pb(OCH3)2,which facilitates the transformation of phenyl urea.
Furthermore,solid alkaline catalysts,being prepared through supporting KF on metallic oxide(Al2O3,Nd2O3,La2O3,CeO2,Pr6O11or SiO2),were also demonstrated enable to promote the methanolysis of phenyl urea to MPC.As seen from the result,KF/Al2O3catalyst displayed good catalytic performance,and the conversion of phenyl urea reached 96.5% with MPC selectivity of 86.3%[15].There is a strong interaction between KF and Al2O3,and K3A1F6forms in the calcination process.KF is beneficial to the activation of methanol,while K3A1F6is conducive to the formation of MPC.Thus,the synergistic catalysis promotes the conversion of phenyl urea to MPC.
Methanol is used as both reactant and solvent in phenyl urea alcoholysis method,and the excess of methanol (e.g.the molar ratio of phenyl urea/methanol up to 1:40) is unfavorable to industrial production.Due to the equivalent amount of NH3generated during the reaction,the atom economy is relatively low,and meanwhile there is much difficulty in the separation process of methanol.Additionally,the competitive reaction in this system results in the formation of aniline and carbamate,which leads to the difficulty in separation and simplification of equipment.At the same time,the production cost increases.In the process of phenyl urea alcoholysis,the efficiency of Pb(OCH3)2catalyst is very high,but the metal used is not friendly to the environment.In addition,the cost of the raw material phenyl urea is also high,and now the method only stays in the laboratory stage and is reported rarely.
1.2 Diphenyl urea alcoholysis
MPC can also be synthesized through the reaction of diphenyl urea and methanol in the presence of suitable catalyst (eq.(4)),and fortunately diphenyl urea is easily obtained through the reaction of aniline with CO2[16,17].In addition,it is worth noting that the yield of diphenyl urea is enhanced after the introduction of CO2into the system.Firstly,aniline can react with CO2and give diphenyl urea (eq.(5)).Secondly,the removing of the aniline in eq.(4) through eq.(5) will promote the equilibrium shifting to the direction of MPC generation.At last,the synergistic effect further stimulates the conversion of methanol,and as a result,both the conversion of diphenyl urea and the selectivity of MPC are greatly improved.
Comparing with the system without any catalyst,both the conversion of diphenyl urea and selectivity of MPC increased in the presence of metal catalyst[18].As for the mechanism,the MPC is formed through the exchange reaction between phenylamino group of diphenyl urea and -CH3O of methanol along with the generation of by-product aniline.In this work,the catalysts supported on various metal compounds including Pb,Zn,Al,Fe or Mo were investigated successively.In the absence of catalysts,diphenyl urea conversion,MPC yield and selectivity reached 70.21%,40.32% and 57.43%,respectively.However,after introducing the Mo catalyst,the conversion of diphenyl urea,the yield and selectivity of MPC correspondingly increased to 85.36%,73.92% and 86.60%.With the addition of PbO,the catalytic activity was enhanced,and the conversion of diphenyl urea and the selectivity of MPC significantly increased.The conversion of diphenyl urea was higher than that of the other catalytic species screened when PbO/Al2O3was used as the catalyst.Comparing with PbO catalyst,the selectivity of MPC from PbO/Al2O3catalytic system was slightly lower.Nevertheless,the PbO/Al2O3catalyst was more easily recovered from the reaction system than that of PbO catalyst,which was favorable for the technology exploration.Under the conditions of 180 °C for 3 h,the mass ratio ofm(methanol)/m(diphenyl urea) being 10∶1,the conversion of diphenyl urea reached up to 95.68%,and the selectivity of MPC reached 88.16% in presence of PbO/Al2O3catalyst.To inhibit theNmethylation reaction of aniline with methanol to produceN-methylaniline,CO2was introduced into the reaction for consuming the aniline.The activation energy of CO2reacting with aniline to produce diphenylurea is relatively low,however,the methylation reaction of methanol with aniline requires higher activation energy.As a result,noNmethylaniline formation is observed in the product,indicating that the introduction of CO2inhibits the methylation reaction between methanol and aniline.By adjusting the conditions,the furtherN-methylaniline reaction between aniline and methanol was inhibited leading to an improved yield and selectivity of MPC,and a new and effective method for the synthesis of MPC was opened up.
Totally,MPC is obtained through the reaction of diphenyl urea and methanol under relatively mild conditions.The main side reaction is methylation of aniline.The introduction of high pressure of CO2contributes to an improved selectivity of MPC but leads to the new by-product i.e.dimethyl carbonate(DMC) which can also accelerate the formation ofNmethylaniline.As a result,the separation process can also become complicated and the production cost also increases.
1.3 Urea alcoholysis
The urea alcoholysis route contains two steps.Firstly,the reaction of urea with methanol gives a methyl carbamate (eq.(6)).Secondly,methyl carbamate reacts with aniline to form MPC (eq.(7)).Both of them afford a stoichiometric ammonia.In fact,the first step of this route has been widely used,and the yield of methyl carbamate can reach more than 90%[19].Herein,the second step is studied[20],and on the basis of the distribution of products,the main and side reactions occurring in the reaction system are also proposed (eqs.(3) and (4)).
Using ZnCl2as catalyst,MPC was synthesized with high selectivity by the reaction of aniline and methyl carbamate under methanol medium[20].The introduction of excess methanol in the reaction system significantly increased the MPC selectivity.Methanol is beneficial to promoting the eq.(4) shifting to the right side.By contrast,short of methanol,the side eq.(8) is enhanced,and meanwhile DPU and ammonia are also generated.At 160 °C for 4 h,the aniline conversion and MPC selectivity can reach 90.1% and 99.7%,respectively.
Urea alcoholysis method can be used to synthesize MPC conveniently due to the abundant raw materials and simple processes.However,the separation process is relatively complicated and the efficiency is urgently needed to be improved.In addition,coupling the two steps,MPC can be obtained in “one pot” pattern as seen in eq.(9).
Wang et al.[21]reported an effective route to synthesizeN-substituted carbamates from amines,urea and alcohols,and in the work,the developed TiO2-Cr2O3/SiO2catalyst performed the excellent catalytic activity.Several different aliphatic amines (butylamine,cyclohexylamine,isophorone diamine,4,4'-diaminodicyclohexyl methane and 1,4-butanediamine)and various aromatic amines were tested,and meanwhile the alcohols bearing different chain length(from methanol to butanol) were also examined.Under the optimized reaction conditions (n(amine)∶n(urea)∶n(alcohol) being 1∶1.2∶15,0.1 g catalyst,190 °C,8 h),several importantN-substituted carbamates were successfully synthesized in good yields.Under the optimized reaction conditions,the conversions of various aromatic amines are 73%-99% along with 70%-97% yields of the correspondingN-substituted carbamates.The aromatic amines behave a little weaker basicity and nucleophilicity than those of aliphatic amines due to the conjugate effect,thus showing a lower reactivity.
The catalytic performances of some metal oxides(PbO,MgO,CaO,ZnO and γ-Al2O3) and Lewis acids(AlCl3,ZnCl2,Zn(OAc)2,Pb(OAc)2and Pb(NO3)2) on the one-step synthesis of MPC from aniline,urea and methanol were investigated as well as the effects on the generation of by-products DPU,phenyl urea (PU),Nmethylbenzourea (NMA),andN,N-dimethylbenzourea(DMA)[22].Under the conditions of 180 °C,0.6 MPa,2 h,n(aniline)∶n(catalyst)∶n(urea)∶n(MeOH) being 1∶0.12∶5∶75,all of the metal oxides had certain catalytic effect on the reaction.Among them,only γ-Al2O3calcined at400 °C for 2 h improved aniline conversion as well as MPC selectivity up to 88.4% and 84.6%,respectively.Among the investigated Lewis acid catalysts,only in the presence of Zn(OAc)2,the conversion of aniline and the selectivity of MPC reached up to 82.2% and 81.1%,respectively.The catalytic performance of γ-Al2O3had no obvious change after reusing for five times.Afterwards,a reaction mechanism referring to five possible reaction paths namely via DMC,methyl carbamate (MC),1-phenyl biuret (PBU),PU or DPU intermediates was conjectured.In order to verify the feasibility of these pathways,thermodynamic analysis and experimental studies were carried out on the reactions involved in these pathways.The results revealed that in the absence of catalyst,the reaction through PU intermediate was mainly taken place.While in the presence of γ-Al2O3catalyst,the synthesis of MPC went through the MC intermediate[23].
Qin et al.[24]carried out one-step reaction of aniline,urea and methanol to synthesize MPC in a high-pressure reaction kettle,and HY catalyst was screened out with good catalytic performance.Under the conditions ofn(AN)∶n(urea)∶n(MeOH) being 1∶5∶15,180 °C for 5 h,the yield of MPC was 66.5%,and the catalyst retained high catalytic activity after recycling 4 times.They prepared catalysts by impregnating alkaline metals and alkaline earth metals on HY,and found that KNO3/HY showed the best catalytic performance.The aniline conversion and MPC selectivity reached 93.5% and 83.5% respectively under the condition of 180 °C for 5 h[25].In the first step,urea interacts with active surface site of HY and carbonyl group is activated.In the second step,aniline and methanol molecules react with carbonyl of urea and give methylN-phenylcarbamate.This step can occur via two reaction pathways,one goes through a MC intermediate,and another undergoes a phenyl urea intermediate.In detail,methanol or aniline exerts the nucleophilic attack on the urea and gets rid of a NH3at first to form MC or phenyl urea,and then aniline or methanol nucleophilically attacks the intermediate and gets rid of a NH3to form MPC.
1.4 Aminolysis of DMC
DMC being a widely used building block and green chemical has attracted considerable attention in recent years.The mainstream synthetic route to DMC is from sustainable CO2and methanol,and water is the only byproduct (eq.(10))[26].As one of the most important derivatives of CO2,DMC has many advantages as a feedstock to react with aniline for the synthesis of MPC.Comparing with the traditional routes,the avoidance of highly toxic phosgene represents one of the most promising and green methods.
In view of the environmentally friendly advantage,MPC can be directly synthesized through the reaction of DMC and aniline with the assistance of suitable catalysts (eq.(11))[27-29].
Thermodynamic calculation on the synthesis of MPC was carried out through the non-phosgene method from DMC and aniline,and the results showed that it is a spontaneous exothermic reaction.Therefore,the reaction is favorable for the synthesis of MPC under low reaction temperature.With the decrease of reaction temperature,the reaction rate also slows down.In practical application,the synthetic reaction is generally carried out at about 150 °C under the catalytic condition[30].
Zinc acetate catalyst supported on activated carbon (AC) or γ-Al2O3showed good catalytic properties in the reaction of aniline and DMC (Table 1,entry 1).The MPC was obtained in up to 78% yield and 98% selectivity in the Zn(OAc)2/AC catalyst system.The study revealed that the acidic supporter can promote theN-methylation of aniline while the alkaline supporter benefits for the formation of diphenyl urea[31].Baba et al.[32]reported the methoxy carbonylation of 2,4-toluene diamine with DMC using Zn(OAc)2·2H2O as a catalyst.Under the condition of 180 °C for 2 h,dimethyltoluene-2,4-dicarbamate was produced in a yield of 98%.During the study,an induction period was observed in the reaction of aromatic amines with DMC.However,with the addition of methanol,the methoxy carbonylation of aniline with DMC was accelerated.Notably,after pretreating the catalyst with methanol,the induction period was almost completely eliminated.A series of inorganic and organic zinc compounds e.g.ZnX2(X=CH3COO,Cl,SO4,Br or NO3),Zn-MOF and so on were screened[33].Among them,Zn(CH3COO)2showed the best catalytic activity (Table 1,entry 2).In the optimized reaction conditions of molar ratio of DMC to aniline being 9 at 170 °C for 2 h,the conversion of aniline reached 99% and the selectivity of MPC was over 90%.
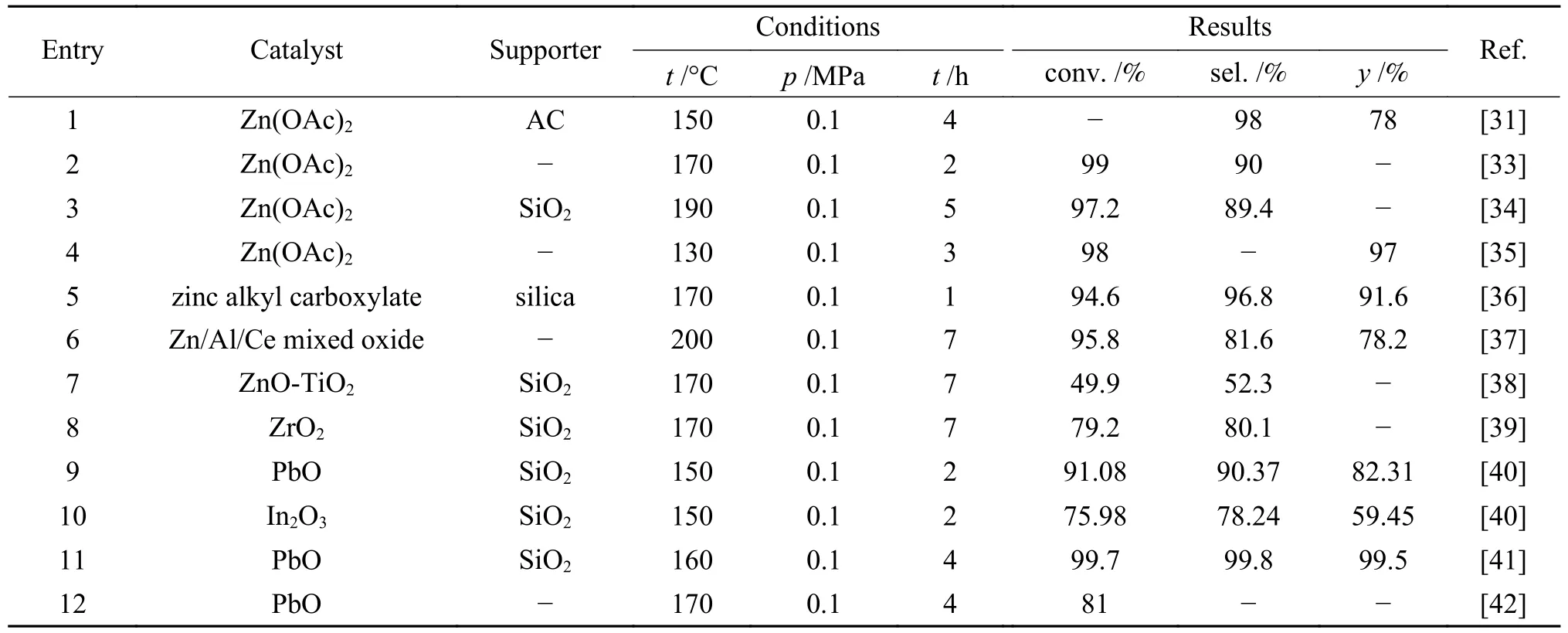
Table 1 Metal catalysts for MPC synthesis from aniline and DMC
Zn(OAc)2/SiO2catalyst was prepared through solvothermal impregnation with DMC as the solvent(Table 1,entry 3)[34].Under the conditions of 190 °C for 5 h,n(DMC):n(aniline) being 20:1 andm(aniline):m(Zn(OAc)2/SiO2) being 0.2,the aniline conversion and MPC selectivity reached 97.2% and 89.4%,respectively.Zn(OAc)2/SiO2catalyst showed good stability.After reusing 7 times,the aniline conversion and MPC selectivity correspondingly decreased to 79.19% and 79.29%.The explanation is that DMC as the solvent is easily hydrolyzed during the process of solvothermal impregnation.Furthermore,the presence of Zn(OAc)2will accelerate the hydrolysis of DMC.Moreover,the adsorbed water on the surface of SiO2will be hydrolyzed thus releasing a large number of free silica hydroxyl group,which is conducive to the dispersion of the active component on the SiO2.As the dispersion of Zn(OAc)2on SiO2surface increases,the density of Si-O-Zn bonds on SiO2supporter rises.Meanwhile,the steric-hindrance effect of Zn2+enhances,which inhibits the reaction of Zn(OAc)2with methanol and avoids the conversion of Zn(OAc)2into invalid ZnO.
Furthermore,in the Zn(OAc)2-catalyzed reaction of aniline and DMC (Table 1,entry 4),Zn(OAc)2could be converted into Zn4O(OAc)6which also showed excellent catalytic activity for carbamate synthesis[35].Besides,some other catalysts containing Zn components also displayed good catalytic performance.For example,a new type of heterogeneous salt catalyst was designed and prepared through zinc alkyl carboxylate covalently bonding on silica by a special grafting method in aqueous media[36],and it was demonstrated highly active and recyclable for MPC synthesis from aniline and DMC (Table 1,entry 5).In addition,Zn/Al/Ce mixed oxide was also acting as an effective and recoverable heterogeneous catalyst for MPC synthesis via DMC aminolysis[37].The introduction of appropriate amount of cerium could form the strong interaction within the mixed oxide.Thus,Zn/Al/Ce2.5oxide showed high catalytic activity with 95.8% conversion of aniline and 78.2% yield of MPC (Table 1,entry 6).Moreover,the catalyst also exhibited a good stability with retained nanoplate structure after five cycles.During the 5 repeated runs,the conversion of aniline was kept at about 95%,but the selectivity of MPC decreased slowly.Factually,the supporter acted as a key role on the catalytic activity improvement of metal species.Comparing with ZnOTiO2catalyst in synthesis of MPC,MPC selectivity obtained through ZnO-TiO2/SiO2catalyst was improved (Table 1,entry 7)[38].In addition,the ZnOTiO2/SiO2showed higher MPC selectivity than ZnOTiO2/HZSM-5 and ZnO-TiO2/γ-Al2O3.Aniline conversion and MPC selectivity reached 49.9% and 52.3% under conditions ofn(aniline)∶n(DMC)=1∶20 andm(catalyst)∶m(DMC)=0.013,respectively.The catalytic activity of ZrO2and ZrO2/SiO2were also investigated.As a result,ZrO2supported on SiO2could greatly improve the catalytic performance (Table 1,entry 8)[39].This attributes to the formation of Zr-O-Si bond through the interaction between ZrO2and SiO2,which significantly increases the proportion of weak acid sites on ZrO2/SiO2surface,and the weak Lewis acid sites are in favor of MPC synthesis.
Based on the systematic study on the catalytic performance of PbO catalyst for the synthesis of MPC through the reaction of aniline and DMC,the catalytic performance of the supported PbO catalysts with different carriers was investigated[40,41].Among them,the SiO2carrier is demonstrated with the highest catalytic performance (Table 1,entry 9).Subsequently,the catalytic ability of potential active components for MPC synthesis was investigated using SiO2as the supporter,and finally the In2O3/SiO2catalyst exhibits the highest catalytic activity among the selected candidates.Under the conditions of Pb compound(PbO/SiO2) catalyst with 5∶1 molar ratio of DMC/aniline,10% Pb (based on aniline) at 150 °C for 2 h,the conversion of aniline reaches 91.08%,and the yield of MPC is 82.31% with 90.37% selectivity.Considering the toxicity of Pb compounds and the easy deactivation of PbO catalyst,In2O3/SiO2catalyst is selected as a substitute.Under the same reaction conditions,the catalyst also shows good catalytic activity,and the aniline conversion,MPC yield and selectivity are 75.98%,59.45% and 78.24%,respectively (Table 1,entry 10).With the increased numbers of catalyst recycling experiments,the aniline conversion and MPC selectivity show a downward trend.Totally,In2O3/SiO2catalyst shows a relatively long lifetime and its deactivation is not significant after recycling 8 times.Meanwhile,the complex posttreatment process is avoided during the catalyst regeneration stage.
The catalytic activity of Sn-,Ti-,Zn-and Pbbased catalysts for the reaction of DMC and aniline was investigated,and the PbO catalyst displayed the outstanding catalytic activity (Table 1,entry 12)[42].Under the reflux condition at 170 °C for 4 h with 7.5%PbO,the conversion of aniline reaches 81%.In addition,the study also reveals the catalytic activity of Pb3O4-ZnO composite catalyst is higher than that of Pb3O4or ZnO alone,which indicates a certain synergistic effect.
In summary,on the one hand,this synthetic method leads to the generation of equivalent methanol,which reduces the atom economy and increases the complexity of product separation.On the other hand,methanol can easily react with aniline to generate byproductN-methylaniline which reduces the chemoselectivity and meanwhile increases the difficulty of separation.Therefore,the development of highly efficient catalyst is indispensable to obtain the specificity of the reaction.
1.5 The coupling of DMC and diphenyl urea
Among the above-mentioned methods,aminolysis of DMC and alcoholysis of diphenyl urea are the relatively better methods that focusing on the synthesis of carbamate by non-phosgene method with good industrial prospects.However,equal mole of methanol or aniline is generated as by-products in both methods,and therefore,the atom economy of the reaction is very low.Delightedly,the synthesis of MPC is achieved by the coupling reaction of DMC and diphenyl urea(eq.(12)).This process is much simpler than the previous routes.In this transformation,only the target product MPC is afforded,which greatly increases the utilization ratio of raw materials and meanwhile reduces the difficulty of the separation procedure.The method represents a very promising non-phosgene route featuring as environmentally friendly and high atom economy[43].
Di-n-butyltin oxide (DBTO) was reported as a catalyst for the synthesis of carbamates from substituted urea and organic carbonates.The results verify that basic homogeneous catalysts such as tin complexes,particularly DBTO show excellent catalytic activity towards carbamate synthesis.In aminolysis of substituted carbonates,the reactivity depends upon the basicity of nucleophilic amine.Aliphatic ureas show higher reactivity than aromatic ureas.Till now,the reports on the route to MPC from DMC and diphenyl urea are few,and the study still stays in the laboratory stage.
Based on the previous reports on the role of PbO species,the supported PbO catalyst was investigated in the reaction of diphenyl urea and DMC[44].After the screening of different supporters,Al2O3is found to be the potential high activity supporter.Under the conditions of 160 °C for 4 h with 4% catalyst relative to diphenyl urea as well as the mass ratio of DMC and DPU being 15,MPC is afforded in up to 98.2% yield.Liu et al.[45]prepared a new alcoholic Pb catalyst HOPb-OCH3.For the synthesis of MPC from DMC and DPU,the alcoholic Pb catalyst shows good catalytic activity under the reaction pressure of 0.5 MPa,and the catalytic activity remains stable after four cycles.Under the conditions of 120 °C for 2 h,the raw material ration(DMC) andn(DPU) being 5,catalyst dosage being 1.0%,both DPU conversion and MPC selectivity reach 99%.There is a -PbOH group in this catalyst which performs high catalytic activity.Moreover,the catalyst reacts with DMC and trace water from the system to obtain basic lead carbonate 2PbCO3·Pb(OH)2with the same good catalytic performance.The nucleophilic group -PbOH in 2PbCO3·Pb(OH)2attacks the carbonyl carbon of DMC,causing demethylation of DMC and subsequently forming -PbOCOOCH3.Then,this unit interacts with DPU via (Ph-NH-PbOCH3)+intermediate to give the target product MPC.In addition,NaOCH3is also demonstrated to be an excellent catalyst in a nonphosgene route for the synthesis of MPC from DMC andN,N'-diphenyl urea,and the reaction proceeds smoothly at atmospheric pressure[46].At 90 °C,the yield of MPC exceeds 80%.By comparison,under the conditions of 3.4 MPa and 150 °C,the yield of MPC only exceeds 77% using highly poisonous homogenous Bu2SnO catalyst.For this route,there is nearly no side reaction happening,and meanwhile the target product is single which is convenient for products separation,purification and industrial production.The emphasis of this path is to develop the powerful catalysts to boost the efficiency and the easily recovered catalysts to facilitate the catalyst separation and recycling from the reaction system.
2 Direct CO2 route to carbamates and MPC:one-pot reaction of various amines,CO2 and alcohols
Indirect methods such as CO2equivalents and derivatives used for preparation of MPC with special advantages especially bearing thermodynamic favorable process have been introduced above,however,the most ideal route to MPC is the direct method using CO2as a carbon resource.One-pot cascade reaction of aniline,CO2and methanol represents the most simple and green route to MPC,which uses the widely available commercial raw materials.Currently,most of the carbamates synthesized by this method are limited to the aliphatic carbamates from aliphatic amines and CO2.The reactivity of aromatic amine substrates is very low.Therefore,one-pot synthesis of MPC from aniline,methanol and CO2is generally confronted with very low reactivity (eq.(13)),which makes this method face a great challenge.Herein,the advances on insights into efficient methods to carbamates/MPC direct from aliphatic amines/aniline and CO2in the past several years are reviewed[47].Especially,the CeO2-catalyzed effective scheme for MPC synthesis from aniline,CO2and CH3OH with the assistance of 2-cyanopyridine as dehydrating agent is highlighted here.
Firstly,thermodynamic calculations were carried out for the reaction of aniline,CO2and methanol.The results showed that the three-component reaction is exothermic (ΔrH=-35.27 kJ/mol) but it is nonspontaneous (ΔrG=12.53 kJ/mol)[48,49].In addition,three routes via the intermediates of DPU,DMC and DPU/DMC,respectively,were proposed (Figure 2).Among the routes,the route via DPU intermediate was demonstrated to be the most favorable manner[50].

Figure 2 Proposed routes to MPC via three different processes [50]
A series of alkaline catalysts were examined for the synthesis of MPC through aniline,CO2and methanol[51].As seen from the results,the bicyclic amidine DBU showed outstanding catalytic ability in acetonitrile which contributed to the good solubility of CO2.Besides,acetonitrile also acted as a dehydrating agent to shift the reaction equilibrium to the right.Under the conditions of initial 1 MPa CO2at 180 °C for 5 h,volume ratio of methanol to aniline being 1:4,and volume ratio of acetonitrile to aniline being 1:1,the conversion of aniline and the selectivity of MPC were 7.1% and 25.3%,respectively.Similarly,in the presence of DBU and CH2Br2as both solvent and indirect dehydrating agent,carbamates were obtained from aromatic amines,CO2,and CH3OH under mild conditions[52].Two possible routes referring to the amine or alcohol activation through DBU were proposed.The active [DBUH+][] and [DBUH+][] intermediates were converted into the target products through reacting with the corresponding amines and alcohols with the assistance of CH2Br2.In the metal-contained system,Cu-Fe/ZrO2-SiO2catalyst was investigated and showed catalytic activity in the synthesis of MPC using acetonitrile as a dehydrating agent[53].Under 10% metal loading,14% conversion of aniline and 65.7% selectivity of MPC were obtained,which were much higher than the values reported.Nevertheless,there remain some shortcomings being confronted in these systems.Firstly,both the reactivity of aniline and selectivity of MPC are very low.Additionally,the chemical dehydrating method adopted still brings much byproduct and increases the complexity in the separation procedure.
In 2018,Tomishige's group[54]reported a CeO2-catalyzed scheme for MPC synthesis from aniline,CO2and CH3OH with the assistance of 2-cyanopyridine as dehydrating agent (Figure 3).To date,this is the best example reported for highly efficient MPC synthesis using CO2as direct carbon source.The reaction proceeds smoothly,and the MPC is afforded in more than 99% conversion and 98% selectivity under the mild conditions.

Figure 3 Possible route of CeO2-catalyzed four-component reaction
In this work,various metal oxides and nitrile dehydrating agents were investigated,and CeO2combining with 2-cyanopyridine was demonstrated to be the best protocol.Based on the experimental study,the synthetic mechanism of MPC via DPU intermediate was proposed.Initially,aniline reacts with CO2to form DPU and H2O,and meanwhile the H2O molecule is consumed through hydration reaction of 2-cyanopyridine.Moreover,the intermediate DPU reacts with methanol to produce the target product MPC with the generation of aniline.In the system,CeO2offering the active catalytic species can catalyze both the coupling reaction for MPC synthesis and hydration of 2-cyanopyrdine under the same reaction conditions[55].
At present,the reports on the synthesis of carbamates/MPC by one-pot method from amines,CO2and alcohols are relatively few,and the target products are mostly concentrated on aliphatic carbamates.Under the conditions of 30 MPa CO2at 200 °C for 24 h,the reaction of tert-butylamine (t-BuNH2),ethyl alcohol and CO2can take place smoothly in the presence of Bu2SnO and two equivalents of 2,2-diethoxypropane with 100% conversion oft-BuNH2and 84% selectivity of urethane[56].Under the same conditions,using Ni(OAc)2as catalyst,4,4-dimethyldipyridine as additive and dimethylacetal as dehydrant,the conversion oft-BuNH2reaches 49% and the selectivity oftert-butylcarbamate is up to 98%[57].
Basic catalysts were found suitable to convert a broad variety of amines and alcohols into the corresponding carbamates using CO2as a carbonyl source.Under the conditions of 2.5 MPa CO2at 200 °C for 24 h,Cs2CO3acting as catalyst,and the molar ratio ofn-octylamine ton-PrOH being 1∶15,the conversion of amine and the selectivity of carbamate reached 56%and 79%,respectively[58].The catalytic scheme is able to convert both linear and branched aliphatic amines into their corresponding carbamates under mild reaction conditions.For the amine substrate with steric hindrance such ast-BuNH2,the introduction of added dehydrating agents such as dimethoxypropane or diethoxypropane is required,and as a result the corresponding carbamate is obtained with over 90%selectivity.
Using CeO2as a heterogeneous catalyst,one-pot synthesis of aliphatic carbamates from CO2,benzylamine and alcohols was also achieved[59].In the absence of any dehydrating agent,the reactivity of different amines was detailedly investigated.Under the CeO2-catalyzed reaction conditions of initial 5 MPa CO2at 150 °C for 2 h,n(amine)∶n(alcohol) being 5∶900,the amine conversion and carbamate selectivity were up to 99% and 89%,respectively.The protocol was also compatible with the other aliphatic amines(e.g.benzylamine,amino methylcyclohexane and methylamine) and alcohols (e.g.methanol,ethanol,1-propanol and 2-propanol) in good yields and high selectivity.Nevertheless,the reactivity of aniline is still very low.During the process,the CeO2catalyst is deactivated because of the poisonous adsorption of the products.Delightedly,the experimental results show that the CeO2catalyst can be reused after the calcination at 600 °C.
Based on the previous study[54],Tomishige et al.[60]continued to expand the research on synthesis of alkylN-alkylcarbamates from anilines,CO2and various alcohols through CeO2and 2-cyanopyridine system.In the work,alcohol substrates were extended to the branched alcohols such as 2-propanol and cyclohexanol.As a result,the undesirable side reaction of alcohols with CO2to dialkyl carbonates was remarkably suppressed probably due to the steric effect of branched alcohols,and the formation of picolinamide was minimized.High conversion of aniline (97%) and yield of isopropylN-phenylcarbamate (94%) were obtained under 1 MPa CO2,and low CO2pressure (0.5 MPa) was also applicable in 90% yield of isopropylNphenylcarbamate.In this study,various alkylNalkylcarbamates could be synthesized from CO2,anilines,and alcohols in good product yield and high selectivity.
Comparing with aliphatic carbamates,the synthesis of methylN-phenylcarbamate by one-pot reaction of aniline,methanol and CO2has many disadvantages e.g.low reactivity and poor selectivity.This may be due to the hyper conjugation effect of the aromatic ring,which reduces the electron density of N atom in the amino group and subsequently weakens its alkalinity and nucleophilicity.Therefore,it is necessary to improve the yield of MPC prepared by one-pot reaction of aniline,methanol and CO2from two aspects:one is to develop a robust catalyst enable to activate the substrates and CO2,the other is to remove the water generated from the reaction in time to break the chemical equilibrium and promote the reaction to take place in the direction of product generation.
3 Summary and outlook
With the continuous increase of diphenylmethane diisocyanate requirement during recent years,the synthesis of methylN-phenylcarbamate,a key raw material for the synthesis of MDI,is becoming more and more important,and the green and sustainable preparation methods are urgently needed.Currently,the effectively indirect routes to MPC are dependent on the coupling of methanol with CO2equivalents or derivatives acting as carbon/nitrogen source.These efforts contribute to much important foundation in the process of technology exploration.Although great advances are made,most of the catalysts rely on the limited metal species e.g.Pb compounds.In addition,the selectivity is still very low in most systems,and the process is also complicated which restricts the exploration of industrial technology.Moreover,the thermal stability of the current supported catalysts is not well enough to meet the practical production.In particular,the most ideal route to MPC is the reaction of commercial aniline,methanol and CO2used as carbonyl group source.However,the thermodynamic limitation bottleneck is confronted,thus leading to very low efficiency.At present,the effective schemes are still depended on a great deal of chemical dehydrating agents which produces much waste.At the same time,the reactivity and selectivity are still insufficient.
In the near further,to solve the problems in the synthesis of MPC,the development of new and efficient catalysts is inevitable.Firstly,expanding the newly efficient and non-toxic metal catalyst in place of Pb and the other metal species is needed to boost the efficiency of the process.Secondly,it is necessary to develop the heterogeneous catalysts e.g.supported catalysts with high catalytic activity and stability.Furthermore,although the great challenge confronted in the direct method for CO2conversion to MPC,the breakthrough is urgently needed to make and it is one of the most promising trends from the viewpoint of green and sustainable chemistry.The hopeful fields refer to the exploration of functional catalytic materials in combination with the water removal process and the highly efficient technology on the regeneration of dehydration agent.Last but not least,the exploration of the simple and efficient separation methods is indispensable for the economic industrial technology.
