Template synthesis of copper azide primary explosive through Cu2O@HKUST-1 core-shell composite prepared by “bottle around ship” method
2023-07-31XuwenLiuYanHuJiahengHuJiaxinSuCaiminYangYinghuaYeRuiqiShen
Xu-wen Liu ,Yan Hu ,* ,Jia-heng Hu ,Jia-xin Su ,Cai-min Yang ,Ying-hua Ye ,Rui-qi Shen
a School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China
b Micro-Nano Energetic Devices Key Laboratory, Ministry of Industry and Information Technology, Nanjing, 210094, China
Keywords:Composite energetic materials Copper azide Carbonization Template method Core-shell composite Electrostatic safety
ABSTRACT Copper azide (CA),as a primary explosive with high energy density,has not been practically used so far because of its high electrostatic sensitivity.The Cu2O@HKUST-1 core-shell structure hybrid material was synthesized by the “bottle around ship” methodology in this research by regulating the dissolution rate of Cu2O and the generation rate of metal-organic framework (MOF) materials.Cu2O@HKUST-1 was carbonized to form a CuO@porous carbon(CuO@PC)composite material.CuO@PC was synthesized into a copper azide(CA)@PC composite energetic material through a gas-solid phase in-situ azidation reaction.CA is encapsulated in PC framework,which acts as a nanoscale Faraday cage,and its excellent electrical conductivity prevents electrostatic charges from accumulating on the energetic material's surface.The CA@PC composite energetic material has a CA content of 89.6%,and its electrostatic safety is nearly 30 times that of pure CA(1.47 mJ compared to 0.05 mJ).CA@PC delivers an outstanding balance of safety and energy density compared to similar materials.
1.Introduction
Traditional pyrotechnics can no longer meet the requirements of advanced miniaturization weapons and equipment as modern weapon technology develops.Modern pyrotechnics are characterized by miniaturization,intelligence,and a high degree of integration [1].The energy density of lead-based primary explosive commonly used in conventional detonating systems is not high enough that they can no longer meet the requirements of sophisticated pyrotechnic devices.Furthermore,leftover lead ions after initiation are detrimental to the environment in practical applications,necessitating the development of a new green high-energy primary explosive.
CA is an eco-friendly and sustainable primary explosive with great initiation performance [2-5],with six times the capacity to initiate PETN than lead azide (LA) [6].If it could be employed as a primary explosive in advanced pyrotechnics,the output energy of the energetic device could be increased while the input energy required decreased.It's particularly intriguing that CA can be prepared in-situ by reacting copper (or copper oxide) with azido acid gas,which is perfectly tailored to the needs of Micro-Electro-Mechanical System (MEMS) technology for the safe filling of explosives.However,CA's extreme electrostatic sensitivity is the key problem preventing it from being used in weapon charges [6,7].
Over the last ten years,researchers have focused on compounding CA with a range of carbon materials and utilizing carbon materials'superior conductivity to increase the electrostatic safety of composite energetic materials based on CA.The following three categories are by far the most typical ideas: filling CA into carbon nanotubes (CNTs) [8-11],using copper-containing MOF materials or other polymers as precursors,and then synthesizing CA coated with carbon framework through carbonization and azidation[12-16],and composite CA with graphene and other carbon materials[17-19].CA composite energetic materials synthesized with MOF as a template perform the best among these concepts.
In recent years,researchers have focused a great deal of attention on energetic MOF[12-14].Yang Li et al.[13]first proposed the approach of synthesizing CA by carbonizing HKUST-1 and then reacting with hydrogen azide gas,resulting in an electrostatically insensitive composite energetic material.HKUST-1,also known as MOF-199 [20,21],is a material in the MOF class,which was first synthesized by Chui et al.at the Hong Kong University of Science and Technology.The ligands in HKUST-1 were carbonized into PC framework at high temperature,while its metal center Cu2+was converted to copper.During the gas-solid azide reaction,the porous structure of the composite allows it to react more thoroughly[22-24].The carbon skeleton separates CA molecules on a microscopic level,and this structure can diminish the composite material's sensitivity to weak external stimuli [12-14].Despite the fact that numerous investigations on carbon-based CA energetic materials have been conducted in recent years,the composite energetic materials synthesized with carbonized MOF as a template remain the most impressive.It provides the optimal balance of high energy density and electrostatic safety [13].
Nevertheless,the metal center Cu2+in the copper-containing MOF is the only Cu source in the carbonized MOF,limiting the amount of CA in the energetic material.As a result,it is viable to explore incorporating metal nanocrystals as the MOF's core to raise the Cu content when constructing the precursor.Controllable integration of functional components to construct composite materials has always been a reasonable technique to implement material multi-functionalization,and it is an inevitable trend in material development [25-31].For inorganic nanoparticles@MOF multifunctional materials,according to the order of synthesis of inorganic nanoparticles and MOF,the synthetic approaches can be divided into two types: assembling the inorganic nanoparticles within the pores of the MOF,known as “ship in bottle” approach;assembling the MOF around the inorganic nanoparticles,also known as “bottle around ship” or “templated synthesis” approach[32].An intriguing idea is to try to embed Cu2O(as the ship)in the copper-containing MOF material Cu-BTC (HKUST-1,as the bottle).For Cu2O nanoparticles,a strong size effect of surface energy density occurs when the particle size is on the nanometer scale.The coating of MOF can overcome this problem and reduce the agglomeration of nanoparticles.The PC skeleton formed after the carbonization of the MOF material can also contribute to isolating CA,lowering the energetic material's sensitivity.This method should enable carbon-based CA materials to attain a better balance of high energy density and high electrostatic safety.
The“bottle around ship”method was used to self-assemble the Cu2O@HKUST-1 hybrid system in this work by precisely matching the dissolution rate of Cu2O with the generation rate of HKUST-1.Cu2O@HKUST-1 is carbonized to form CuO@PC,which is then used to synthesize CA@PC via an azide reaction.CA@PC has a distinctive framework structure that supports exceptional electrostatic safety (1.47 mJ) and a high CA content (89.6%).
2.Materials and methods
2.1.Materials
Sodium Dodecyl Sulfate,A.R.,Nanjing Shengjianquan Company,China.Copper chloride,1,3,5-Benzenetricarboxylic acid(BTC),N,NDimethylformamide,A.R.,Shanghai Bailingwei Company,China.Acetone,absolute ethanol,sodium azide,sodium hydroxide,hydroxylamine hydrochloride,benzyl alcohol,A.R.,Sinopharm Chemical Reagent Company,China.High-purity nitrogen,99.999%,Nanjing Wenda Special Gas Co.,Ltd,China.
2.2.Characterization and performance
Transmission Electron Microscopy (TEM),Talos F200X STEM,FEI.X-ray powder diffractometer(XRD),Rigaku smartlab 9,Rigaku,Japan.Scanning Electron Microscope (SEM),Nova NanoSEM 450,FEI.X-ray Photoelectron Spectroscopy (XPS),ESCALAB 250Xi,Thermo Scientific.Fourier Transform Infrared Spectroscopy (FTIR),Nicolet iS5,Thermo Fisher.Inductively coupled plasma emission spectrometer(ICP),PE Optima 7000 DV,Perkin Elmers.Differential Scanning Calorimeter (DSC),DSC204F1 Phoenix,NETZSCH.Electrostatic sensitivity tester,JGY-50III,No.213 Research Institute of China Ordnance Industry.High-speed camera,UX50,Photron.Vacuum tube furnace,SGL-1700,Shanghai Institute of Optics and Fine Mechanics.
2.3.Preparation of Cu2O nanocrystals
Micheal H.Huang's method [33] was slightly improved to prepare Cu2O nanocubic crystals.107.5 mg (0.8 mmol) of CuCl2was dissolved in 150 mL of deionized water,sonicated at 20 kHz power for 20 min,and then 1.4 g of surfactant SDS was added.The mixed solution was placed in a water bath and continuously stirred at 40°C until a uniform clear solution was formed.128 mg(3.2 mmol)of NaOH was poured into the CuCl2/SDS mixed solution system and stirred quickly.After the solution turned blue,44 mg(0.64 mmol)of NH2OH·HCl was added into the system and stirred at constant temperature for 1 h until the solution turned orange,then it was cooled to room temperature and centrifuged at 1000 rpm to obtain a precipitate.The precipitated product was washed 3 times with absolute ethanol to remove impurities such as SDS on the surface and dried in a vacuum oven to obtain an orange powder sample.
2.4.Preparation of Cu2O@HKUST-1
210 mg (1 mmol) of trimesic acid was dissolved in 10 mL of absolute ethanol and ultrasonicated for 20 min.107 mg(0.75 mmol) of Cu2O nanocrystal samples were weighed and dissolved in 50 mL of benzyl alcohol solution (or DMF,ethanol) and heated to 80°C in an oil bath.The H3BTC solution was slowly added to the Cu2O solution,magnetically stirred for a certain period of time,and then naturally cooled to room temperature,and centrifuged to obtain a precipitate.The precipitate was washed 3 times with absolute ethanol and dried in a vacuum oven.The samples obtained at different reaction times were separately characterized to gain an in-depth understanding of the growth process of the hybrid material Cu2O@HKUST-1.At the optimal reaction time,the Cu2O content in Cu2O@HKUST-1 was about 31.3 wt%.
2.5.Carbonize Cu2O@HKUST-1 to prepare CuO@PC
The prepared Cu2O@HKUST-1 samples were carbonized in a tube furnace.The specific process of pyrolytic carbonization is as follows: the sample was placed in a porcelain dish,and the porcelain dish was pushed into the middle of the tube furnace.The heating started after the parameter setting was completed,the heating rate was set to 5°C/min,and the carbonization temperature was 400°C.Carbonization was carried out under an argon atmosphere for 1 h,and then the temperature was set to 250°C.When the temperature dropped to the set temperature,the air was introduced to replace the argon atmosphere and the temperature was continued to be maintained,then the instrument was turned off,and the samples were taken out after natural cooling.
2.6.Gas-solid phase azide reaction
The CuO@PC was converted into CA@PC through an in-situ gassolid phase azide reaction.The chemical reaction equations involved are as follows.
Reactions were performed using a homemade one-piece apparatus.The integrated reaction device was more airtight,which was crucial for reactions involving dangerous gases like hydrogen azide.In a glass dish at the bottom of the reactor,2 g of sodium azide was dissolved in 50 mL of ultrapure water.2 g of concentrated nitric acid was diluted in 50 mL of ultrapure water and slowly poured into the glass dish via the funnel.The CuO@PC was placed in a container above the polyethylene mesh.Nitrogen gas should be progressively introduced before and after the reaction to discharge air and hydrogen azide.
3.Results and discussion
3.1.Characterization of Cu2O nanocrystals
By comparing the standard spectrum (JCPDS card 34-1354),it can be noted that the product's diffraction peak is consistent with the standard characteristic peak of Cu2O(Fig.1),and the peak of the(111) crystal plane is the strongest.The (110),(111),(200),(220),and (311) crystal planes of Cu2O are represented by the diffraction peaks at 29.981°,37.009°,42.611°,62.444°,and 74.402°,respectively.There are no additional peaks.The product is reasonably pure Cu2O without CuO or copper,according to the XRD result.
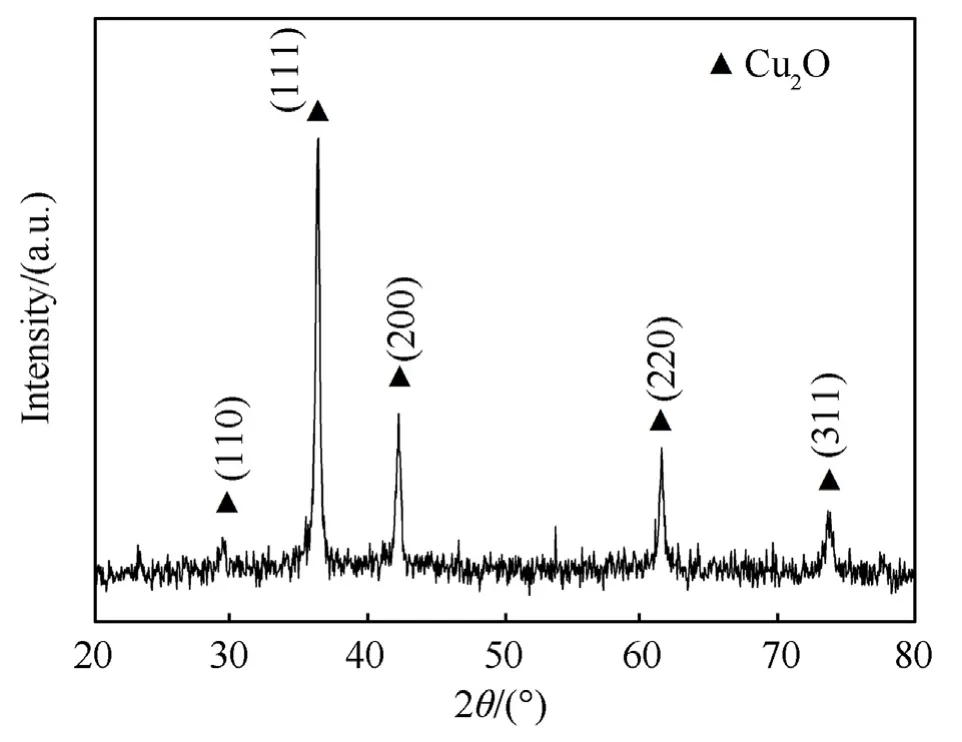
Fig.1.XRD pattern of Cu2O.
3.2.The growth process and mechanism of Cu2O@HKUST-1
The key to successfully constructing nano core-shell hybrid materials using the “bottle around ship” method is to precisely match the dissolution rate of metals or metal oxides in the reaction system with the generation rate of MOF materials to achieve hybrid system self-assembly and controllable preparation.The coordination environment has a significant impact on this process.For that purpose,the morphology of the core-shell structure hybrid in different polarities of solvent was investigated.The products produced in an ethanol/water volume ratio of 1:1 (Fig.2(a)),2:1(Fig.2(b)),and DMF (Fig.2(d)) solvents display significant aggregation.Cu2O nanoparticles are clustered together and coated by HKUST-1 on a huge scale.The scale of the entire hybrid reaches the micron level when taken as a whole.C element is uniformly distributed throughout the HKUST-1 shell,and Cu and O elements are enriched at the Cu2O core,according to the TEM-EDS image(Fig.2(c)).The core-shell structure hybrids with a diameter of about 200 nm are dispersed in benzyl alcohol(Fig.2(e)),and the interface between the core and shell is clear(Fig.2(f)).This is due to the extra polarity of solvents like ethanol,water,and DMF,which facilitates the dissociation of the organic ligand H3BTC,raising the system's acidity.In this case,the divalent Cu2+ions generated by Cu2O dissolution immediately interact with a large number of ligands,resulting in the HKUST-1 shell's self-nucleation.To better control the dissociation of Cu2O into Cu2+ions,a less polarity benzyl alcohol was chosen as the solvent environment for heterogeneous hybrid nucleation.
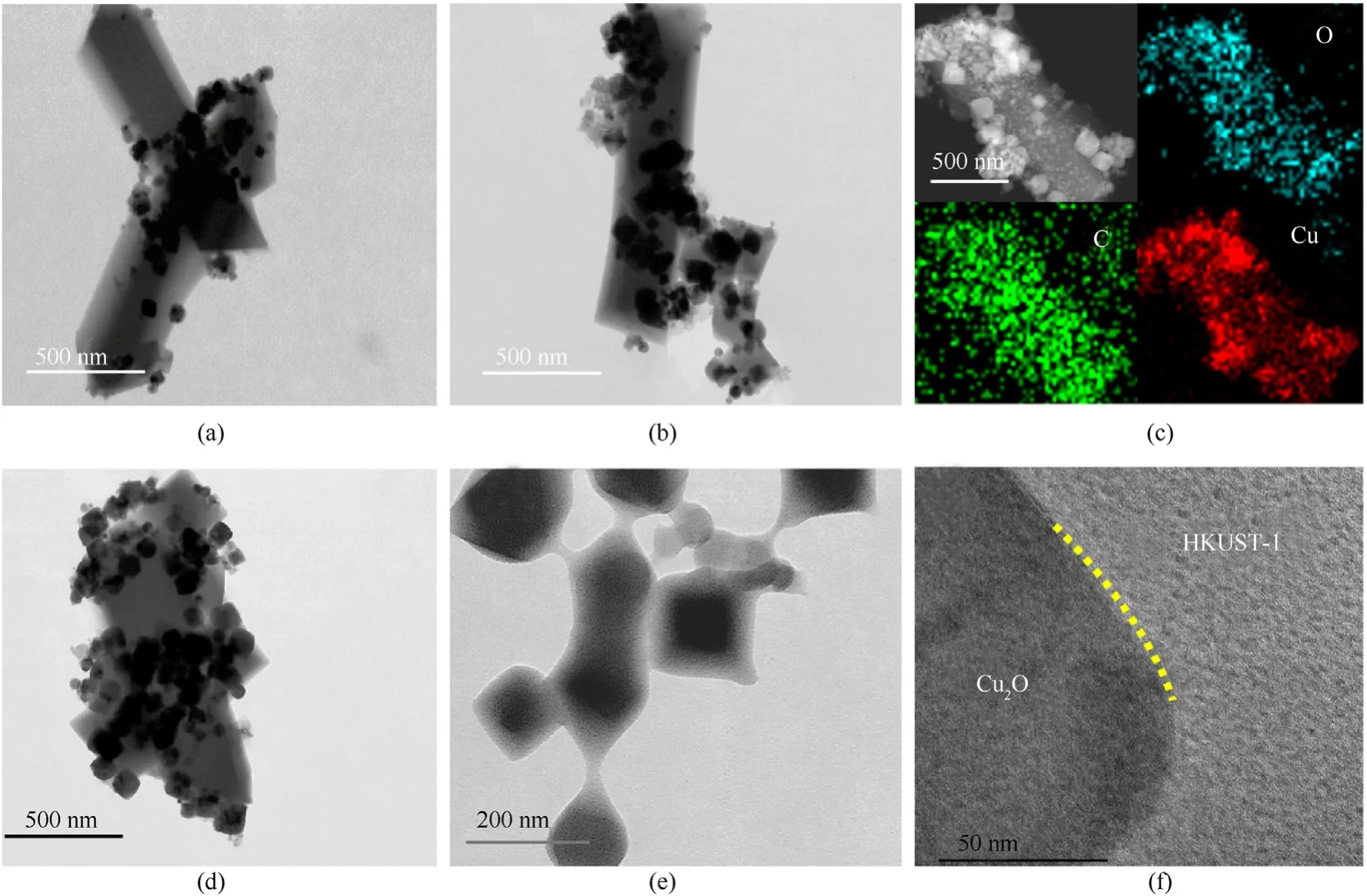
Fig.2.TEM images of Cu2O@HKUST-1 obtained under different solvent conditions:(a)Ethanol and water at a volume ratio of 1:1;(b),(c)Ethanol and water at a volume ratio of 2:1;(d) DMF;(e),(f) benzyl alcohol.
The products obtained under various reaction durations were characterized to better understand the Cu2O@HKUST-1 growth process.When the reaction advances to 1 h,the result shows characteristic peaks of HKUST-1,suggesting that the HKUST-1 shell layer has begun to develop,as shown in Fig.3.The relative strength of the characteristic peak of HKUST-1 relative to the diffraction peak of the(111)and(200)crystal plane of Cu2O increases as the reaction advances through 2 h and 3 h,showing that the shell layer is thicker.The typical peak of Cu2O on the XRD spectrum of the product is nearly disappeared after 6 h of reaction.The product's diffraction peak is compatible with the standard spectrum of HKUST-1 determined by simulation,showing that the product is substantially pure HKUST-1 at this time.
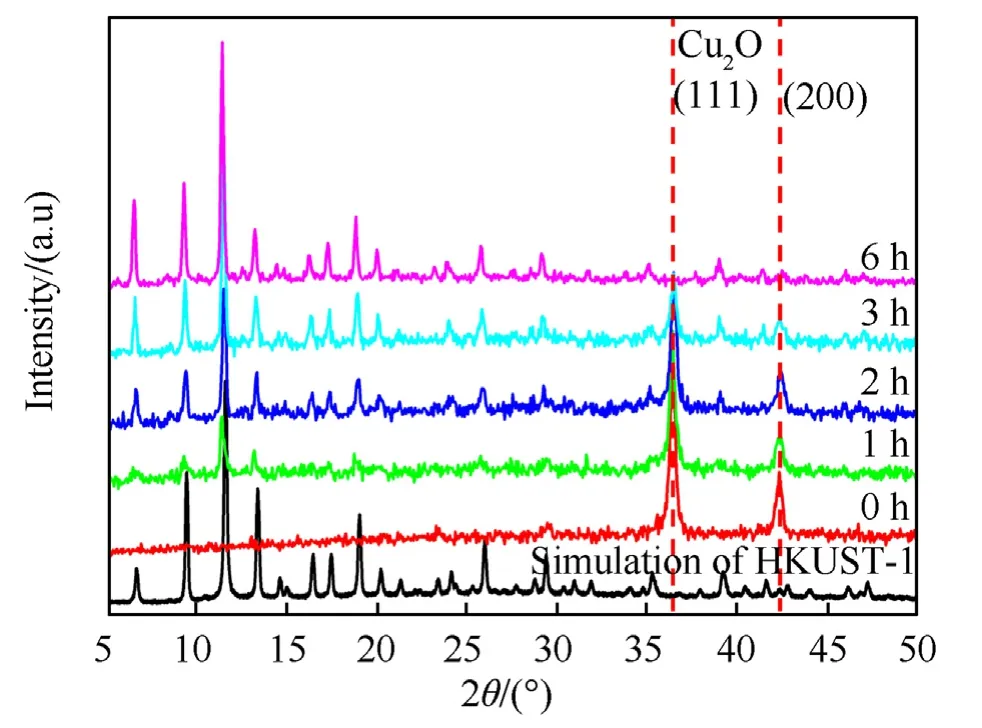
Fig.3.XRD patterns of the Cu2O@HKUST-1 products obtained under different reaction times.
The microscopic morphology of the product obtained under varied reaction durations was characterized in order to better understand the growth mechanism of the core-shell structure hybrid.The aggregation of the hybrid material could already be seen after 1 h of reaction,and the morphology's uniformity was poor(Fig.4(a)).The size of the monomer's hybrid particles grow larger as the reaction advances to 2 h,and the morphology becomes more regular than it was at 1 h(Fig.4(b)).The particles grow larger,and the shape becomes more regular as the reaction advances to 3 h(Fig.4(c)).When the reaction reaches 6 h,the typical octahedral crystal shape of HKUST-1 can clearly be seen(Fig.4(d)).Because the SEM penetration of the HKUST-1 shell is weak,no clear core-shell structure can be detected.After 3 h of reaction,EDS Mapping of the product reveals that the distribution of Cu,O,and C in the area is comparable,which could be attributable to the thick shell of HKUST-1(Fig.4(e)).The mass percentages of Cu,C,and O in HKUST-1 and Cu2O@HKUST-1 obtained by the molecular formula and EDS(Fig.4(f)) are shown in Table 1.From Table 1,it can be calculated that the Cu2O content in Cu2O@HKUST-1 is about 31.3 wt%.It can be seen that from 100%(0 h)to 31.3%(3 h),to 0%(6 h),the content of Cu2O varies with the reaction time,so the content of Cu2O in Cu2O@HKUST-1 can be adjusted by controlling the reaction time.

Table 1 The mass percentage of Cu,C and O elements in HKUST-1 and Cu2O@HKUST-1.
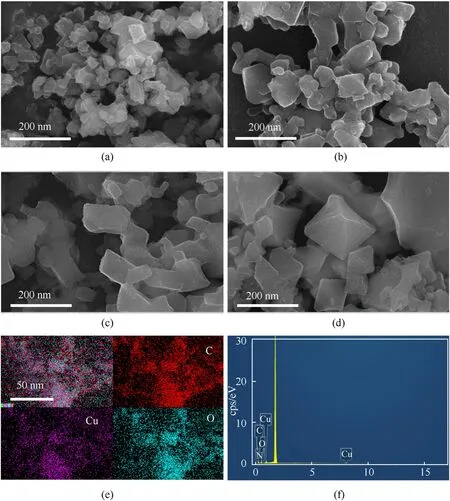
Fig.4.SEM and EDS images of Cu2O@HKUST-1 obtained under different reaction times:(a)-(d)SEM images at 1 h,2 h,3 h,and 6 h of reaction;(e)TEM mapping and(f)EDS at 3 h.
The Cu2O@HKUST-1 hybrid material was further studied by TEM because SEM photographs could not clearly show the core-shell structure of the hybrid material.The cubic structure of the original Cu2O crystal is regular (Fig.5(a)).A thin HKUST-1 shell begins to form on the surface of the Cu2O crystal after 20 min of reaction(Fig.5(b)).As the reaction progresses,the HKUST-1 shell grows larger and larger within 1-3 h(Fig.5(c)-Fig.5(e)).It grows thicker and more regular in morphology.At the same time,due to dissolution,the Cu2O in the core shrinks.Only very few Cu2O grains remained after 6 h of processing(Fig.5(f)).The HRTEM image of the core-shell interface is shown in Fig.5(g).The grain boundary shows an evident gradient transition,indicating that Cu2O dissociates layer by layer from the edge of the crystal grain during the hybrid's heterogeneous nucleation process.Cu2O as the core retains good crystallinity during the dissolution process,with clear lattice stripes and interplanar spacing of 0.21 nm and 0.24 nm,respectively,corresponding to Cu2O(200)(111)crystal plane,as shown in Fig.5(h).The TEM-EDS image of the product after 3 h of reaction shows that C and N elements are uniformly distributed on the entire hybrid particle,and the proportion of Cu and O elements contained in the shell is relatively high (29.1 wt and 36.6 wt,respectively),so the enrichment of Cu and O elements at the core is not obvious from the TEM-EDS image (Fig.5(i)).
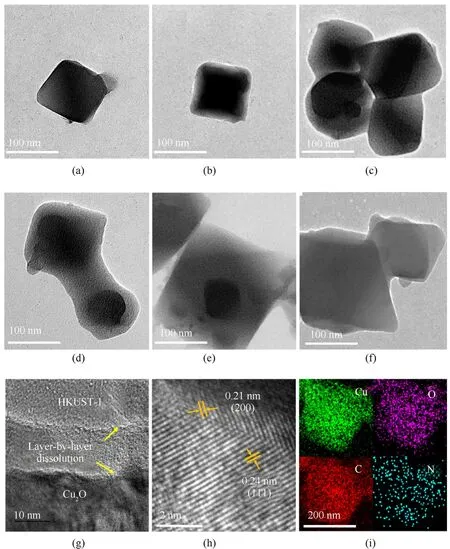
Fig.5.TEM images and mapping of Cu2O@HKUST-1 obtained at different reaction times:(a)-(f)TEM images of the product at 0 min,20 min,1 h,2 h,3 h,and 6 h;(g),(h)HRTEM at 2 h;(i) TEM mapping at 3 h.
As shown in Fig.6,the Cu2O@HKUST-1 hybrid material's growth mechanism can be deduced from the following discussion: H3BTC is primarily used as an organic ligand in the metal-organic framework material HKUST-1.It also creates an acidic environment in the reaction system.Cu2O dissociated to form Cu2+in this acidic environment.The Cu2+formed by the dissociation becomes the metal center ion of HKUST-1,which is coordinated with the organic ligand BTC in the system,and a heterogeneous nucleation reaction takes place on the Cu2O surface.As a result,a layer of HKUST-1 shell progressively forms over the Cu2O core,creating a core-shell structure.Due to the constant oxidation-reduction reaction,the Cu2O core dissociates layer by layer during this process,and the diameter gradually decreases.The reaction yields relatively pure HKUST-1 after 6 h.The product with a reaction time of 3 h is chosen for the follow-up experiment because it requires the Cu concentration in the precursor and the thickness of the HKUST-1 shell to reach the optimal equilibrium.
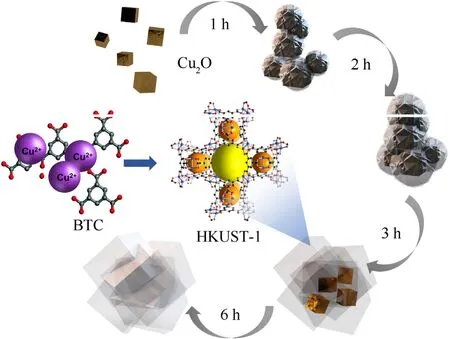
Fig.6.Schematic diagram of the growth process of Cu2O@HKUST-1.
3.3.Characterization of Cu2O@HKUST-1
The products obtained under the optimal synthesis time (3 h)were characterized.The nitrogen adsorption isotherm measurement confirms that Cu2O@HKUST-1 and HKUST-1 materials exhibit type I adsorption curve features(Fig.7(a)).As can be observed from the pore size distribution curve obtained by the Horvath-Kawazoe(HK) method (Fig.7(b)),HKUST-1 and Cu2O@HKUST-1 have the similar pore size distribution.Cu2O@HKUST-1 has a Brunauer-Emmett-Teller (BET) specific surface area of 729.8 m2/g,which is somewhat less than HKUST-1's 1129.1 m2/g.The non-porous Cu2O core in the hybrid material's center is responsible for decreasing specific surface area.

Fig.7.(a)N2 adsorption-desorption curves and(b)pore size distribution of HKUST-1 and Cu2O@HKUST-1;(c)FTIR spectrum and of Cu2O@HKUST-1;(d)TG curve of HKUST-1 and Cu2O@HKUST-1.
Cu2O@HKUST-1 has an FTIR spectrum (Fig.7(c)) that is quite comparable to HKUST-1 material published in the literature[34-36].The C-H and C-O functional groups of HKUST-1 are shown by the two peaks at 731 cm-1and 1109 cm-1,respectively.The stretching vibration of the C=O bond is responsible for the peaks seen around 1374 cm-1and 1643 cm-1.The existence of BTC as an organic ligand is generally confirmed by these peaks found between 700 cm-1and 1700 cm-1[37].The peak at 608 cm-1is usually attributed to Cu-O,indicating the presence of Cu2O nuclei in the hybrid.
Thermogravimetric analysis was used to investigate the thermal stability of Cu2O@HKUST-1 (Fig.7(d)).The weightlessness step before 100°C,like with HKUST-1,is mostly due to the loss of free molecules filled by physical adsorption in the material's micropores[38].The mass loss between 320°C and 400°C is due to the organic ligands in the material components being calcined into amorphous carbon.Its weightlessness step is less than that of HKUST-1 due to the existence of a Cu2O core in the center of the core-shell material.According to TG's results,the calcination temperature of the hybrid material should be 400°C to carbonize the material thoroughly.
3.4.Carbonization of Cu2O@HKUST-1 and characterization of CuO@PC
XRD was used to determine the composition of the product formed when Cu2O@HKUST-1 was carbonized at a high temperature(Fig.8(a)).It can be noticed that CuO particles are formed in the product when compared to a standard CuO spectrum (JCPDS card 48-1548).Raman spectroscopy characterization was used on the CuO@PC composite material to study the carbon framework(Fig.8(b)).As illustrated in the image,a typical D peak reflecting the lattice defects of C atoms in carbon materials appears at 1350 cm-1,whereas a typical G peak indicating the in-plane stretching vibration of sp2hybridization of C atoms appears at 1601 cm-1.The intensity ratios are minimal(ID/IG=0.386),showing that the C atom crystal in PC has fewer flaws.
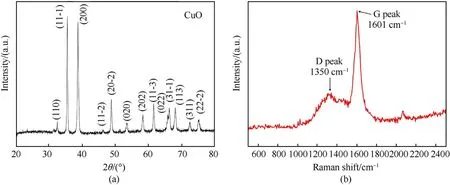
Fig.8.(a) XRD and (b) Raman spectra of the carbonized products of Cu2O@HKUST-1.
CuO@PC was studied by SEM to learn more about the microscopic morphology of the carbonized product.The product is a core-shell material of CuO well coated by PC,as illustrated in Fig.9(a)and Fig.9(c).PC is calcined from the organic ligand in the shell HKUST-1.CuO is mainly calcined and oxidized by Cu2O as the core and partly formed by calcining the original Cu element in HKUST-1.The latter and the former are fused and merged to form a complete monomer.This structure not only increases the Cu content in the precursor,but also ensures the independence and dispersion between CuO particles,which provides a structural basis for the excellent performance of the composite energetic material synthesized based on the CuO@PC precursor.Almost no exposed CuO particles overflowing PC are found in CuO@PC,which is conducive to the electrostatic safety performance of composite energetic materials after following azidation.Table 2 shows the mass proportion of each element based on SEM-EDS data(Fig.9(b)).The EDS mapping of CuO@PC is shown in Fig.9(d).C and O elements are extensively scattered on the surface of the precursor material,and this could be because the PC's surface contains oxygen-containing functional groups.Cu elements are abundant at the CuO core.

Table 2 The mass percentage of Cu,C and O elements in CuO@PC.
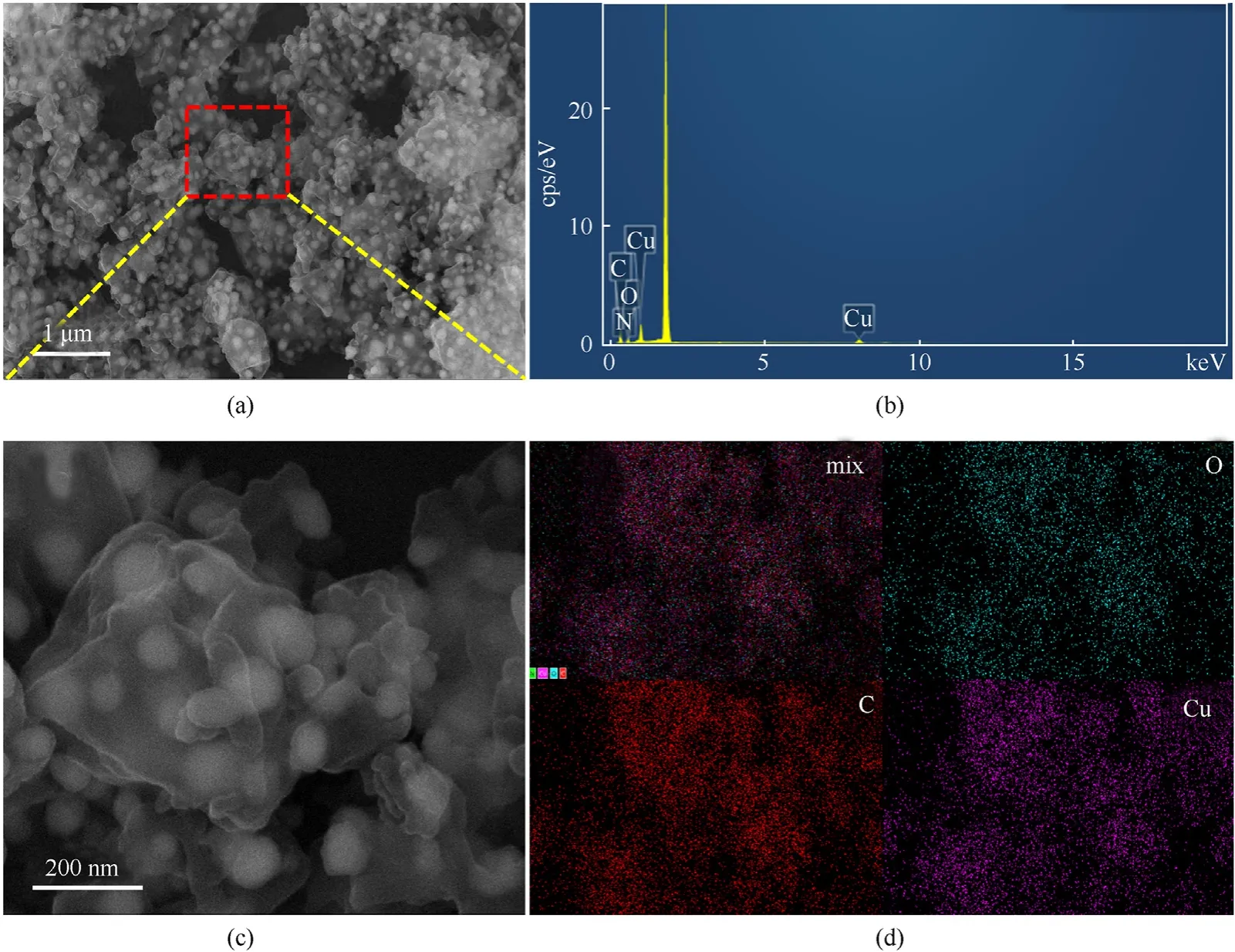
Fig.9.(a) (c) SEM and (b) (d) SEM-EDS of CuO@PC.
The carbonization process of Cu2O@HKUST-1 is depicted in Fig.10(a).The CuO@PC precursor material was studied by TEM to get further information about the microstructure of the core-shell structure precursor.
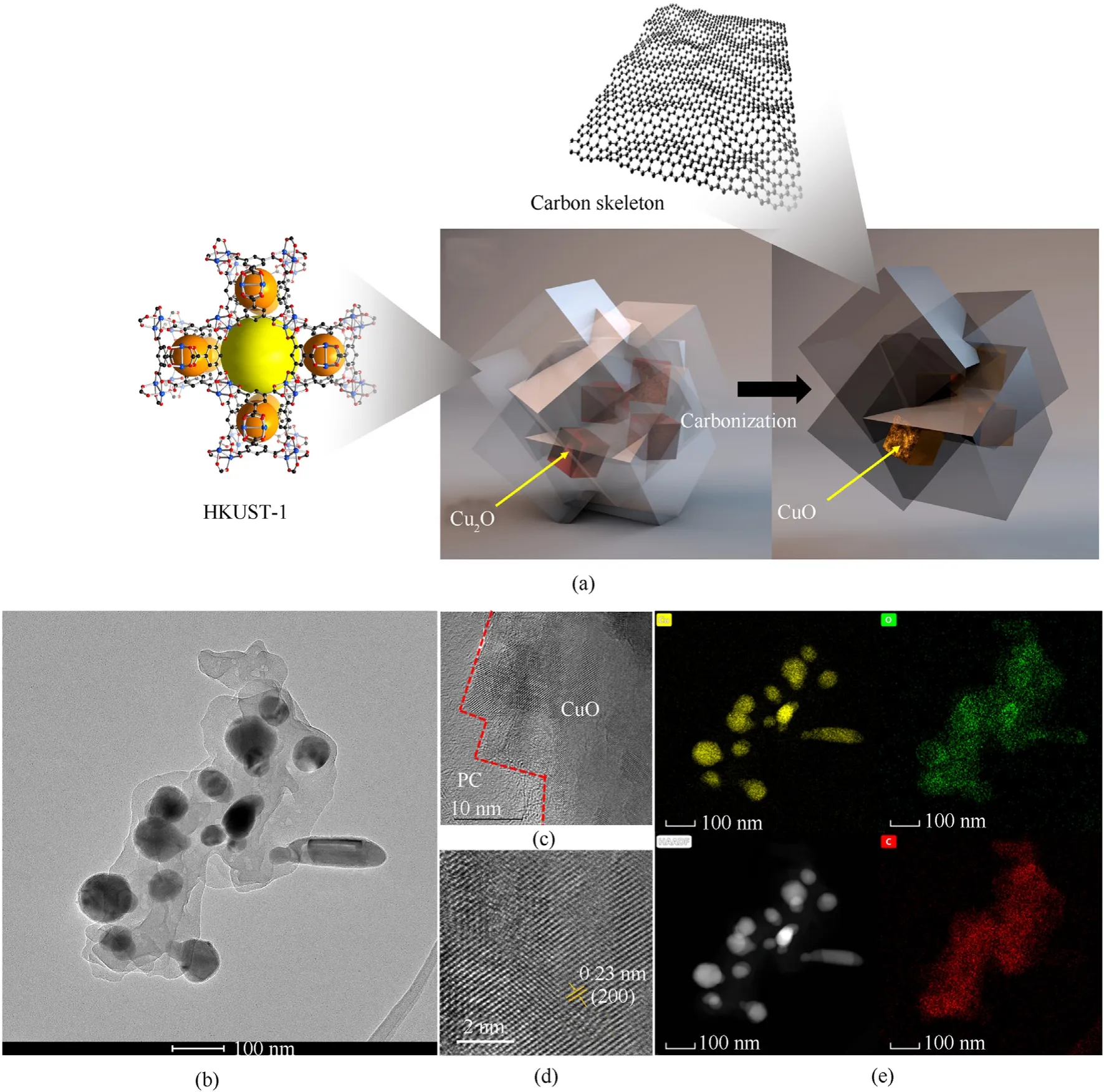
Fig.10.(a) Schematic diagram of Cu2O@HKUST-1 carbonization process;(b)-(d) HRTEM of CuO@PC;(e) TEM-mapping of CuO@PC.
Fig.10(b) shows how PC functions as a nanocage to coat CuO particles,which are incorporated in the carbon framework.The CuO core and the PC have a clear interface (Fig.10(c)).The crystallinity of the CuO formed by calcination is demonstrated by the distinct lattice fringes.The measured area has an interplanar spacing of around 0.23 nm (Fig.10(d)),which corresponds to the CuO crystal plane (222).The results of the TEM-EDS (Fig.10(e))demonstrate that the O element,like the C element,is broadly distributed in the scanned area,owing to oxygen-containing functional groups on the PC's surface,whereas the Cu element is clearly enriched at the CuO core.
3.5.Characterization and performance of CA@PC composite energetic materials
CA@PC composite energetic materials produced by gas-solid phase azide reaction were characterized by XRD and FTIR.The XRD data demonstrate that the azide product is pure Cu(N3)2,suggesting that the azide reaction has been completed entirely,as illustrated in Fig.11(a).The product's XRD spectrum matches the Cu(N3)2standard spectrum (JCPDS card 21-0281).
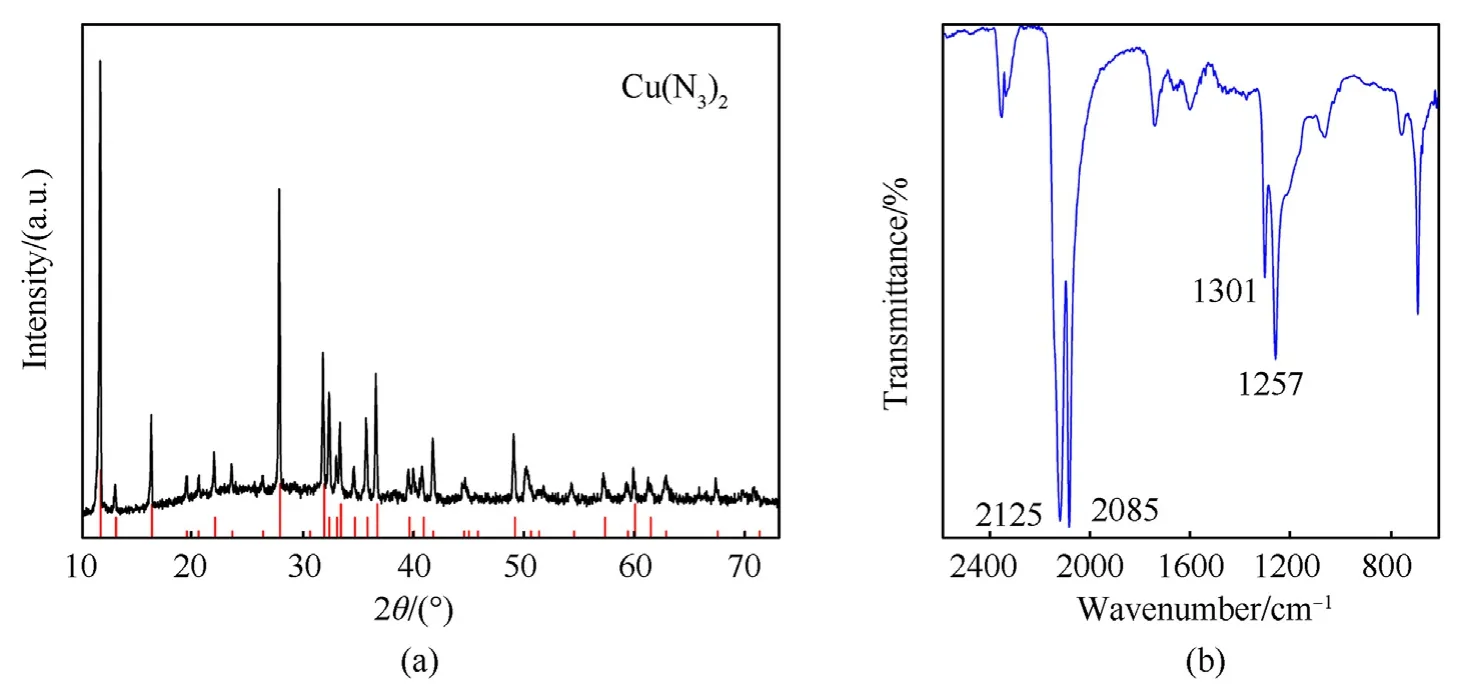
Fig.11.(a) XRD and (b) FTIR spectra of CA@PC.
Typical asymmetric peaks of azide can be seen at 2085 cm-1and 2125 cm-1in the FTIR spectra of the azide product (Fig.11(b)),while typical symmetric vibration peaks of azide are observed at 1257 cm-1and 1301 cm-1,proving the existence of[16].
The TEM image(Fig.12(a))shows that CA is dispersed in the PC in a diffuse fashion in the CA@PC composite energetic material,rather than preserving a clear interface between the core and the shell like CuO@PC.The flocculent distribution of copper azide is caused by the diffusion of HN3gas in the PC and the erosion of the CuO core during the gas-solid phase azidation reaction.As a result,there is no clear interface between PC and CA (Fig.12(b) and Fig.12(c)).
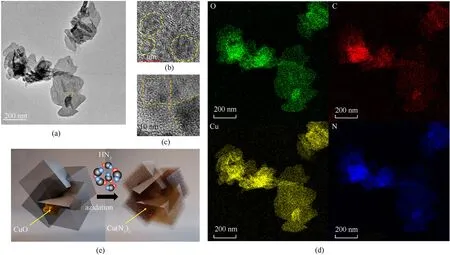
Fig.12.(a) (b) (c) HRTEM and (d) TEM-EDS of CA@PC;(e) Schematic diagram of the azide reaction process.
In PC,in addition to the large-scale CA produced by the azide reaction of CuO as the core,there are also some nano-CA with a diameter of less than 5 nm(Fig.12(b)).These particles are azidized from Cu or Cu oxide produced by the calcination of Cu2+in HKUST-1.Cu and N elements are enriched in the flocculent CA and distributed in the PC,as can be seen from the TEM mapping(Fig.12(d)).C and O elements were also found to be abundant at CA.This is because PC compresses toward CA during the HN3gas diffusion phase.This structure is better for ensuring CA@PC composite energetic materials' electrostatic safety.
The synthesis of CA@PC composite energetic material from CuO@PC via gas-solid phase azide reaction is graphically depicted in Fig.12(e).
On CA@PC,XPS characterization analysis was performed to analyze the sample's components.Fig.13 depicts the sample's XPS high-resolution spectrum.The binding energy difference between Cu 2p's orbital spin splitting peaks(Cu 2p3:Cu 2p1)is 19.75 eV,and the peak area ratio is approximately 2:1(Fig.13(a)).Copper can be determined to be divalent based on the binding energy coordinates and peak characteristics (satellite peak) of the Cu 2p peaks.It has also been confirmed that the product contains no monovalent cuprous azide(CuN3).N 1s can be divided into three chemical states based on their peak shape and binding energy coordinates: Azide groups are responsible for the two N 1s peaks with binding energies of 403.27 eV and 404.78 eV.Pyrrolic-N doped in PC is responsible for N 1s with a binding energy of roughly 400.5eV.N-Cu in CA is responsible for the peak near 398.94 eV(Fig.13(b)).The peak of C 1s with binding energy near 283.56eV is attributed to inorganic carbon,the peak of adsorbed carbon and organic carbon C-C/C-H is near 285eV,the C-O/C-N chemical bond has binding energy near 285.98 eV,and the C=O chemical bond has binding energy near 287.45 eV(Fig.13(c)).The C=O chemical bond leads to the O 1s peak,which has a binding energy of 531.1 eV.The C-O peak on the carbon skeleton has a binding energy of approximately 532.82 eV.Adsorbed O or H2O is represented by the peak at 534.13 eV (Fig.13(d)).The XPS results support the effective construction of CA@PC and demonstrate that CA exists as pure copper azide.The reactivity of the composite energy material is ensured by the abundance of functional groups on the PC surface.
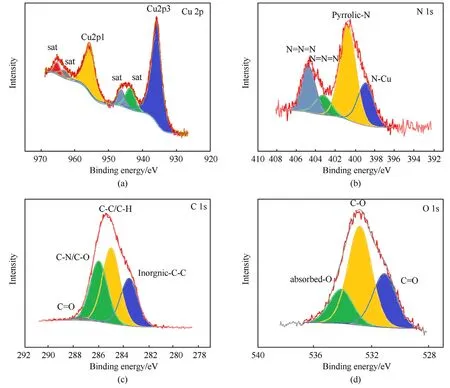
Fig.13.High-resolution XPS spectra of CA@PC: (a) Cu 2p;(b) N 1s;(c) C1s;(d) O 1s.
DSC was performed at a heating rate of 2.5 K/min over a temperature range of 100-300°C under a N2flow of 30.0 mL/min and the exothermic process of CA@PC composite energetic material reveals a steep exothermic peak at 210.97°C (Fig.14),which corresponds to the typical exothermic process of primary explosives.CA@PC did not explode or decompose after being heated to 150°C for 24 h,showing that it may have good long-term thermal storage stability.
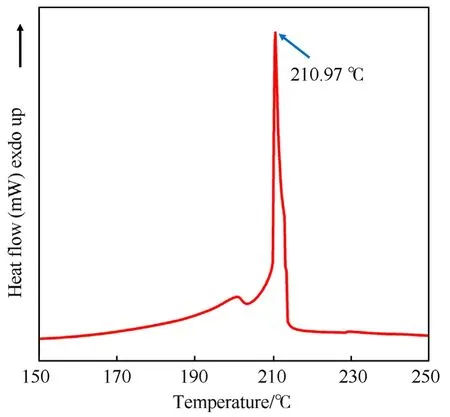
Fig.14.DSC analysis of CA@PC.
From the XRD pattern of CA@PC,it can be seen that Cu was completely converted into CA.The Cu content in CA@PC was determined by ICP and the CA content can be calculated to be 89.6 wt%.Using the electrostatic sensitivity tester to determine the electrostatic safety of energetic materials.The test used a capacitance of 500 pF,and the sample's ignition energy may be calculated through the capacitor energy storage formula:
CA@PC has an electrostatic sensitivity(E50)of 1.47 mJ,nearly 30 times lower than pure CA (0.05 mJ).Although MOF Templated-CA(MOFT-CA) has an electrostatic sensitivity of 1.6 mJ,CA@PC has a substantially greater CA concentration (89.6%) than MOFT-CA(75.8%).CA@PC offers an outstanding balance between high electrostatic safety and high energy density compared to recently reported carbon-based CA energetic materials.Compared with CA materials with a similar copper azide content,the electrostatic sensitivity of CA@PC still has advantages[16].While compared with other primary explosives reported in the literature,such as commercial LS(E50=0.25 mJ),mercury fulminate(MF)(E50=0.51 mJ),porous CA film (E50=0.098 mJ) [23],and two graphene-modified LSs composites(labeled GLS(I) and GLS(II)) (E50=0.4/0.5 mJ) [39](as shown in Fig.15(a)),the electrostatic safety advantage of CA@PC is more obvious.
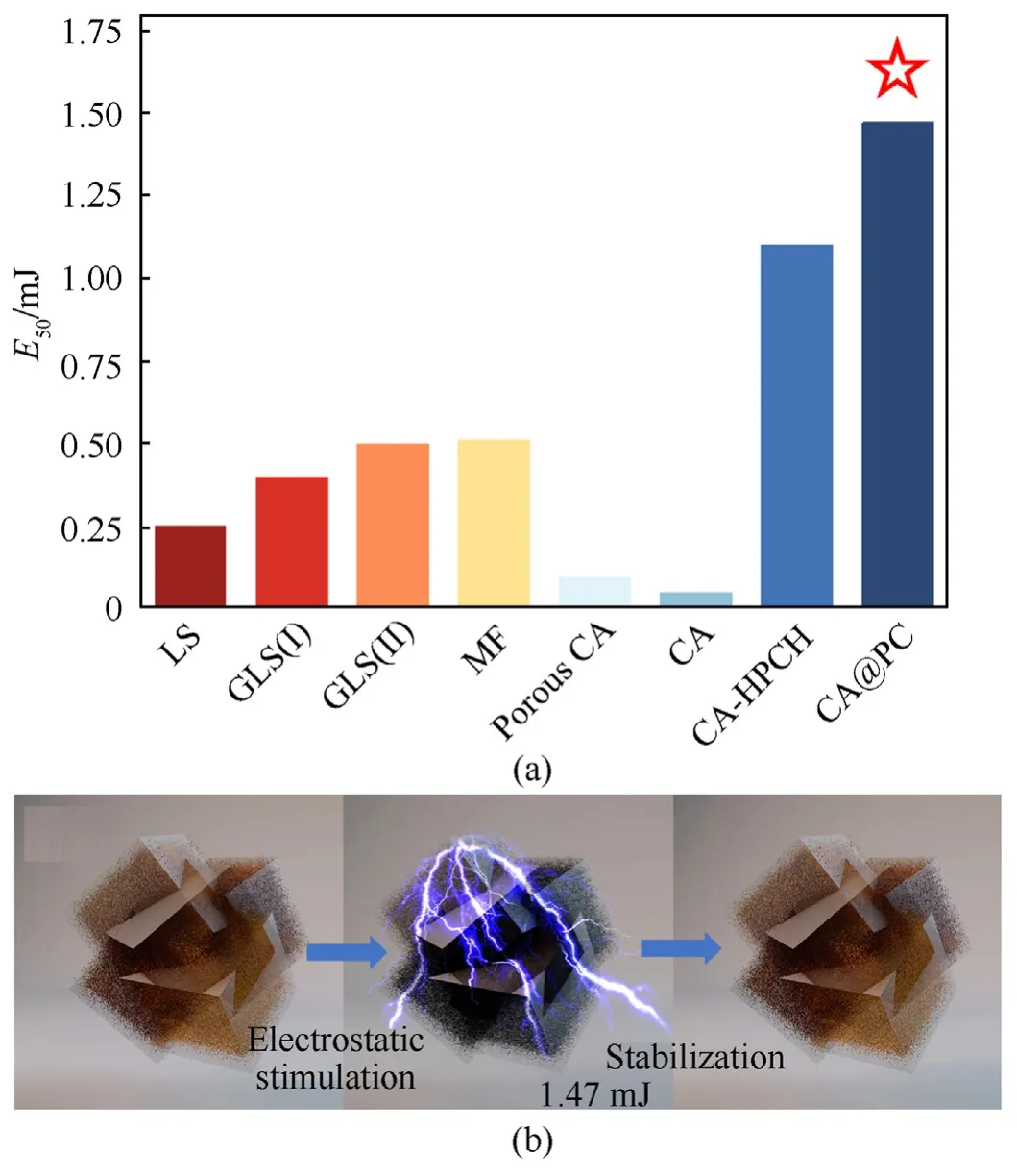
Fig.15.(a) Comparison of electrostatic sensitivity of CA@PC with other primary explosives;(b) Illustration of CA@PC's electrostatic safety.
Fig.15(b) shows a schematic diagram of CA@PC's electrostatic stimulation.The dense core of Cu2O@HKUST-1 increases the copper content of the hybrid material,allowing CA@PC to enhance CA content while sacrificing little electrostatic safety.Furthermore,the CuO@PC precursor material produced by calcination retains the Cu2O@HKUST-1 core-shell structure well.As a result,the CA@PC energetic composite material obtained by the gas-solid phase azide reaction has a greater energy density,and no CA is exposed outside the PC.A nanocage encases the sensitive copper azide.The high conductivity of PC prevents electrostatic charges from accumulating on the surface of energetic materials.The electrostatic shielding effect of the carbon nanocage is similar to that of a nanosized Faraday cage.The surface of the PC forms an equipotential during electrostatic stimulation,which protects the CA in the framework from being detonated by electrostatic stimulation,and has exceptional electrostatic safety.
In order to further study the detonation performance of CA@PC energetic materials,a nickel-chromium bridge wire electric igniter was used to detonate it,the loading mass was about 1 mg,and high-speed photography was used to record the ignition and detonation process.It can be seen from Fig.16 that the ignition process of the ignition device integrated with the CA@PC energetic material is in sharp contrast with the nickel-chromium bridge wire without the energetic material.It can be seen that a strong and well-shaped flame appears during the ignition and detonation of the micro-igniter integrated with energetic materials.Under the action of heat,the CA@PC was detonated to release a large amount of energy,and at the same time,N2was released rapidly,so the energy output of the micro-igniter was significantly improved.
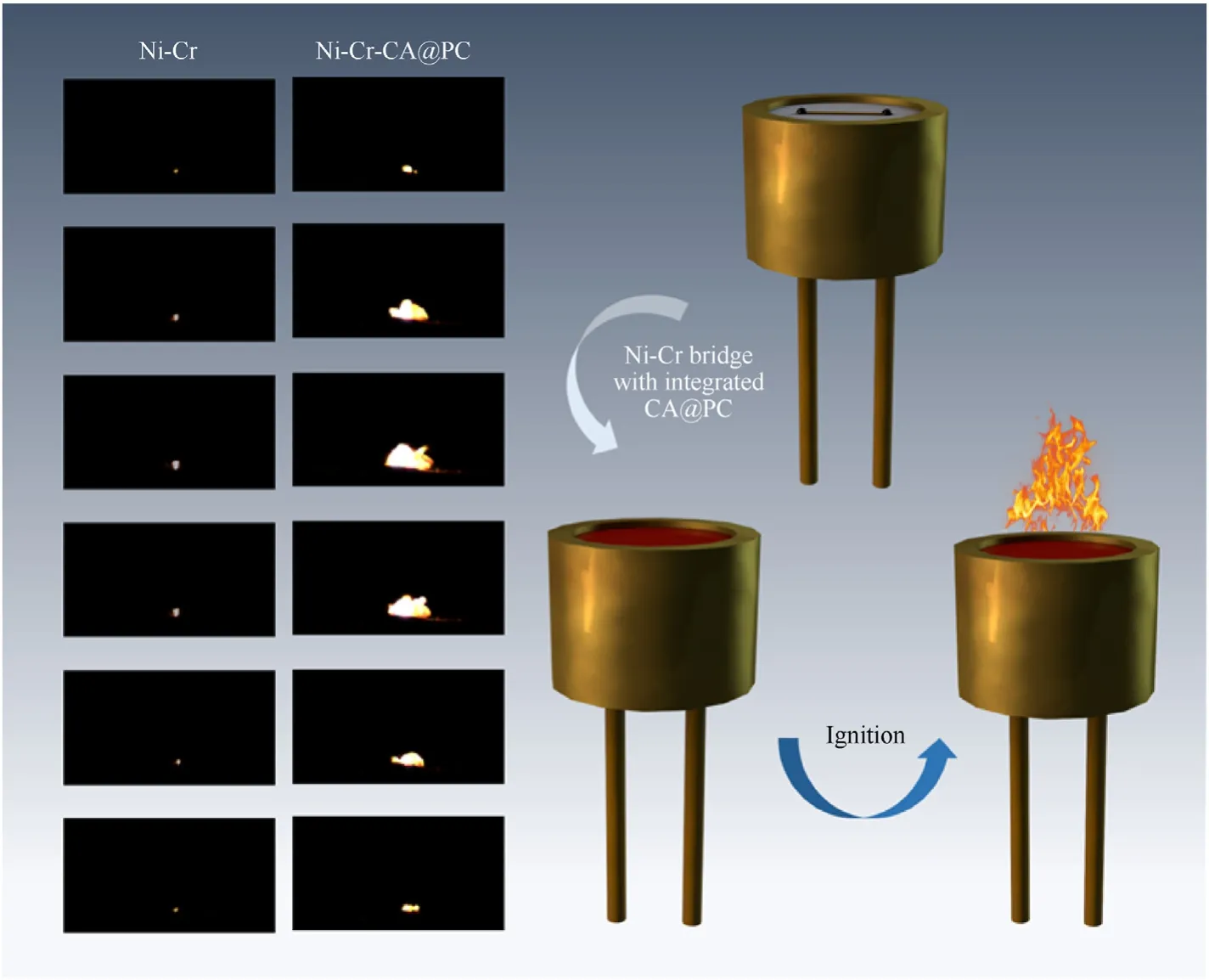
Fig.16.The corresponding ignition process of the Ni-Cr microbridge igniter and the Ni-Cr microbridge integrated with CA@PC.
4.Conclusions
CA@PC composite energetic materials were devised and manufactured to improve the content of energetic components in carbon-based CA energetic materials while increasing electrostatic safety.The dissolution rate of the CuO core in the reaction system and the generation rate of the HKUST-1 shell are precisely matched using the “bottle around ship” method to achieve self-assembly preparation of the Cu2O@HKUST-1 hybrid system.The effect of solvent polarity and reaction time on the product was investigated,as well as the suitable low-polarity solvent(benzyl alcohol)for the reaction system and the best reaction time (3 h).By calcining the Cu2O@HKUST-1 hybrid material,the CuO@PC precursor material was synthesized.CuO nanoparticles were found to be uniformly embedded in the carbon framework,with no exposed CuO particles leaking out of the carbon framework.CA@PC was prepared using CuO@PC as a precursor through a gas-solid phase azide reaction.CA was flocculently diffused throughout the PC.The energetic material's exothermic peak temperature is 210.97°C.The CA content was as high as 89.6%.It has great electrostatic safety with electrostatic ignition energy of 1.47 mJ,which is 30 times higher than pure CA.CA@PC achieves a wonderful combination between high electrostatic safety and high energy density.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the financial support by Postgraduate Research &Practice Innovation Program from Jiangsu Science and Technology Department under Grant number KYCX19_0320.We are grateful to Jingwei Li and Jiang Lin for his support in visualization.We acknowledge the China Scholarship Council,which funds Xuwen Liu scholarship.
杂志排行
Defence Technology的其它文章
- In-plane and out-of-plane quasi-static compression performance enhancement of 3D printed re-entrant diamond auxetic metamaterial with geometrical tuning and fiber reinforcement
- Flame behavior,shock wave,and instantaneous thermal field generated by unconfined vapor-liquid propylene oxide/air cloud detonation
- Frequency domain analysis of pre-stressed elastomeric vibration isolators
- Burning surface formation mechanism of laser-controlled 5-aminotetrazole propellant
- Blast disruption using 3D grids/perforated plates for vehicle protection
- Nonlinear tight formation control of multiple UAVs based on model predictive control
