盐胁迫下欧李叶片叶绿素代谢与超微弱发光的关系
2023-07-27孙聪郭金丽
孙聪 郭金丽

摘 要:【目的】解析鹽胁迫下欧李叶片叶绿素代谢与超微弱发光(ultraweak luminescence, UWL)的变化规律及二者之间的关系。【方法】以蒙原金秋欧李[Cerasus humilis (Bge.) Sok.]盆栽苗为试材,采用浓度为400 mmol·L-1、800 mmol·L-1的NaCl分别进行轻度和重度盐胁迫处理,测定UWL强度和叶绿素代谢相关指标的变化规律并进行相关性分析。【结果】(1)不同程度盐胁迫下,与对照相比,轻度和重度盐胁迫下欧李叶片7种叶绿素合成前体物质(ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-protoⅨ、Pchl)含量均下降,主要合成过程酶(ALAD、MgCH)及叶绿素酶(Chlase)含量均表现为上升,叶绿素(Chla、Chlb、Chla+b)含量均下降;同时叶片UWL强度也持续下降。(2)2种盐胁迫下,重度胁迫导致叶绿素代谢各指标及UWL强度的下降或上升幅度均较轻度胁迫更大。(3)相关分析显示,2种盐胁迫下,叶片UWL强度均与叶绿素合成前体物质含量及叶绿素含量呈显著正相关,与叶绿素酶含量呈显著负相关。【结论】欧李叶片UWL与叶绿素代谢密切相关:盐胁迫下,随着叶片叶绿素合成前体物质含量下降及叶绿素酶含量的上升,叶绿素合成代谢减弱而降解代谢加强,引起叶绿素含量下降;以上叶绿素代谢变化导致叶片UWL强度降低。
关键词:蒙原金秋欧李;盐胁迫;叶片;超微弱发光;叶绿素代谢
中图分类号:S661.2 文献标志码:A 文章编号:1009-9980(2023)07-1411-10
The relationship between chlorophyll metabolism and ultraweak luminescence of leaves under salt stress in Cerasus humilis
SUN Cong, GUO Jinli*
(College of Horticulture and Plant Protection, Inner Mongolia Agricultural University, Hohhot 010010, Inner Mongolia, China)
Abstract: 【Objective】 Ultraweak luminescence (UWL) is a natural luminescence phenomenon in all living organisms. However, the understanding of the mechanism of luminescence is still limited. In order to analyze the excitation mechanism of UWL in plants, this study investigated the changes in UWL intensity, chlorophyll metabolism, and chlorophyll content in leaves of Cerasus humilis under salt stress, and carried out correlation analysis. The purpose was to reveal the relationship between plant physiological status and UWL, with focus on chlorophyll metabolism so as to provide understandings related to physiology of UWL emission in plants. 【Methods】 The potted seedlings of biennial C. humilis were taken as the materials in this study. The seedlings were subjected to mild and severe stress treatments with 400 mmol·L-1 and 800 mmol·L-1 NaCl, respectively. Each potted seedling was irrigated with 400 mL of salt solution at different concentrations, and irrigation with the same amount of water was taken as the control. The UWL intensity, main precursor substances of chlorophyll (ALA, PBG, UroⅢ, CopⅢ, ProtoⅨ, Mg-protoⅨ, and Pchl), main chlorophyll synthetases (ALAD and MgCH), enzymes related to chlorophyll degradation, and chlorophyll contents (Chla, Chlb, and Chla+b) in the leaves of C. humilis were measured every 2 days. The correlation between the indexes of chlorophyll metabolism and UWL was analyzed. About 10-20 mature leaves were selected from the base of the branches for measurement of the UWL intensity using an UWL test system (BPCL-2-SH, Beijing). Take 5 leaves from each treatment, and take samples from the top, middle, and bottom three parts of each leaf to measure UWL. Then, take a sufficient amount of leaves that were washed with distilled water and dried. After removal of the main leaf veins, they were quickly frozen with liquid nitrogen and stored at -80 ℃ to measure chlorophyll metabolism and contents. The tests were repeated three times, each with three biological repeats. 【Results】 (1) With the extension of stress time, the UWL intensity of C. humilis leaves under different levels of salt stress showed a decreasing trend, which was 40.80% and 83.26% lower in mild and severe stresses than that before the stress, respectively. During the whole stress period, UWL in severe stress treatment decreased more rapidly compared with that in the mild stress treatment, and at the end of the experiment, it decreased by 35.93% and 81.88%, compared with the control respectively. (2) With the extension of stress time, the contents of seven chlorophyll synthesis precursors (ALA, PBG, Uro Ⅲ, Cop Ⅲ, Proto Ⅸ, Mg-proto Ⅸ, and Pchl) in C. humilis leaves under different salt stress treatments showed a decreasing trend. Among them, Cop Ⅲ decreased fastest under mild stress with a reduction of 57.97% compared with that before stress, and Pchl decreased fastest under severe stress with a reduction of 67.17% compared with that before stress. The contents of the main synthetase (ALAD and MgCH) and the degrading enzymes chlorophyllase (Chlase) showed an increasing trend. Under mild and severe stress treatments, ALAD increased the fastest and was 1.59 times and 1.27 times higher than before stress, respectively. Chlorophyll (Chla, Chlb, and Chla+b) contents showed a decreasing trend, among which Chlb decreased fastest under mild stress with a reduction of 39.47% compared with that before stress, and Chla decreased fastest under severe stress with a reduction of 76.66%. At the same time, the severe stress led to greater changes in chlorophyll metabolism indicators than mild stress. (3) Correlation analysis showed that under the two salt stress treatments, the UWL intensity of leaves was significantly positively correlated with the contents of chlorophylls and their precursors, and was significantly negatively correlated with the content of chlorophyll metabolism enzymes. Under mild stress, the intensity of UWL was significantly positively correlated with the chlorophyll precursors, ALA, PBG, and Mg-proto Ⅸ, as well as Chla and Chla+b, but significantly negatively correlated with metabolism-related enzymes, ALAD and Chlase. Under severe stress, UWL intensity was significantly positively correlated with chlorophyll precursor PBG, Uro Ⅲ, and Cop Ⅲ, as well as Chla, Chlb and Chla+b, but negatively correlated with metabolic enzyme Chlase. 【Conclusion】 Salt stress blocked the chlorophyll synthesis process of leaves in C. humilis, enhanced the degradation enzymes, and decreased the chlorophyll content. The UWL intensity decreased with the decrease in chlorophyll synthesis precursors, chlorophyll enzyme activity, and chlorophyll content. The above changes in chlorophyll metabolism may lead to changes in leaf UWL intensity. Therefore, the UWL of leaves in C. humilis is closely related to chlorophyll metabolism. Salt stress led to the decrease in UWL emission, and the decrease becomes faster under more severe salt stresses.
Key words: Cerasus humilis; Salt stress; Leaves; Ultraweak luminescence; Chlorophyll metabol
任何生物组织或细胞在生命活动的代谢过程中,都自发地辐射出一种超弱电子流,其強度仅为在1 s内1 cm2上几个至几千个光子(100~103 hv·s-1·cm-2),波长范围为180~800 nm[1],称为生物超微弱发光(ultraweak luminescence,UWL;ultraweak photon emission,UPE)。UWL是一种来自细胞内的本源信号,检测这种信号并破译其所携带的与生命活动相关的信息,可以了解各种生命过程的真实现象;未来UWL可能是研究植物信号识别、信息传递、细胞衰老等基本生命过程的重要工具[2]。1923年苏联细胞生物学家最早在“洋葱实验”中发现了UWL现象[3],一直到20世纪80年代,随着超高灵敏度的弱光图像探测器的发展,UWL的研究进入到一个新的阶段,开始在生命科学、医学、食品等领域开展研究[4-5]。自20世纪90年代UWL进入到农业领域开始,多数研究集中在UWL与环境因素及植物抗逆性的关系,如董家伦等[6]研究沙生植物的UWL,发现其与树木品种之间的抗旱性有关;在低温和高温条件下,抗性强的品种具有更高的UWL强度[7],同时种子的发光强度也随着温度的升高而增强[8];盐胁迫会导致植物UWL强度的降低,与抗性弱的品种相比,抗性强的品种种子的发芽率和UWL强度较高[9];因此,UWL也有望作为抗性品种鉴定和评价的有力工具。另外,有少数研究初步探索了UWL与植物部分生长发育进程的关系[10-12],但植物整个生命周期与UWL的关系如何?以及UWL产生的来源和机制均不够清楚完整,仍有待于更多的、大量的试验来研究验证。
关于UWL产生机制,生物化学的观点认为,UWL有可能来源于能级跃迁、活性氧发光、DNA发光和能量转换发光等方面,对于以上假设观点,已进行部分研究加以验证。张新华等[13]对植物体外线粒体UWL的初步探索发现,线粒体提取液的UWL强度与线粒体浓度呈正相关。前人[14-19]对草莓(Fragaria × ananassa Duch.)果实采后衰老过程中线粒体及其呼吸作用中能量代谢及活性氧与UWL的关系进行了研究,发现UWL强度可以反映果实的衰老程度;线粒体呼吸代谢的能量水平和生成效率与UWL的强度呈显著正相关,活性氧主要通过影响线粒体功能而影响UWL强度,活性氧爆发导致线粒体功能下降,从而导致UWL强度下降。以上研究进一步验证了线粒体是UWL产生的来源之一,能量代谢和活性氧水平与UWL激发有关。
那么,作为植物细胞中进行光合作用和能量转换主要细胞器的叶绿体,从其承担的作用与功能来看,应与植物中UWL的产生来源有关。针对该假设,笔者团队前期以欧李[Cerasus humilis (Bge.) Sok.]和德景天(Sedum hybridus L.)作为材料研究发现,干旱胁迫下两者叶片的净光合速率、蒸腾速率、胞间二氧化碳浓度、气孔导度均与UWL强度显著相关[20-21],初步说明植物光合作用与UWL有关,而叶绿体主要光能吸收色素—叶绿素及其代谢在其中扮演怎样的角色仍未可知。另外,光合作用为植物生长发育提供能量和物质,是对盐胁迫最敏感的生理过程之一;同时,欧李具有耐盐碱的特点,但有关盐胁迫下欧李叶片叶绿素代谢与UWL激发关系的研究还鲜见报道。故笔者在本研究中以欧李为试验材料,在前期初步探索的基础上,进一步对盐胁迫下欧李叶片叶绿素代谢及UWL的变化规律进行研究,解析植物叶绿素代谢与UWL发生的关系,为揭示逆境胁迫下植物光合作用与UWL的关系及植物中UWL产生的来源提供理论依据。
1 材料和方法
1.1 试验材料
试验以内蒙古农业大学欧李科研基地的2年生蒙原金秋欧李(C. humilis)盆栽苗为材料。
1.2 试验方法
选择生长正常、长势一致的欧李盆栽苗进行盐胁迫处理。根据预试验结果,采用NaCl浓度为400 mmol·L-1、800 mmol·L-1分别进行轻度和重度盐胁迫。两种盐胁迫均以浇灌法进行处理,每盆一次性浇400 mL不同浓度盐溶液,重复将浇灌后流出的盐溶液倒回盆内直至达到完全吸收;对照(Control)浇等量清水。各处理材料按完全随机排列,每处理每重复各10盆,5次重复。分别于胁迫0、2、4、6、8、10、12 d取样,取样时选取欧李植株当年基生枝由基部向上10~20枚之间的成熟叶片,用于UWL测定的叶片用冰盒带回,进行叶绿素代谢相关指标测定的叶片以蒸馏水洗净擦干去除主叶脉后,液氮速冻带回。
1.3 试验指标及测定方法
1.3.1 UWL的测定 使用超微弱发光测试系统(BPCL-2-SH,北京)进行测定。开机后调制高压1100 V,预热30 min,用打孔器(10 mm)对所取欧李叶片进行打孔,迅速将打孔部分叶片平铺于测量杯中,打开光窗立即测定。每个处理每次取5枚叶片,每片叶取样3次。以15次减去本底值的最大值的平均值表示UWL强度。
1.3.2 叶绿素代谢试验指标的测定 叶绿素合成前体物质的测定:δ-氨基乙酰丙酸(ALA)含量测定参照金鑫[22]的方法;胆色素原(PBG)、尿卟啉原Ⅲ(UroⅢ)和粪卟啉原Ⅲ(CopⅢ)含量的测定按照Bogorad[23]的方法;原卟啉Ⅸ(ProtoⅨ)、Mg-原卟啉Ⅸ(Mg-ProtoⅨ)和原叶绿素酸酯(Pchl)含量的测定参照Liu等[24]的方法。
叶绿素代谢酶含量的测定:用购自睿信生物科技有限公司(泉州)的Elasa试剂盒测定δ-氨基酮戊酸脱水酶(ALAD)、镁螯合酶(MgCH)、叶绿素酶(Chlase)含量。具体方法按操作说明进行。
叶绿素含量的测定参照李合生[25]的方法。
以上指标测定均重复3次试验,每次试验3个生物重复。
1.4 数据处理与方法
采用Excel统计软件进行数据处理,采用Origin软件绘图,采用SPSS软件进行相关性分析。
2 结果与分析
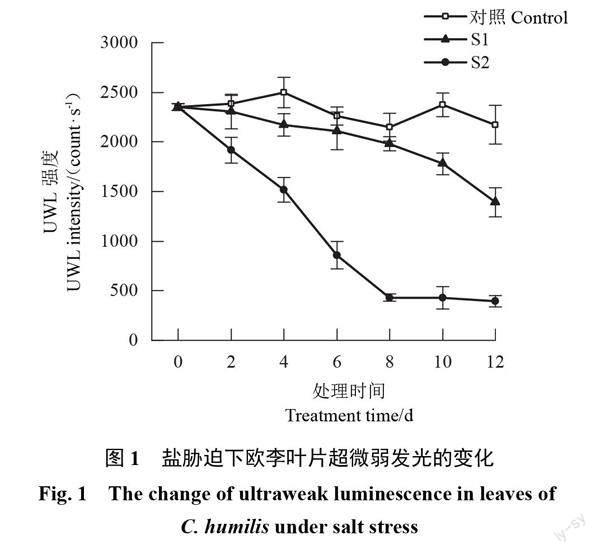
2.1 盐胁迫下欧李叶片超微弱发光的变化
随着胁迫时间的延长,对照叶片的UWL强度基本保持不变,2种盐胁迫下叶片的UWL强度均整体呈下降趋势。轻度胁迫下UWL强度缓慢下降,胁迫结束时UWL强度为1 392.47 count·s-1,比胁迫前降低了40.80%;重度胁迫下UWL强度于前8 d快速下降,之后基本不变,胁迫结束时UWL强度为393.81 count·s-1,比胁迫前降低了83.26%。整个胁迫期间,2种盐胁迫下UWL强度均明显低于对照,且重度胁迫的UWL强度明显低于轻度胁迫;胁迫结束时轻度胁迫下比对照降低了35.93%,重度胁迫下比对照降低了81.88%(图1)。可见盐胁迫会导致欧李叶片UWL强度下降,盐浓度越大UWL强度下降幅度越大。
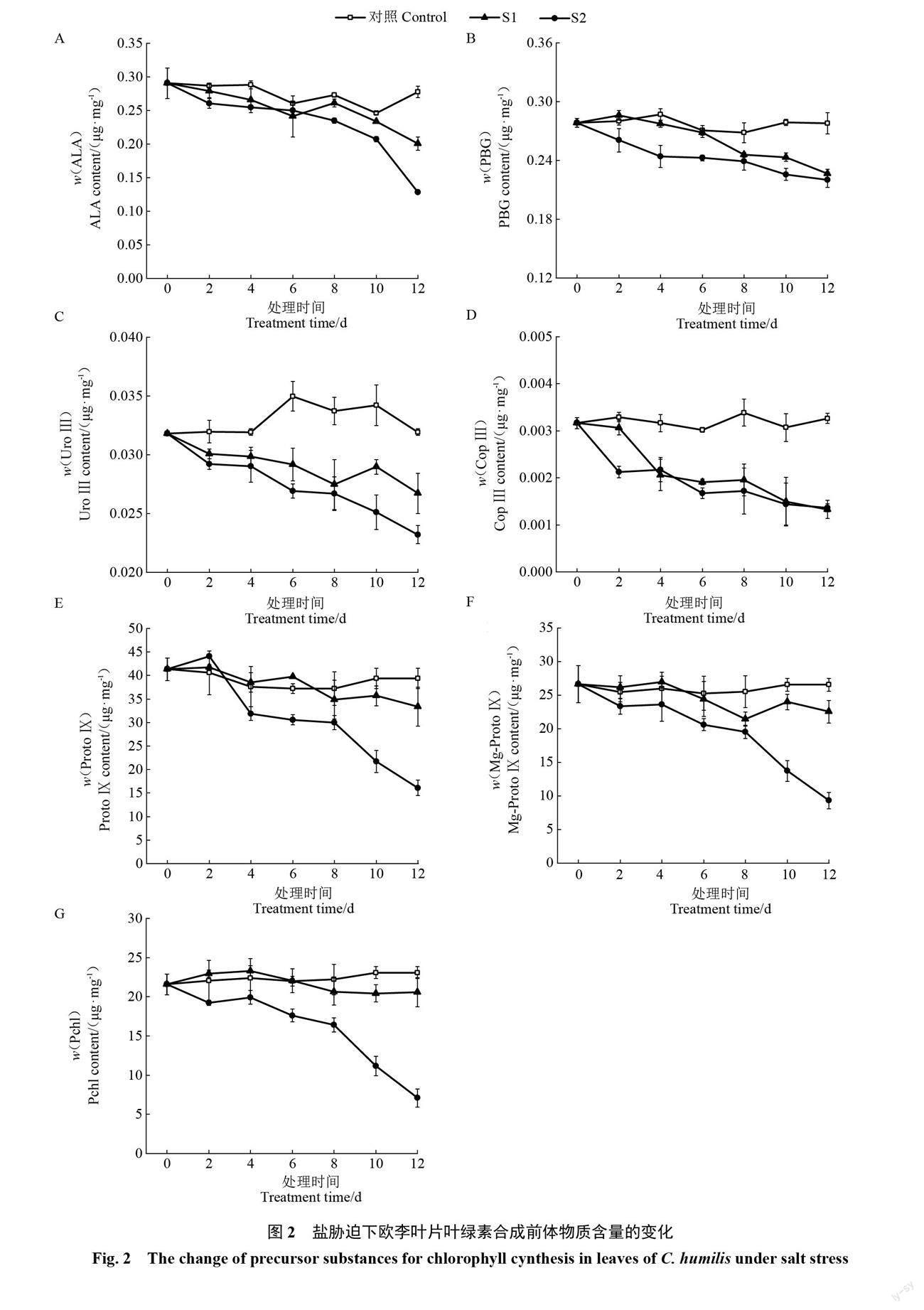
2.2 盐胁迫下欧李叶片叶绿素代谢及叶绿素含量的变化
2.2.1 叶绿素代谢合成前体物质含量的变化 随着胁迫时间的延长,对照叶片的ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-ProtoⅨ及Pchl含量均基本保持不变,2种盐胁迫下叶片的以上7项指标值均整体呈下降趋势。轻度胁迫下叶片7项指标均下降较为缓慢,重度胁迫下7项指标的下降幅度均不同程度地大于轻度胁迫。整个胁迫期间,2种盐胁迫下叶片的7项指标均低于对照,且重度胁迫下7项指标均低于轻度胁迫;其中,2种盐胁迫下叶片的UroⅢ和CopⅢ含量均明显低于对照,重度胁迫下叶片的ProtoⅨ、Mg-ProtoⅨ及Pchl含量均明显低于对照(图2)。以上不同程度盐胁迫下7项指标的变化表明,盐胁迫会导致欧李叶片叶绿素代谢过程中主要合成前体物质含量下降,盐浓度越大合成前体物质含量下降幅度越大。
2.2.2 叶绿素代谢过程中代谢相关酶含量的变化 随着胁迫时间的延长,对照叶片的叶绿素代谢合成过程酶ALAD、MgCH及Chlase含量均基本保持稳定,2种盐胁迫下叶片的以上3种酶含量均整体呈上升趋势。轻度胁迫下叶片3种酶含量均上升较为缓慢,重度胁迫下3种酶含量的上升幅度均不同程度地大于轻度胁迫。整个胁迫期间,2种盐胁迫下叶片的3种酶含量均明显高于对照,且重度胁迫下3种酶含量均不同程度地高于轻度胁迫(图3)。以上不同程度盐胁迫下叶绿素代谢合成过程酶及叶绿素酶含量的变化表明,盐胁迫下欧李叶片叶绿素代谢2种合成过程酶及叶绿素降解酶的含量均表现为增加趋势,盐浓度越大以上各种酶的含量上升幅度越大。
2.2.3 叶绿素含量的变化 随着胁迫时间的延长,对照叶片的Chla、Chlb及Chla+b含量的变化小有起伏,整体上保持平稳;2种盐胁迫下叶片的3种叶绿素含量均整体呈下降趋势。轻度胁迫下叶片3种叶绿素含量均下降较为缓慢,胁迫结束时Chla、Chlb及Chla+b含量比胁迫前分别降低了26.38%、39.47%、29.73%;重度胁迫下3种叶绿素含量的下降幅度均不同程度地大于轻度胁迫,比胁迫前分别降低了76.66%、72.16%、75.51%。整个胁迫期间,2种盐胁迫下3种叶绿素含量均低于对照,且重度胁迫下3种叶绿素含量均低于轻度胁迫;胁迫结束时轻度胁迫下Chla、Chlb及Chla+b含量比对照分别降低了34.73%、39.84%、35.93%,重度胁迫下比对照分别降低了79.31%、72.33%、77.67%(图4)。以上不同程度盐胁迫下叶绿素含量的变化表明,盐胁迫会导致欧李叶片叶绿素含量下降,盐浓度越大叶绿素含量下降幅度越大。
2.3 盐胁迫下欧李叶片叶绿素代谢与UWL的关系
盐胁迫下,与对照相比,不同程度盐胁迫欧李叶片叶绿素代谢过程中的7种合成前体物质含量均下降,2种合成过程酶及叶绿素酶含量均表现为上升,Chla、Chlb及Chla+b含量均下降;同时叶片UWL强度也下降。且盐浓度越高,以上叶绿素代谢指标值及UWL强度的下降或上升的幅度越明显。叶片叶绿素代谢与UWL的相关性分析显示,对照叶片的UWL强度与合成前体物质PBG含量及合成过程酶MgCH含量呈显著正相关。轻度胁迫下,UWL强度与合成前体物質ALA、PBG、Mg-protoⅨ含量呈极显著正相关,与UroⅢ、CopⅢ含量呈显著正相关;与代谢相关酶ALAD、Chlase含量极显著负相关,与MgCH含量呈显著负相关;与Chla及Chla+b含量呈极显著正相关,与Chlb含量呈显著正相关。重度胁迫下,UWL强度与合成前体物质PBG、UroⅢ、CopⅢ含量呈极显著正相关,与ProtoⅨ、Mg-protoⅨ、Pchl含量呈显著正相关;与代谢酶Chlase含量呈极显著负相关,ALAD与MgCH含量呈显著负相关;与Chla、Chlb和Chla+b含量均呈显著正相关(表1)。以上盐胁迫下叶绿素代谢与UWL强度的相关性分析表明,叶片UWL强度与叶绿素合成前体物质、相关代谢酶及叶绿素含量密切相关。
综合盐胁迫下叶绿素代谢和UWL的变化规律及两者之间的相关性关系,表明欧李叶片UWL强度与叶绿素代谢密切相关。盐胁迫下,随着叶片叶绿素合成前体物质含量下降、叶绿素酶含量的上升,叶绿素合成代谢减弱而降解代谢占据优势,从而引起叶绿素含量下降;以上叶绿素代谢变化导致了叶片UWL强度降低。
3 讨 论
植物在逆境胁迫中表现出不同的UWL现象已有一些报道。如接玉玲等[26]研究干旱胁迫下湖北海棠[Malus hupehensis (Pamp.) Rehder]幼苗UWL的变化发现,随着胁迫程度的加深,叶片UWL强度逐渐降低。卜令豪等[27]研究发现,盐胁迫下罗布麻(Apocynum venetum L.)叶片UWL强度随胁迫时间的延长而降低,且胁迫程度越重,UWL强度下降幅度越大。类似地,笔者在研究中发现,轻度和重度盐胁迫下欧李叶片的UWL强度均低于未胁迫处理,随着胁迫时间延长,UWL强度持续下降;且重度胁迫下UWL强度的下降幅度更大。以上研究结果显示,植物受到逆境胁迫时会导致UWL强度下降,胁迫程度越重UWL强度下降幅度越大,UWL强度能够反映植物受到的逆境胁迫的程度。
李德红等[28]早期对白菜(Brassica rapa var. glabra Regel)体外叶绿体的UWL进行了研究,发现叶绿体的UWL不能简单地归因于该过程的自由基,植物的UWL应与植物光形态建成和光合作用过程相关。但具体与植物光合作用过程中哪些因素有关的研究还未见报道。叶绿素作为叶绿体光合作用中的聚光色素和反应中心色素,承担着光能的吸收和传递、并通过光化学反应将光能转变为电能的职能,开启了光合作用的第一步。闫妮等[29]发现盐胁迫会导致番茄(Solanum lycopersicum L.)叶绿素含量下降,进而使光合作用效率降低。叶绿素代谢反映了叶绿体功能及其光合作用的效能[30],其过程分为叶绿素合成与降解两部分,合成与降解的动态平衡决定了叶绿素的含量。叶绿素的生物合成主要是通过谷氨酸(Glu)→ALA→PBG→UroⅢ→CopⅢ→ProtⅨ→Mg-ProtoⅨ→Pchl→Chla→Chlb途径完成[31]。其中,ALA的合成和Mg离子插入ProtⅨ是叶绿素合成的2个主要控制点;合成途径中任何一步发生异常都会使叶绿素合成受阻,导致叶绿素含量下降[32]。Chlase是叶绿素降解的关键酶,催化Chla转化为Chlidea,由此开启了叶绿素的降解。毛晶晶等[33]研究发现,低温胁迫下叶绿素合成途径中ALA和Mg-Proto Ⅸ的积累量显著上升,PBG、Urogen Ⅲ、CoprogenⅢ、Proto Ⅸ、Mpe、Mpde、Pchlide的积累量低于常温,推测ALA向PBG的转化及Mg-Proto Ⅸ向Mpe的转化过程受到低温胁迫抑制,从而导致玉米(Zea mays L.)叶绿素含量下降,抑制了玉米的转绿过程。王颖等[34]研究发现,盐胁迫使菠菜(Spinacia oleracea L.)叶片ALA和PBG含量升高,而UroⅢ、ProtoⅨ、Mg-ProtoⅨ、Pchl、Chla、Chlb及Chla+b含量均降低,说明盐胁迫下叶片叶绿素合成受阻位点在PBG向UroⅢ的转化过程中。上述研究表明不同环境因素对叶绿素合成途径的影响有所差异,这种差异可能由植物种类及环境因子不同所致。笔者在本试验中,不同程度盐胁迫下,与对照相比,欧李叶片7种叶绿素合成前体物质ALA、PBG、UroⅢ、CopⅢ、ProtoⅨ、Mg-protoⅨ、Pchl的含量均下降;主要合成过程酶ALAD、MgCH的含量均表现为上升,Chlase含量大幅度上升;同时Chla、Chlb、Chla+b的含量均下降。其中,叶绿素合成首要前体ALA含量降低,因而造成后续叶绿素合成原料不足,应该是盐胁迫下叶绿素合成代谢受阻的主要原因;兼之Chlase含量明显上升,加快了叶绿素的分解进程。以上两方面综合作用引起盐胁迫下欧李叶片叶绿素含量下降。同时,试验中盐胁迫下欧李叶片叶绿素合成主要酶ALAD、MgCH的含量均上升,且上升幅度均为重度胁迫大于轻度胁迫,猜测该结果应该是盐胁迫下欧李本身的一种应激反应,但仍有待于进一步研究验证。盐胁迫下,在以上叶绿素代谢变化的过程中,叶片UWL强度也持续下降;相关分析结果显示,不同程度盐胁迫下,UWL强度均与叶绿素合成前体物质含量及叶绿素含量呈显著正相关,与叶绿素酶含量呈显著负相关。上述结果表明欧李叶片叶绿素代谢与UWL密切相关。
4 结 论
盐胁迫下,随着叶片叶绿素合成前体物质含量下降及叶绿素酶含量的上升,叶绿素合成代谢减弱而降解代谢加强,从而引起叶绿素含量下降,以上叶绿素代谢变化导致叶片UWL发生受阻,发光强度降低,表明盐胁迫下欧李叶片叶绿素代谢与UWL激发密切相关。
参考文献References:
[1] POPP F A,LI K H,GU Q. Recent advances in biophoton research and its applications[M]. Singapore:World Scientific Publishing Conpany,1992:1-46.
[2] 程海鹏,王君晖,池浩超,朱睦元. 豌豆种子萌发过程中超微弱发光的研究[J]. 浙江大学学报(理学版),2001,28(6):682-685.
CHENG Haipeng,WANG Junhui,CHI Haochao,ZHU Muyuan. Study on ultraweak luminescence of Pisumsativumseeds at the stage of germination[J]. Journal of Zhejiang University (Sciences Edition),2001,28(6):682-685.
[3] GURWITSCH A. Die natur des spezifischen erregers der zellteilung[J]. Archiv FüR Mikroskopische Anatomie und Entwicklungsmechanik,1923,100:11-40.
[4] 王暢,蒋礼林,王乐新,朱文霞. 奶牛血清微弱发光的分析与应用研究[J]. 江苏农业科学,2013,41(2):187-189.
WANG Chang,JIANG Lilin,WANG Lexin,ZHU Wenxia. Analysis and application of weak luminescence in dairyserum[J]. Jiangsu Agricultural Sciences,2013,41(2):187-189.
[5] 岳霞丽,刘永红,胡先文,董元彦. 水华鱼腥藻的超弱发光研究[J]. 光谱实验室,2008,25(4):673-676.
YUE Xiali,LIU Yonghong,HU Xianwen,DONG Yuanyan. The study of ultraweak luminescence of Anabaena flos-aquae[J]. Chinese Journal of Spectroscopy Laboratory,2008,25(4):673-676.
[6] 董家伦,李树真,刘生龙. 一些沙生植物苗期超弱发光特征研究[J]. 中国沙漠,1990,10(2):29-34.
DONG Jialun,LI Shuzhen,LIU Shenglong. A stuay on the characteristics of ultra-weak luminescence of some psammophytes during seedling stage[J]. Journal of Desert Research,1990,10(2):29-34.
[7] 杨起简. 几种作物籽粒萌发时超弱发光与其抗逆性关系[J]. 生物化学与生物物理进展,1993,20(4):315-317.
YANG Qijian. Relationship between ultra-weak luminescence and stress resistance of several crops during grain germination[J]. Progress in Biochemistry and Biophysics,1993,20(4):315-317.
[8] 习岗. 植物超弱发光及其在农业上的应用[J]. 物理,1994,23(9):548-552.
XI Gang. Ultra-weak luminescence of plants and its application in agriculture[J]. Physics,1994,23(9):548-552.
[9] 杨起简,周禾,ПОГОСЯН C И,ЯКОВЛЕВ А Ф. 盐胁迫下豌豆幼苗的超弱发光[J]. 激光生物学报,2001,10(4):265-268.
YANG Qijian,ZHOU He,ПОГОСЯН C И,ЯКОВЛЕВ А Ф. Study on superweak luminescence of pea seedlingunder the different Na-salt stress[J]. Acta Laser Biology Sinica,2001,10(4):265-268.
[10] 侯仙慧,廖祥儒,李穎,张晓晴,卜文娟,贾燕,李光. 苋菜种子萌发过程的超微弱发光及其机理研究[J]. 种子,2004,23(7):24-27.
HOU Xianhui,LIAO Xiangru,LI Ying,ZHANG Xiaoqing,BU Wenjuan,JIA Yan,LI Guang. Ultraweak biophoton emission and its mechanism during seed germination of Amaranthus hypochondriacus[J]. Seed,2004,23(7):24-27.
[11] 林桂玉,黄在范,张翠华,郑成淑. 菊花花芽分化期超微弱发光及生理代谢的变化[J]. 园艺学报,2008,35(12):1819-1824.
LIN Guiyu,HUANG Zaifan,ZHANG Cuihua,ZHENG Chengshu. Changes in ultraweak luminescence intensity,respiration rate and physiological metabolism of Chrysanthemum during floral differentiation[J]. Acta Horticulturae Sinica,2008,35(12):1819-1824.
[12] 赵丹莹,生吉萍,丁洋,申琳,范蓓,刘灿. 超微弱发光用于番茄果实冷害发生程度的无损监测[J]. 光谱学与光谱分析,2010,30(9):2493-2495.
ZHAO Danying,SHENG Jiping,DING Yang,SHEN Lin,FAN Bei,LIU Can. Nondestructive examination of tomato chilling injury by Ultraweak Luminescence[J]. Spectroscopy and Spectral Analysis,2010,30(9):2493-2495.
[13] 张新华,杨洪强. 植物叶绿体和线粒体的超微弱发光[J]. 植物生理学通讯,2004,40(1):111-114.
ZHANG Xinhua,YANG Hongqiang. Ultraweak bioluminescence of chloroplast and mitochondria in plants[J]. Plant Physiology Communications,2004,40(1):111-114.
[14] 郭金丽,刘欢,梁爽,朱冠宇,白杨,李连国. 活性氧调控下草莓果实衰老过程中活性氧与超微弱发光的关系[J]. 果树学报,2017,34(3):363-369.
GUO Jinli,LIU Huan,LIANG Shuang,ZHU Guanyu,BAI Yang,LI Lianguo. Relationship between reactive oxygen species and ultraweak luminescence in strawberry fruit during senescence under various reactive oxygen regulation treatments[J]. Journal of Fruit Science,2017,34(3):363-369.
[15] 刘欢,梁爽,闫宇彤,白杨,郭金丽. 活性氧和能量调控下草莓果实衰老与超微弱发光的关系[J]. 西北植物学报,2017,37(6):1182-1188.
LIU Huan,LIANG Shuang,YAN Yutong,BAI Yang,GUO Jinli. Relationship between senescence and ultraweak photon emission under controlling of reactive oxygen and energy in strawberry fruit[J]. Acta Botanica Boreali-Occidentalia Sinica,2017,37(6):1182-1188.
[16] GUO J L,ZHU G Y,LI L G,LIU H,LIANG S. Ultraweak photon emission in strawberry fruit during ripening and aging is related to energy level[J]. Open Life Sciences,2017,12(1):393-398.
[17] GUO J L,LIU H,BAI Y,YAN Y T,LI L G. Manipulation of cellular energy reveals the relationship between ultraweak luminescence and cellular energy during senescence of strawberry (Fragaria × ananassa) fruits[J]. Acta Physiologiae Plantarum,2018,40(7):134.
[18] 孙聪,白杨,李连国,郭金丽. 草莓果实线粒体呼吸代谢与超微弱发光的关系[J]. 西北植物学报,2019,39(10):1805-1811.
SUN Cong,BAI Yang,LI Lianguo,GUO Jinli. Relationship between mitochondrial respiratory metabolism and ultraweak luminescence in strawberry fruit[J]. Acta Botanica Boreali-Occidentalia Sinica,2019,39(10):1805-1811.
[19] SUN C,LIU J C,LIU H,GUO J L. Reactive oxygen species mediate the relationship between mitochondrial function and delayed luminescence during senescence of strawberry (Fragaria ananassa) fruits[J]. Acta Physiologiae Plantarum,2022,44(2):25.
[20] 郭金麗,梁爽,邵长芬,白杨,闫宇彤,李连国. 干旱胁迫下景天植物光合作用与超微弱发光的关系[J]. 西北植物学报,2017,37(9):1789-1796.
GUO Jinli,LIANG Shuang,SHAO Changfen,BAI Yang,YAN Yutong,LI Lianguo. Relationship between photosynthesis and ultraweak luminescence in Sedum hybridum under drought stress[J]. Acta Botanica Boreali-Occidentalia Sinica,2017,37(9):1789-1796.
[21] 孙聪,任鹏达,李连国,李晓艳,郭金丽. 干旱胁迫下欧李叶片叶绿体光合参数与超弱光子辐射的关系[J]. 北方园艺,2021(9):25-31.
SUN Cong,REN Pengda,LI Lianguo,LI Xiaoyan,GUO Jinli. Relationship between ultraweak photon emission and photosynthetic parameters of Cerasus humilis leaves chloroplast under drought stress[J]. Northern Horticulture,2021(9):25-31.
[22] 金鑫. 柑橘黄化脉明病毒对柠檬、柚和甜橙叶绿素代谢影响的研究[D]. 重庆:西南大学,2017.
JIN Xin. Effect on chlorophyll metabolism in lemon,pummelo and sweet orange by Citrus yellow vein clearingvirus[D]. Chongqing:Southwest University,2017.
[23] BOGORAD L. [122] Porphyrin synthesis[J]. Methods in Enzymology,1962,5:885-895.
[24] LIU J,WANG J Y,YAO X Y,ZHANG Y,LI J Q,WANG X X,XU Z J,CHEN W F. Characterization and fine mapping of thermo-sensitive chlorophyll deficit mutant1 in rice (Oryza sativa L.)[J]. Breeding Science,2015,65(2):161-169.
[25] 李合生. 植物生理生化实验原理和技术[M]. 北京:高等教育出版社,2000.
LI Hesheng. Principles and techniques of plant physiological biochemical experiment[M]. Beijing:Higher Education Press,2000.
[26] 接玉玲,赵海洲,张伟,杨洪强,李德全,束怀瑞. 甜菜碱对干旱胁迫下湖北海棠超微弱发光及抗氧化能力的影响[J]. 应用生态学报,2006,17(12):2394-2398.
JIE Yuling,ZHAO Haizhou,ZHANG Wei,YANG Hongqiang,LI Dequan,SHU Huairui. Effects of glycinebetaine on ultraweak luminescence and anti-oxidative capability of Malus hupehensis under drought stress[J]. Chinese Journal of Applied Ecology,2006,17(12):2394-2398.
[27] 卜令豪,陈忠祥,刘志华.盐胁迫对罗布麻生理生化指标及超微弱发光的影响[J/OL].分子植物育种,2022:1-15. http://kns.cnki.net/kcms/detail/46.1068.S.20220822.1657.004.html.
BU Linghao,CHEN Zhongxiang,LIU Zhihua. Effects of salt stress on physiological and biochemical lndices of rob hemp and ultra-faint Luminescence[J/OL]. Molecular Plant Breeding,2022:1-15. http://kns.cnki.net/kcms/detail/46.1068.S.20220822.1657.004.html.
[28] 李德红,唐永红,何永红,邢达. 白菜叶绿体的超弱发光机理初探[J]. 激光生物学报,2002,11(1):64.
LI Dehong,TANG Yonghong,HE Yonghong,XING Da. Preliminary study on ultra-weak luminescence mechanism of chloroplast in Chinese cabbage[J]. Acta Laser Biology Sinica,2002,11(1):64.
[29] 闫妮,冯棣,杨凤娟,张敬敏,桑茂鹏,祝海燕. γ-氨基丁酸浸种对盐分胁迫下番茄出苗及幼苗生长的影响[J]. 中国瓜菜,2022,35(10):58-63.
YAN Ni,FENG Di,YANG Fengjuan,ZHANG Jingmin,SANG Maopeng,ZHU Haiyan. GABA soaking affects tomato emergence and seedling growth under salt stress[J]. China Cucurbits and Vegetables,2022,35(10):58-63.
[30] 周振翔,李志康,陈颖,王志琴,杨建昌,顾骏飞. 叶绿素含量降低对水稻叶片光抑制与光合电子传递的影响[J]. 中國农业科学,2016,49(19):3709-3720.
ZHOU Zhenxiang,LI Zhikang,CHEN Ying,WANG Zhiqin,YANG Jianchang,GU Junfei. Effects of reduced chlorophyll content on photoinhibition and photosynthetic electron transport in rice leaves[J]. Scientia Agricultura Sinica,2016,49(19):3709-3720.
[31] 孙锦,贾永霞,郭世荣,李娟. 海水胁迫对菠菜(Spinacia olerancea L.)叶绿体活性氧和叶绿素代谢的影响[J]. 生态学报,2009,29(8):4361-4371.
SUN Jin,JIA Yongxia,GUO Shirong,LI Juan. Effects of seawater stress on metabolism of reactive oxygen species and chlorophyll in chloroplasts of spinach (Spinacia olerancea L.)[J]. Acta Ecologica Sinica,2009,29(8):4361-4371.
[32] 靳晓青. 外源γ-氨基丁酸调控活性氧和叶绿素代谢增强甜瓜幼苗盐碱胁迫耐性[D]. 杨凌:西北农林科技大学,2019.
JIN Xiaoqing. Exogenous γ-aminobutyric acid regulates reactive oxygen species and chlorophyll metabolism to enchance salinity-alkalinity stress tolerance in muskmelon seedlings[D]. Yangling:Northwest A & F University,2019.
[33] 毛晶晶,李泽娇,赵雨晴,袁澍,袁明. 低温胁迫对玉米转绿过程中叶绿素生物合成的影响[J]. 四川农业大学学报,2019,37(5):617-622.
MAO Jingjing,LI Zejiao,ZHAO Yuqing,YUAN Shu,YUAN Ming. The effects of low temperature on chlorophyll synthesis during greening of maize[J]. Journal of Sichuan Agricultural University,2019,37(5):617-622.
[34] 王颖,郭世荣,束胜,刘芳,刘涛,孙锦. 外源亚精胺对盐胁迫下菠菜叶绿素合成前体含量的影响[J]. 西北植物学报,2015,35(10):2026-2034.
WANG Ying,GUO Shirong,SHU Sheng,LIU Fang,LIU Tao,SUN Jin. Effects of exogenous spermidine on chlorophyll precursors content of spinach plants under salt stress[J]. Acta Botanica Boreali-Occidentalia Sinica,2015,35(10):2026-2034.
