不同缝隙连接蛋白与线粒体动力学蛋白在肝移植术后早期急性肾损伤中的变化
2023-05-27麦康凤李佳嫒魏靖茹陈潮金李晓芸
麦康凤?李佳嫒?魏靖茹?陈潮金?李晓芸
【摘要】目的 探索不同縫隙连接蛋白在肝移植术后早期急性肾损伤中与线粒体动力学失衡及线粒体损伤可能的关系。方法 将24只SPF级SD雄性健康大鼠随机分为假手术组(sham组,n = 6),肝移植手术再灌注2 h、4 h、8 h组
(M2、M4、M8组,每组n = 6)。sham组只开腹进行血管分离,M组进行原位肝移植手术,各组检测肾功能、肾病理及肾皮质缝隙连接蛋白32(Cx32)、缝隙连接蛋白43(Cx43)、线粒体动力相关蛋白1(Drp1)、线粒体融合蛋白1(Mfn1)、线粒体融合蛋白2(Mfn2)、融合相关蛋白视神经萎缩相关蛋白A1(Opa1)、内质网应激相关蛋白C/EBP 同源蛋白(CHOP)的表达,于电镜下观察线粒体形态变化。结果 与sham组相比,M2组肾组织损伤病理评分增高(P < 0.01),并随时间增加趋势增高,M8组达到高峰(P < 0.001),但血清肌酐(SCr)和血清尿素氮(BUN)仅M8组增高(P < 0.001,P < 0.05);与sham组相比,M2组大鼠Cx32表达增加(P < 0.01),M4组Cx43表达增加(P < 0.001),M8组均达到峰值(P < 0.001);与sham组比较,M2组线粒体动力学蛋白Drp1、Mfn1、Mfn2、OPA1表达均增加(P < 0.001);电镜下M2组即出现线粒体损伤,可见线粒体自噬和溶酶体自噬,粗面内质网轻度肿胀。线粒体损伤程度随肝移植再灌注时间延长而增加,M2、M4、M8组均可见自噬线粒体及内质网-线粒体接触位点。结论 SD大鼠经历自体原位肝移植术后早期就出现肾病理及线粒体损伤,SCr及BUN诊断肾损伤具有延迟性;在肝移植术后早期肾皮质Cx32表达增高,线粒体分裂融合表现均活跃,提示Cx32传递伤害性信号与线粒体损伤及线粒体动力学失衡相关,可能为肝移植术后早期急性肾损伤的重要作用机制。
【关键词】急性肾损伤;肝移植;缝隙连接蛋白;线粒体动力学
Changes of different connexins and mitochondrial dynamins in early acute kidney injury after liver transplantation Mai Kangfeng, Li Jiaai, Wei Jingru,Chen Chaojin, Li Xiaoyun. Department of Anesthesiology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China
Corresponding author, Li Xiaoyun, E-mail: lixyun@mail.sysu.edu.cn
【Abstract】Objective To explore the potential relationship between different connexins and mitochondrial dynamic imbalance and mitochondrial injury in early acute kidney injury after liver transplantation. Methods 24 healthy specific pathogen-free (SPF) SD male rats were randomly divided into the sham operation group (sham group, n = 6), and 2-, 4- and 8-h reperfusion liver transplantation groups (M2, M4 and M8 groups, n = 6 each). In the sham group, open surgery alone was performed for vessel separation. In the M2, M4 and M8 groups, orthotopic liver transplantation was conducted. Renal function and renal pathological examination were determined. The expression levels of connexin 32 (Cx32), Cx43, dynamin-related protein 1 (Drp1), mitofusin-1 (Mfn1), Mfn2, optic atrophy 1 (Opa1) and C/EBP homologous protein (CHOP), an endoplasmic reticulum stress-related protein, were detected in each group. The morphological changes of mitochondria were observed under electron microscope. Results Compared with the sham group, the pathological score of kidney tissue injury in the M2 group was increased (P < 0.01), and gradually increased over reperfusion time, and reached the peak in the M8 group (P < 0.001). However, serum creatinine (SCr) and blood urea nitrogen (BUN) levels were up-regulated only in the M8 group (P < 0.001, P < 0.05). Compared with the sham group, Cx32 level was increased in the M2 group (P < 0.01), Cx43 level was increased in the M4 group (P < 0.001), and reached the peak in the M8 group (both P < 0.001). Compared with the sham group, the expression levels of Drp1, Mfn1, Mfn2 and OPA1 were up-regulated in the M2 group (all P < 0.001). Under electron microscope, mitochondrial injury, mitochondrial autophagy, lysosomal autophagy and slightly swollen rough endoplasmic reticulum were observed in the M2 group. The degree of mitochondrial injury was aggravated over reperfusion time during liver transplantation. Mitochondrial autophagy and endoplasmic reticulum - mitochondria contact sites were observed in the M2, M4 and M8 groups. Conclusions Kidney pathological injury and mitochondrial injury occur early after autogenous orthotopic liver transplantation in SD rats. The diagnosis of kidney injury by SCr and BUN levels can be delayed. The expression level of Cx32 in the renal tissues is up-regulated early after liver transplantation. Active mitochondrial division and fusion can be seen, suggesting that the transmission of injury signals by Cx32 is associated with mitochondrial injury and mitochondrial dynamic imbalance, which may be an important mechanism of early acute kidney injury after liver transplantation.
【Key words】Acute kidney injury; Liver transplantation; Connexin; Mitochondrial dynamics
急性肾损伤(AKI) 是肝移植围术期常见且严重的并发症,发生率高达40.7%,AKI不仅是术后早期主要死亡原因之一,还是引发慢性肾衰竭及影响长期预后的独立危险因素,且目前尚无特效药,因此关注肝移植术后早期AKI是防止肝移植术后肾功能继续恶化的关键[1]。线粒体动力学失衡所致肾小管细胞能量代谢紊乱和细胞损伤是早期AKI激活机制。肾小管细胞含有丰富的线粒体,由线粒体动力学维持的线粒体稳态对于正常的肾功能至关重要[2]。缝隙连接(GJ)是细胞间直接通信的重要方式,由缝隙连接蛋白(Cx)组成。Cx32、Cx43等在肾脏中的表达丰富,本课题组的前期研究发现,Cx32及Cx43与肾小管上皮细胞损伤凋亡及肝移植术后AKI恶化有关[3-4]。
本研究将关注点提前至肝移植术后早期,观察不同的Cx及线粒体动力学蛋白变化特点,探索Cx32、Cx43在肝移植术后早期AKI中与线粒体动力学失衡及线粒体损伤可能的关系,为肝移植术后早期肾保护策略的制定提供理论依据。
材料与方法
一、材 料
1.实验动物
24只SPF級5~6周龄的雄性SD大鼠来源于湖南斯莱克景达实验动物有限公司[SCXK(湘)2019-0004],体重200~220 g。动物建模及取材均在华南农业大学实验动物中心内完成[SYXK(粤)2022-0136]。按照每笼3只群养,温度约25℃,相对湿度约60%,昼夜间隔12 h,允许动物自由摄食饮水。本研究方案经华南农业大学实验动物伦理委员会审查批准(伦理编号:2022D092)。
二、方 法
1. 建立大鼠自体原位肝移植模型
大鼠常规禁食不禁饮12 h,将其麻醉后消毒、开腹。阻断肝门前在尾静脉注射1 mL肝素生理盐水(50 U/mL),分别结扎左膈上静脉、脾胃底静脉、右肾上腺静脉;阻断肝门后经门静脉推注3 mL常温肝素盐水(25 U/mL)将肝内血驱回心脏;再经门静脉冷灌注0~4℃肝素生理盐水(12.5 U/mL)20 mL。待肝脏颜色均匀变为土黄色时结束无肝期(20±1)min,缝补静脉血管穿刺点,给予48℃ 0.0002%肾上腺素和去甲肾上腺素混合冲洗腹腔以复温,阻断肝门后在尾静脉注射1 mL鱼精蛋白(1 mg/mL),然后缝合腹部切口并复苏大鼠[5]。
2. 动物实验分组及处理
采用随机数表法将24只大鼠随机分为4组,每组6只,分别为假手术组(sham组)以及肝移植模型M2组、M4组、M8组。sham组只开腹进行血管分离,肝移植模型M组则进行原位肝移植手术。在肝脏再灌注后2 h(M2组)、4 h(M4组)、8 h(M8组) 分别麻醉大鼠,处死大鼠后取其血及肾组织,采用生化仪检测血清肌酐(SCr)、尿素氮(BUN)水平。以上各组分别随机选取3只大鼠,采用蛋白质印迹法检测其肾组织中Cx32、Cx43、线粒体动力相关蛋白1(Drp1)、线粒体融合蛋白1(Mfn1)、线粒体融合蛋白2(Mfn2)、融合相关蛋白视神经萎缩相关蛋白A1(Opa1)和内质网应激相关蛋白C/EBP同源蛋白(CHOP)的表达情况;用石蜡包埋肾切片,经HE染色在光镜下观察并进行病理评分,参照文献[6]评分标准评估肾小管损伤情况,包含肾小管扩张、肾小管上皮损伤和管腔形成,采用4分法,0分:无变化;1分:影响<25%的视野;2分:影响25%~50%的视野;3分:影响51%~75%的视野;4分:影响>75%的视野;经4%戊二醛固定肾组织包埋切片,行2%醋酸铀饱和乙醇溶液和枸橼酸铅染色后在透射电镜下观察线粒体内质网超微结构。
三、统计学处理
采用SPSS 25.0处理数据。多组间的比较用单因素方差分析,各组与对照组的多重比较采用 Dunnett-t检验,P < 0.05为差异有统计学意义。
结果
一、肾功能与肾病理改变
1. SCr与BUN水平的变化
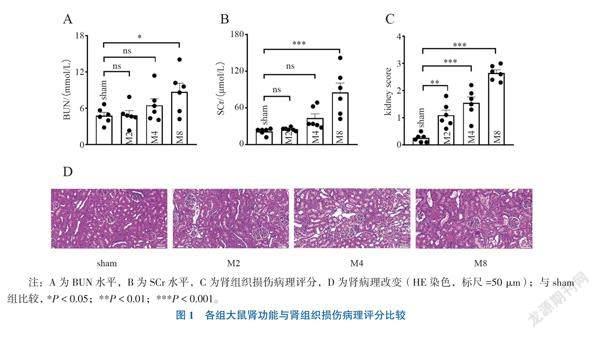
与sham组比较,M8组SCr、BUN水平增高(Dunnett-t = 5.274, P < 0.001; Dunnett-t = 2.737, P = 0.033),见图1A、B。
2. 肾组织损伤病理评分及病理改变特点
与sham组比较,M2、M4,M8组肾组织损伤病理评分均增高,高峰出现在M8组(Dunnett-t = 3.935, P = 0.002; Dunnett-t = 6.018, P < 0.001; Dunnett-t = 11.110, P < 0.001),见图1C。sham组肾小管病理形态正常,无明显肿胀;M2组可见轻度肾小管上皮细胞水肿,肾小管轻度扩张;M4组可见肾小管上皮细胞明显水肿,肾小管明显扩张;M8组可见部分肾小管上皮细胞细胞核固缩、变性、坏死、脱落,较多管型形成,见图1D。
二、Cx、线粒体动力学蛋白及线粒体形态结构变化
1.肝移植术后再灌注不同时间点肾皮质组织Cx表达
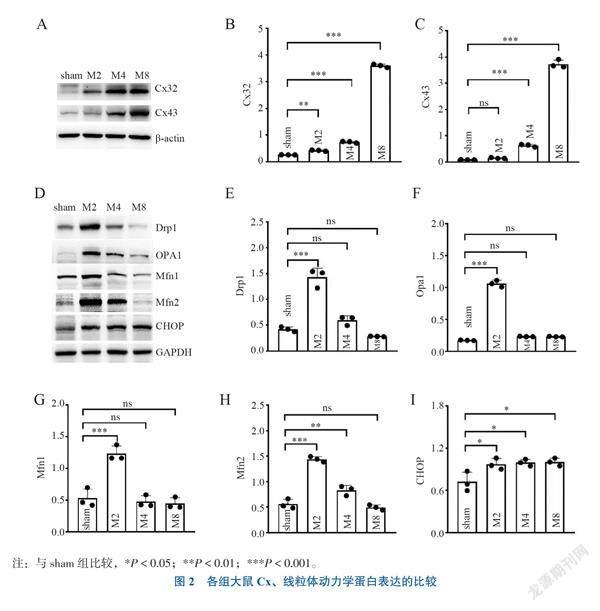
与sham组比较, Cx43表达在M2组无变化(Dunnett-t = 0.930, P = 0.710),在M4、M8组增加(Dunnett-t = 8.493, P < 0.001; Dunnett-t = 57.502, P < 0.001);Cx32表达在M2、M4、M8组均增加(Dunnett-t = 6.187, P = 0.002; Dunnett-t = 18.481, P < 0.001; Dunnett-t = 129.028, P < 0.001),见图2A~C。
2.肝移植術后再灌注不同时间点肾皮质线粒体动力学蛋白表达
与sham组相比,M2组Drp1、Mfn1、Mfn2、Opa1表达均增加(Dunnett-t = 11.742, P < 0.001; Dunnett-t = 7.580,P < 0.001; Dunnett-t = 13.949,P < 0.001; Dunnett-t = 34.298,P < 0.001),M4、M8组线粒体动力学蛋白表达与sham组比较差异无统计学意义,见图2D~I。
3.肝移植术后再灌注不同时间点肾皮质组织线粒体形态结构
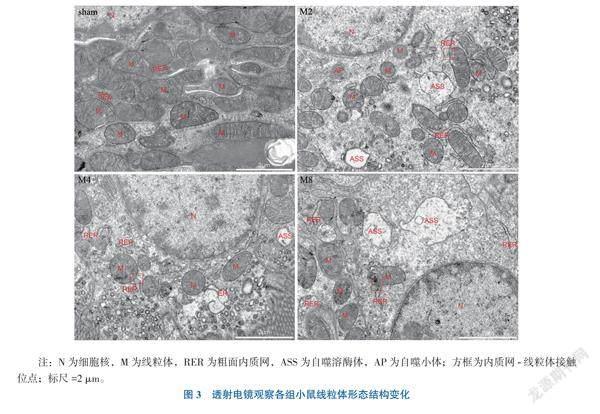
透射电镜检测大鼠肾皮质区线粒体结构情况,sham组大鼠肾皮质线粒体呈椭圆形,嵴结构紧密、清晰、排列整齐,M2组大鼠出现肾皮质线粒体肿胀、变形成圆点状、内膜嵴丢失,结构紊乱,粗面内质网轻度肿胀;M4组线粒体嵴结构疏松、紊乱,可见自噬溶酶体,内质网中度肿胀,线粒体被吞噬消化;M8组线粒体肿胀、嵴结构疏松、紊乱,线粒体外膜破裂损伤,可见自噬溶酶体,内质网明显肿胀;M2、M4、M8组均可见自噬线粒体及内质网-线粒体接触位点,见图3。
讨论
尽管目前有多种治疗策略和药物减轻肾损伤,但肝移植术后部分AKI患者肾功能呈恶化趋势,损伤不可逆。而目前临床上诊断肾损伤的金标准仍然是肾病理,但由于伦理限制,在临床实际操作中很难对所有患者进行创伤性肾穿刺,尤其是早期AKI患者。AKI的诊断仍然以SCr为标准,而影响SCr的因素众多,具有滞后性及偏差性,并不适用于评估肝移植患者的肾功能,不利于AKI的早期发现及防治[7-8]。
本研究以动物模型模拟肝移植主要病理生理过程:夹闭血管后肠腔血液回流受阻至淤血缺血、开放血管后炎症因子和内毒素大量释放入血、循环剧烈波动,肝肾发生再灌注损伤等[9-10]。研究结果也证实了SCr、BUN的延后性,虽然肾病理显示再灌注2 h已出现肾损伤,但再灌注8 h才显示SCr、BUN较sham组明显增高。本研究设立了再灌注2 h及4 h的组别,旨在探索AKI的早期作用机制,为早期AKI的潜在干预靶点提供参考依据。
研究表明Cx32的表达随器官损伤加重而增加[11]。既往的研究显示Cx32及Cx43表达在肝移植术后再灌注4 h已出现上调,再灌注后8 h达到峰值,抑制Cx32、Cx43功能可通过减少氧化应激和细胞凋亡来减轻肝移植术后AKI,但以上研究均未进行更早期的观察[3-4, 12-13]。本研究结果与上述研究一致,同时证实更早期即再灌注2 h时不仅肾病理显示肾损伤,电镜结果亦显示线粒体损伤明显,而此时 Cx32表达增加、Cx43无变化,与此同时,线粒体动力学相关蛋白Drp1、Mfn1、Mfn2、OPA1表达均增加,线粒体分裂融合表现均活跃,提示线粒体损伤在肝移植术后早期已出现,可能与线粒体动力学失衡相关。
Chen等[14]发现由Cx32组成的GJ控制活性氧的生成和相邻细胞之间的分布,Cx32缺乏可减轻肾缺血诱导的AKI,抑制核因子κB/肿瘤抑制基因p53/ p53上调凋亡因子(NF-κB/p53/PUMA)介导的线粒体凋亡途径,表明在AKI中Cx32可能与线粒体损伤有关。而线粒体动力学是保持线粒体在生理和病理条件下内稳态的重要机制[15]。一项研究记录了叠氮化物诱导的肾小管细胞损伤情况,在2 h
时线粒体迅速碎裂,但在12 h时才检测到细胞凋亡,表明在AKI中,线粒体动力学失衡早于细胞凋亡等其他形式的表型出现[16]。本研究结果提示AKI早期即出现了线粒体动力学失衡,即线粒体分裂融合均增强及线粒体损伤,Cx32传递伤害性信号可能与AKI的早期作用机制相关。
研究显示,在早期阶段内质网内未折叠蛋白的积累可导致应激诱导的线粒体过度融合,内质网应激与肾缺血再灌注损伤早期发病机制有关[17-18]。本研究结果显示肝移植术后早期线粒体融合蛋白Mfn2及内质网应激相关蛋白CHOP表达均增加,与此同时电镜下观察到肝移植术后早期即出现肿胀的粗面内质网,肝移植各组均存在内质网-线粒体接触位点。本研究结果提示肝移植术巨大创伤引起的内质网应激可能使线粒体与内质网之间产生联系,线粒体融合增强。本课题组的前期研究也证实Cx32可介导内质网凋亡信号通路激活,在AKI中起重要作用[19]。因此本研究结果证实Cx32传递伤害性信号时也可能参与调节内质网应激,并在肝移植术后早期AKI的过程中扮演重要的角色。
综上所述,关注Cx32、线粒体动力学、内质网应激的变化对于肝移植术后早期AKI机制探索具有重要的意义,Cx32传递伤害性信号与线粒体损伤及线粒体动力学失衡相关,可能为肝移植术后早期AKI的重要作用机制,值得进一步的深入研究。
参 考 文 献
[1] Thongprayoon C, Kaewput W, Thamcharoen N, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta-analysis. J Clin Med, 2019, 8(3): 372.
[2] Cleveland K H, Brosius F C 3rd, Schnellmann R G. Regulation of mitochondrial dynamics and energetics in the diabetic renal proximal tubule by the β2-adrenergic receptor agonist formoterol. Am J Physiol Renal Physiol, 2020, 319(5): F773-F779.
[3] Luo C, Yuan D, Li X, et al. Propofol attenuated acute kidney injury after orthotopic liver transplantation via inhibiting gap junction composed of connexin 32. Anesthesiology, 2015, 122(1): 72-86.
[4] Yuan D, Su G, Liu Y, et al. Propofol attenuated liver transplantation-induced acute lung injury via connexin43 gap junction inhibition. J Transl Med, 2016, 14(1): 194.
[5] Meng Q, Wu W, Zhang W, et al. IL-18BP improves early graft function and survival in lewis-brown Norway rat orthotopic liver transplantation model. Biomolecules, 2022, 12(12): 1801.
[6] Wang Y, Tian J, Qiao X, et al. Intermedin protects against renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress. BMC Nephrol, 2015, 16(1): 169.
[7] Molinari L, Del Rio-Pertuz G, Smith A, et al. Utility of biomarkers for sepsis-associated acute kidney injury staging. JAMA Netw Open, 2022, 5(5): e2212709.
[8] Pan H C, Yang S Y, Chiou T T Y, et al. Comparative accuracy of biomarkers for the prediction of hospital-acquired acute kidney injury: a systematic review and meta-analysis. Crit Care, 2022, 26(1): 349.
[9] Nemeth N, Peto K, Magyar Z, et al. Hemorheological and microcirculatory factors in liver ischemia-reperfusion injury-an update on pathophysiology, molecular mechanisms and protective strategies. Int J Mol Sci, 2021, 22(4): 1864.
[10] 吴永东, 汪华林, 林蓉宇, 等. 肾胺酶预防大鼠缺血再灌注致急性肾损伤的机制研究. 新医学, 2016, 47(1): 17-21.
[11] Chen Z Q, Sun X H, Li X J, et al. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharmacol Sin, 2020, 41(12): 1587-1596.
[12] Yuan D, Li X, Luo C, et al. Inhibition of gap junction composed of Cx43 prevents against acute kidney injury following liver transplantation. Cell Death Dis, 2019, 10(10): 767.
[13] Wu S, Yao W, Chen C, et al. Connexin 32 deficiency protects the liver against ischemia/reperfusion injury. Eur J Pharmacol, 2020, 876: 173056.
[14] Chen C, Yao W, Wu S, et al. Crosstalk between connexin32 and mitochondrial apoptotic signaling pathway plays a pivotal role in renal ischemia reperfusion-induced acute kidney injury. Antioxid Redox Signal, 2019, 30(12): 1521-1538.
[15] Tang C, Cai J, Yin X M, et al. Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol, 2021, 17(5): 299-318.
[16] Brooks C, Wei Q, Cho S G, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest, 2009, 119(5): 1275-1285.
[17] Fan Y, Simmen T. Mechanistic connections between endoplasmic reticulum (ER) redox control and mitochondrial metabolism. Cells, 2019, 8(9): 1071.
[18] Almanza A, Carlesso A, Chintha C, et al. Endoplasmic reticulum stress signalling-from basic mechanisms to clinical applications. FEBS J, 2019, 286(2): 241-278.
[19] Gu Y, Huang F, Wang Y, et al. Connexin32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injury. J Transl Med, 2018, 16(1): 1-13.
(收稿日期:2022-10-20)
(本文編辑:洪悦民)
