Cyanided Covalent Triazine Frameworks with Enhanced Adsorption Capability Toward Chloranil
2023-05-25JINYingweiXIEPeidongYANGYuyingRONGGaoqiLIPingdengHUYeliCHENGBoZHENGQiWANGChang
JIN Yingwei, XIE Peidong, YANG Yuying, RONG Gaoqi,3, LI Pingdeng,HU Yeli, CHENG Bo, ZHENG Qi, WANG Chang*
(1. Hubei Key Laboratory of Environmental and Health Effects of Persistent Toxic Substances, Institute of Environment and Health, Jianghan University, Wuhan 430056, China; 2. School of Environmental Ecology and Biological Engineering, Wuhan Institute of Technology, Wuhan 430025, China; 3. School of Chemistry and Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, China; 4.Key Laboratory of Optoelectronic Chemical Materials and Devices, Jianghan University, Wuhan 430056, China)
Abstract: Porous polymer (pyrrolopyrrole) was successfully prepared via domino-ring-formation reaction. The chemical-physical properties of cyanided covalent triazine frameworks (CTF-CN) were characteriazed by fourier transform infrared spectra (FT-IR), scanning electron microscopy (SEM), nuclear magnetic resonance (NMR), specific surface area analyzer (BET) and thermogravimetric analysis (TGA),respectively. The experimental results of adsorption of chloranil (TCBQ) in aqueous solution indicated that CTF-CN exhibited distinctive adsorption capacity toward TCBQ owing to its large specific surface area.Specifically, the adsorption equilibrium of as-prepared polymer was executed within 5 h and the calculated adsorption capacity was 499.76 mg/g. Furthermore, the adsorption kinetics could be well defined with the linear pseudo-second-order model, which implies that the chemical interaction are relative important in the course of TCBQ removal. Finally, the current studies verify that CTF-CN has unique rigid and nano-porous framework structure, which can be employed for the treatment of a series of harmful aromatic substances.
Key words: disinfection by-products (DBPs); adsorbent; haloquinones; covalent triazine frameworks
1 Introduction
Adsorption has been testified to be an effective way for removing trace contaminants from tap water[1,2]. Currently, nanoporous organic materials have been widely used in adsorption due to their unique properties, such as large surface area, low framework density, unique chemical and physical properties and permanently open pore structure[3]. Inclusively,covalent triazine frameworks (CTFs) not only exhibit outstanding chemical-stability and porosity, but also have excellent photocatalytic activity[4]. In addition,2D/2D heterostructure photocatalyst prepared by electrostatic self-assembly can also be used to further enhance the photocatalytic performance of the organic composites[5]. In general, the combination of above strategies has particular potential for the removal of HBQ (Halogenated benzoquinone) disinfection byproducts.
Chlorination is one of the most effective methods to disinfect tap-water. Currently, the using of chlorine has been confirmed to produce a series of harmful disinfection by-products (DBPs) in the disinfection processes[6]. During conventional tapwater treatment and delivery, chlorine-containing disinfectants can react with natural organic matters to generate large amounts of DBPs such as haloketones,trichloromethane, monobromodichloromethane, and dibromomonochloromethane[7,8]. These harmful D BPs have presented a serious risk to human health.These mutagenic chemicals would likely elevate the occurrence of malignant tumors in human[9]. HBQs were first identified in tap-water in 2010 and it has been classified as a relatively toxic halogenated DBPs according to thein vitroandin vivotests[10,11].In addition, HBQs have been further confirmed to have potential genotoxicity and carcinogenicity by quantitative structure-toxicity analysis[12].Epidemiological findings suggested that the exposure level of DBP has a significant relationship with the increased morbidity of bladder cancer[13]. Previous studies have shown that HBQs can generate excessive reactive oxygen species in organisms and subsequently consume GSH glutathione, leading to oxidative DNA and proteins damage in T24 cells[14]. Recently,in-vitrotoxicity evaluation of HBQs indicated that low dose of HBQs analogues (<10 μM) are cytotoxic to Caco-2 cells after 4 h exposure[15].
Currently, controlling the formation of DBPs precursors has been considered as an effective way to limit the generation of HBQs[16]. Several traditional materials have been employed for the elimination of HBQs precursors,e g, aluminium oxide and activated carbon[17]. The biopolymers and HBQs precursors can be removed by flocculants (aluminium oxide)due to coagulation effects, and activated carbon can be adopted to reduce the generation of DBPs in tapwater[18]. UV spectrophotometer (254 nm) can also effectively degrade 2,3-dibromo-5,6-dimethyl-1,4-benzoquinone (DDBQ) in aqueous solution[19]. Although these treatments can partially remove or degrade the HBQ precursors, HBQ can’t be completely adsorbed or degraded because of the complexity and diversity of its chemical structures. The direct and effective ways for the removal of disinfection by-product are urgently needed.
In this study, the monomer 4CNPP was synthesized from 4-methylaniline, 4-cyanobenzaldehyde and 2,3-butanedione by solvothermal method through domino ring formation reaction and purified by column chromatography to obtain 4CNPP. Then, CTFCN was obtained via a catalyzed polymerization of molten zinc chloride and 4CNPP at 400 ℃ under argon atmosphere. The as-prepared material has a relatively high porosity and abundant microporous structure.Meanwhile, the surface area is 1 513 m2/g. Finally,the as-prepared material exhibited excellent removal performance toward tetrachlorobenzoquinone (a typical HBQ) in aqueous solution.
2 Experimental
2.1 Chemicals and materials
All the chemicals were purchased from commercial sources and used without further purification.1H NMR and13C NMR were recorded in CDCl3 on a Brucker Avance III 400 MHz spectrometer.In addition, 4-formylbenzonitrile, 4-aminobenzonitrile acetate and zinc chloride were purchased from Energy Chemical Co.
2.2 Synthesis of 4CNPP

Fig.1 The reaction formula
4CNPP was prepared using the Gryko’s one-pot domino reaction. 4-aminobenzonitrile (5.9 g, 50 mmol,2.0 eq) and 4-formylbenzonitrile (6.55 g, 50 mmol, 2.0 eq) were added into flask containing acetate acid 70 mL. The mixture was heated at 90 ℃ with continyous for 30 minutes, then 2,3-butanedione (2.2 mL, 25 mmol, 1.0 eq) was slowly added and the resulted solution was stirred at 90 °C for 3 h. The yellow precipitates (3.18 g, 25%) was separated and purified by column chromatography. (DCM:PE = 1:2).
2.3 Synthesis of CTF-CN
4CNPP (435 mg) was evenly mixed with zinc chloride (1.36 g), the mixture was placed into a muffle tube furnace and subsequently heated up to 400℃ at a rate of 5 ℃/min, the high-temperature melt polymerization was executed for 40 h at 400 ℃ under argon atmosphere, and then, the black fluffy material was obtained as a result.
After the fluffy substance was mashed, the residual zinc chloride was further washed with deionized water and hydrochloric acid (1 mol / L).Finally, the black product was dried at 130 ℃ for 10 h.The reaction formula is described as following.
2.4 Characterization
1H NMR spectra were recorded on a Bruker AVANCE III 400 spectrometer. Fourier transform infrared (FTIR) spectra were characterized on a Bruker TENSOR 27 FTIR spectrometer. Thermogravimetric analysis (TGA) measurements were performed with a Netzsch TG209 F3 Jupiter thermal analyzer under nitrogen atmosphere, the temperature range was 40-800℃, and the heating rate was 10 ℃/min. The Brunauer–Emmett–Teller (BET) surface areas and pore size distribution (Barrett–Joyner–Halenda (BJH) model)were measured by an accelerated surface area and porosimetry analyzer ASAP 2020M+C (Micromeritics Instrument Corp, analysis adsorptive: N2and CO2).Scanning electron microscopy (SEM) and highresolution transmission electron microscope (TEM)images was obtained by SU8010 (Hitachi) and JEOL 2100F, respectively.
2.5 Adsorption experiments
For kinetic studies, 4 mg of nitrogen-doped porous conjugated polymer material was added into 200 mL TCBQ solution at an initial concentration of 10 mg/L. And the mixture was vibrated on a shaker at 200 rpm and 25 ℃. At a given series of time intervals, 500 uL of TCBQ solution was sampled and filtered, and its concentration was quantified by high performance liquid chromatography-mass spectrometry(HPLCMS). The adsorption amount at timet,qt(mg / g), was calculated as
whereC0andCt(mg / L) is the initial and instant concentrations of TCBQ respectively,V(L) is the volume of the solution, andW(mg) is the mass of adsorbent used.
3 Results and discussion
3.1 Characterization of CTF-CN
3.1.1 FTIR results
The FT-IR spectrum of polymer CTF-CN is depicted in Fig.2. According to the test data, the vibration bands at 1 590 cm−1was assigned to the cyano group(C≡N). The characteristic peak of cyano does not exist in the infrared spectrum, which indicates that the cyano group (C≡N) ratio and tricyanide structure increased evidently and tricyanide structural unit emerged after domino ring-forming reaction.

Fig.2 The FT-IR spectra of polymer CTF-CN

Fig.3 1H NMR spectrum (400 MHz) of 4CNPP in DMSO-d6
3.1.2 NMR results
1H NMR (400 MHz, (CD3)2SO),δ[ppm]:7.95 (d, J = 8.56 Hz, 4H), 7.77 (d, J = 8.44 Hz, 4H),7.48 (d, J = 8.56 Hz, 4H), 7.39 (d, J = 8.40 Hz, 4H),6.94 (s, 2H). 13C NMR (400 MHz, (CD3)2SO)δ[ppm]:142.92, 137.07, 135.28, 134.38, 133.04, 132.91,128.65, 126.04, 109.36, 109.21, 102.54, 99.58. HRMS(EI): m/z calcd for C34H18N6 510.1593 [M+], found.510.1595.
3.1.3 SEM results
The morphology of the CTF-CN was characterized by using SEM (Fig.4). From the high magnification SEM image (Fig.4(a)), it can be found that the polymer CTF-CN consisted of irregular amorphous block or granular. In addition, the material has uneven surface and it was formed by agglomerated nanoparticles(Fig.4(b)). Generally, the uneven surface of CTFCN is crucial to its excellent adsorption capacity.Such relatively crude external interface can enrich the adsorption active sites and provide potential ability for the removal of pollutants.

Fig.4 SEM images of polymer CTF-CN
3.1.4 Brunauer-Emmett-Teller (BET)
Fig.5 shows the adsorption/desorption isotherms of CTF-CN. Obviously, according to the IUPAC classification, as-prepared material displayed the characteristic of the N2adsorption isotherm (type I).The initial part of the adsorption isotherm represents the filling process of narrow micropores in the adsorbent, and its ultimate adsorption capacity depends on the accessible micropore volume rather than the surface area. Under high relative pressure, the slope of the platform is caused by multi-layer adsorption on non-microporous surfaces (such as mesopore or macropores and outer surfaces). The isotherm in the range ofp/p0= 0.05-0.1 exhibites enhanced adsorption performance, which is attributed to the microporous structure of CTF-CN. As evidence, specific surface area value of CTF-CN shows the high surface area exceeding 1 000 m2/g.
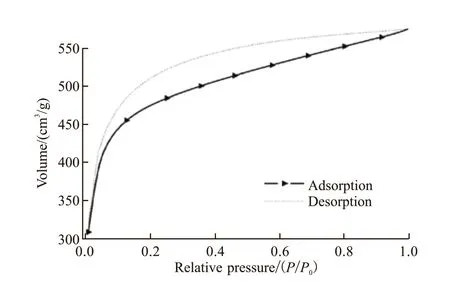
Fig.5 Low pressure nitrogen adsorption-desorption isotherms of CTF-CN under 77 K
3.1.5 TGA results
In order to test the thermal stability of CTF-CN,TGA was executed with a heating rate of 10 ℃/min under a nitrogen atmosphere from room temperature to 800 ℃ (Fig.6). When the temperature rises to 700 ℃,there is still 85% mass residue. Above results suggested the good thermal stability of CTF-CN.

Fig.6 Thermal-gravimetric curve of CTF-CN under nitrogen atmosphere
3.2 The effect of contact time on the TCBQ removal
Fig.7 shows the plot of CTF-CN uptakes against time under the initial concentrations of 10 mg / L.The removal rate of TCBQ was relatively fast at the first stages, and then, the removal rate was decreased.Many unoccupied binding sites are available for adsorption during the initial stage, and then, the remaining unoccupied sites were less and could not uptake the pollutant owing to repulsion between the solute molecules on the liquid phases and solid surface.We can easily comprehend that the adsorption rate of TCBQ by using CTF-CN was relatively higher at initial stages and it gradually decreased and remained stable in 5 h. The adsorption amount was 499.76 mg / g at 25 ℃.

Fig.7 Effect of equilibrium time for absorption of TCBQ at 25 ℃
3.3 Adsorption kinetics of TCBQ
It is necessary to predict the adsorption kinetics of the above mentioned application. The characteristics of the adsorption process depend not only on physical or chemical nature of the material but also on the system conditions. We applied pseudo-first-order, pseudosecond-order, and intra-particle diffusion model as kinetic models for furthr learning the kinetic character of the adsorption process. We also used their linear equations andR2values to assess the applicability of models[20].
Pseudo-first-order:
Pseudo-second-order:
Intraparticle diffusion model:
Table 1 shows the parameters of equations.k1,k2andkintare rate constant for process of the pseudo-firstorder, the pseudo-second-order and the intraparticle diffusion, respectively.qtandqe(mg·g−1) are the absorbed amounts at instant timetand equilibrium,respectively. From the following figure, we can calculateR2of the pseudo-second-order is 0.997 4. Also theqe,calis closer toqe,exp. The fitting results illustrates that the pseudo-second-order can be empolyed to describe the adsorption kinetics of TCBQ on CTFCN. In Fig.8, the linear curves with high correlation coefficients further indicate that the pseudo-secondorder can be used in this case.
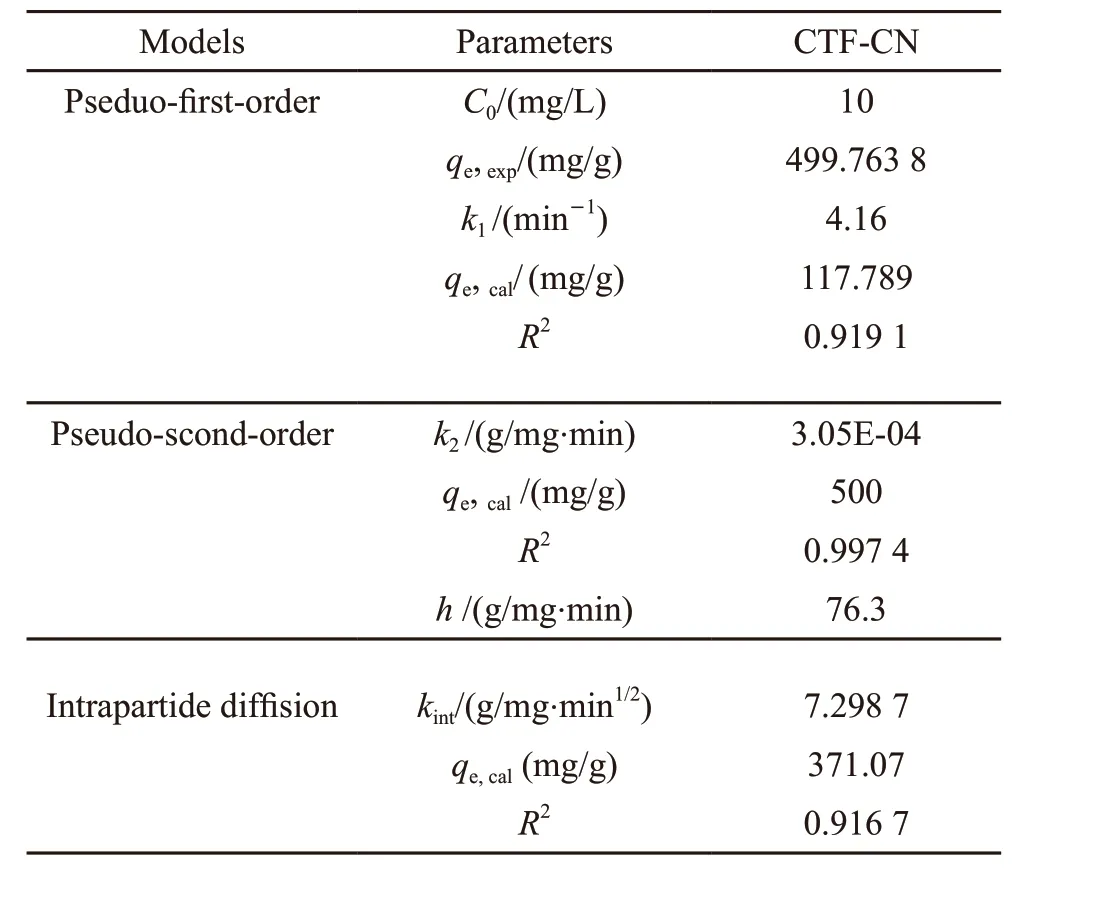
Table 1 Pseudo-first-order, pseudo-second-order, intrapartide diffusion models kinetic parameters for the absorption of TCBQ

Fig.8 (a) Pseudo-first-order model; (b) Pseudo-second-order model;(c) Intraparticle diffusion mode
4 Conclusions
In summary, as a kind of material doped with cyano, CTF-CN were designed and prepared for the removing of TCBQ in aqueous solution. According to the results of nitrogen isothermal adsorption and desorption test, it can be found that the specific surface area of CTF-CN is higher than 1000 m2/g. TGA analysis suggests the excellent thermal stability of CTFCN. Compared with other COFs, the resulting CTFCN materials exhibited superior adsorption capacity for eliminating organic pollutants due to its large specific surface area, aperture size and the covalent connecting junction. In addition, the CTF-CN has unique rigid and nanoporous framework structure to accommodate a series of aromatic organic substances[21]. Generally, the construction of CTF-CN material not only promotes a better comprehension for the structure-activity relationship of COFs as adsorbent, but also provides guidelines for the future application of COFs in the field of HBQs pollutants treatment.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Low-temperature Denitration Mechanism of NH3-SCR over Fe/AC Catalyst
- Influence of Cu doping in Magnesium Hydroxide Nanoparticles for Bandgap Engineering
- Carbon-coated ZnO Nanocomposite Microspheres as Anode Materials for Lithium-ion Batteries
- Influence of Organic Cations on the Crystal and Electronic Structures of Two-dimensional Lead Iodide Perovskites
- Au@Ag Core-shell Nanorods Self-assembled on Polyelectrolyte Multilayers for Ultra-High Sensitivity SERS Fiber Probes
- Effect of Particle Size on Silicon Nitride Ceramic Slurry by Stereolithography
