Simultaneous Morphologies and Luminescence Control of NaYF4∶Yb/Er Nanophosphors by Surfactants for Cancer Cell Imaging
2023-05-18SHENGYangyi盛洋怡CHENGLuSONGYuelin宋岳林WANGZhaojie王兆洁JIANGWeizhong蒋伟忠CHENZhigang陈志钢
SHENG Yangyi(盛洋怡), CHENG Lu(程 璐), SONG Yuelin(宋岳林), WANG Zhaojie(王兆洁), JIANG Weizhong(蒋伟忠), CHEN Zhigang (陈志钢)
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
Abstract:Hydrophilic rare-earth up-conversion nanophosphors (UCNPs) with small sizes and a strong up-conversion luminescence have attracted much interest. Herein the simultaneous control of morphologies and the up-conversion luminescence intensities was reported for NaYF4∶Yb/Er nanophosphors by a facile hydrothermal procedure with different surfactants. With the change of the surfactants from polyvinylpyrrolidone (PVP) to sodium citrate (CIT), edetate disodium (EDTA) or sodium dodecyl benzenesulfonate (SDBS), the morphology of NaYF4∶Yb/Er nanophosphors transformed from nanoparticles with a diameter of about 70.0 nm to hexagonal nanoblocks with a thickness of about 125.0 nm and a length of about 240.0 nm, nanorods with a diameter of about 700.0 nm and a length of about 2.6 μm, or nanowires with a diameter of 250.0 nm and a length of about 3.2 μm. Simultaneously, their up-conversion luminescence intensity went down gradually under laser irradiation at a wavelength of 980 nm due to the increase of photobleaching. PVP-capped NaYF4∶Yb/Er nanoparticles exhibited the smallest size and the strongest up-conversion luminescence intensity. Biological experiment results revealed that NaYF4∶Yb/Er nanophosphors exhibited a high biocompatibility and could be used as biological labels with a perfect signal-to-noise ratio for cancer cell imaging.
Key words:NaYF4; nanophosphor; luminescence; surfactant; adjustable morphology; cancer cell imaging
Introduction
Rare-earth up-conversion nanophosphors (UCNPs) have attracted much interest due to the advantages including low photobleaching, superior stability and sharp absorption/emission. Currently, UCNPs have been widely used in temperature detection, 3D flat-panel displays, optical devices and biomedical applications[1-4]. For biomedical applications, UCNPs with small sizes and a strong up-conversion luminescence are widely used. To prepare UCNPs with small sizes, two kinds of synthesis methods have been well developed. One is a simple hydrothermal method with a liquid-solid-solution (LSS) process[5-6]. For example, with this LSS process, Wangetal.[7]reported the controllable synthesis of NaYF4, YbF3and LaF3nanoparticles with diameters in the range of 4 nm to 12 nm. The other one is the thermolysis of lanthanide trifluoroacetate precursors in a high boiling solvent at 280-330 ℃[8-10]. For example, Maietal.[11]obtained NaREF4(RE∶ Pr to Lu, Y) nanocrystals with adjusted sizes (5.9-155.0 nm). These small-size UCNPs can be well used in many fields, especially in biomedical applications.
There are three kinds of strategies to obtain UCNPs with a strong up-conversion luminescence. The first one is the optimization of host materials. Many kinds of host materials have been developed, including NaYF4[12], NaGdF4[13], KMnF3[14]and CaF2[15]. The second one is the tuning of the crystalline phase. Compared with the cubic-phase NaYF4∶Yb/Er nanophosphors, hexagonal-plase NaYF4∶Yb/Er nanophosphors demonstrate a stronger up-conversion luminescence intensity[16]. The last and the most important one is design and construction of the novel structure with an excellent energy transfer efficiency[1-2,17-18]. For example, researchers have developed various UCNPs with high energy migration, including NaGdF4∶Tb@NaGdF4@NaGdF4∶Yb/Tm[19], NaYF4∶Yb/Tm@NaYF4[20]and NaErF4∶Tm@NaYF4[21]. However, the above methods have some limitations, such as high costs, complex preparation processes and difficulty in the control of morphologies and sizes. Thus, it is still indispensable to explore other novel and facile ways to control morphologies and luminescence intensities.
It is well-known that surfactants can manipulate the crystal growth and thus control the morphologies of nanomaterials[22]. In addition, surfactants may have some effects on the photobleaching process of nanophosphors. These features inspire our interest in developing a simple way to simultaneously control morphologies and the up-conversion luminescence intensities of NaYF4∶Yb/Er nanophosphors by adjusting surfactants. Herein, NaYF4∶Yb/Er nanophosphors are prepared by a simple hydrothermal method assisted with different surfactants, including polyvinylpyrrolidone (PVP), sodium citrate (CIT), edetate disodium (EDTA) and sodium dodecyl benzenesulfonate (SDBS). The effects of surfactants on morphologies and luminescence are analyzed. In addition, cytotoxicity and bioimaging performance of NaYF4∶Yb/Er nanophosphors are also evaluated.
1 Experiments
1.1 Materials
PVP (PVP-K30), CIT (C6H5Na3O7), ethylene-diamine tetra-acetic acid (C10H16N2O8), SDBS(C18H29NaO3S), sodium dodecyl sulfate (C12H25SO4Na), ethylene glycol ((CH2OH)2), sodium fluoride (NaF) and glycerol (C3H8O3) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All the above chemicals are of analytical grades. Rare-earth chlorides (LnCl3, Ln∶Y, Yb, Er) were prepared by dissolving the corresponding oxides (Y2O3, Yb2O3and Er2O3from Beijing Lansu Co., Ltd., China) in a hydrochloric solution (a mass fraction of 10%) and then evaporating the water completely. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were obtained from Beyotime Biotechnology, China. HeLa cells and human umbilical vein endothelial cells (HUVECs) were bought from Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China.
1.2 Material synthesis
PVP, CIT, EDTA or SDBS was added at a mass of 0.5 g to the mixture solution (4 mL water and 4 mL ethylene glycol). LnCl3(0.78 mmol Y3+, 0.20 mmol Yb3+and 0.02 mmol Er3+) was dissolved in the above solution. Then, NaF (7.20 mmol) was dissolved in another solution (2 mL water and 2 mL ethylene glycol), and it was dropped into the LnCl3solution. The resulting solution was agitated for 30 min, and then hydrothermally treated at 180 ℃ for 10 h. After being naturally cooled, NaYF4∶Yb/Er nanophosphors were isolatedviacentrifugation, rinsed with water and dried under vacuum at room temperature.
1.3 Characterization
Morphologies and sizes of NaYF4∶Yb/Er nanophosphors were characterized by a field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Japan) and a high-resolution transmission electron microscope (HR-TEM, JEOL JEM-2010F, Japan). Powder X-ray diffraction (XRD) measurements were performed on a Bruker D4 X-ray diffractometer with Cu Kαradiation (Bruker, Germany). Fourier transform infrared (FTIR) spectra were measured by using an IRPRESTIGE-21 spectrometer (Shimadzu, Japan) from samples in KBr pellets. Up-conversion luminescence spectra were measured by using an FP-6600 spectrometer (JASCO, Japan), but the excitation source was a laser at a wavelength of 980 nm.
1.4 Cytotoxicity assay in vitro
Theinvitrocytotoxicity was measured by using the MTT assay in HeLa cells. Cells growing in a log phase were seeded into a 96-well (5×104/well) cell culture plate in Dulbecoo’s modified eagle medium (DMEM) at 37 ℃ and in the presence of CO2(a volume fraction of 5%) for 24 h. Then the cells were incubated with PVP-capped NaYF4∶Yb/Er nanoparticles at different mass concentrations (0, 0.05, 0.10, 0.15, 0.20 and 0.25 mg/mL) at 37 ℃ for 24 h in the presence of CO2. Subsequently, 10 μL MTT (5 mg/mL) was added to each well of the 96-well cell culture plate and incubated for 4 h at 37 ℃ in the presence of CO2. After the addition of sodium dodecyl sulfate (100 μL/well), the cell culture plate was allowed to stand at room temperature for 12 h. A Multiskan MK3 monochromator-based multifunction microplate reader(Thermo Fisher, USA) was used to measure the absorbance of each well with background subtraction at 492 nm. All of the tests were independently performed three times.
1.5 Bioimaging of cancer cells by PVP-capped NaYF4∶Yb/Er nanoparticles
HeLa cells were incubated in phosphate buffered saline (PBS) containing PVP-capped NaYF4∶Yb/Er nanophosphors(0.20 mg/mL) at 37 ℃ for 3 h in the presence of CO2, and then washed with PBS sufficiently to remove excess nanoparticles. These cells were fixed with paraformaldehyde (a mass fraction of 4%), and their nuclei were stained with 5 μg/mL DAPI in glycerol (a mass fraction of 10%). The multilabeled cells were then imaged by a laser scanning up-conversion luminescence microscope (Olympus FV1000, Japan) and a conventional confocal microscope (Olympus BX51, Japan). These cells were excited by a laser at a wavelength of 980 nm, and up-conversion luminescence signals were detected in two channels: the green channel (500-570 nm) and the red channel (600-700 nm). In addition, these cells were also irradiated by a laser at a wavelength of 405 nm to obtain the fluorescence signals of DAPI for cell nuclei.
2 Results and Discussion
2.1 Characterization of NaYF4∶Yb/Er nanophosphors
NaYF4∶Yb/Er nanophosphors were fabricated through the hydrothermal method[23-25]with different surfactants (PVP, CIT, EDTA and SDBS). The morphologies of these NaYF4∶Yb/Er nanophosphors were characterized (Fig. 1). When PVP is used as the surfactant, the nanophosphors consist of nanoparticles with a diameter of about 70.0 nm (Figs. 1(a), 1(b) and 2(a)). When the surfactant is CIT, the nanophosphors appear to be hexagonal nanoblocks with a thickness of about 125.0 nm and a length of about 240.0 nm (Figs.1(c), 1(d), 2(b) and 2(c)). By using EDTA as the surfactant, the nanophosphors are composed of nanorods with a diameter of about 700.0 nm and a length of about 2.6 μm (Figs.1(e), 1(f), 2(d) and 2(e)). Interestingly, the SDBS surfactant results in the formation of nanowires with a diameter of about 250.0 nm and a length of about 3.2 μm (Figs. 1(g), 1(h), 2(f) and 2(g)). These facts confirm that surfactants can adjust sizes and morphologies of NaYF4∶Yb/Er nanophosphors.
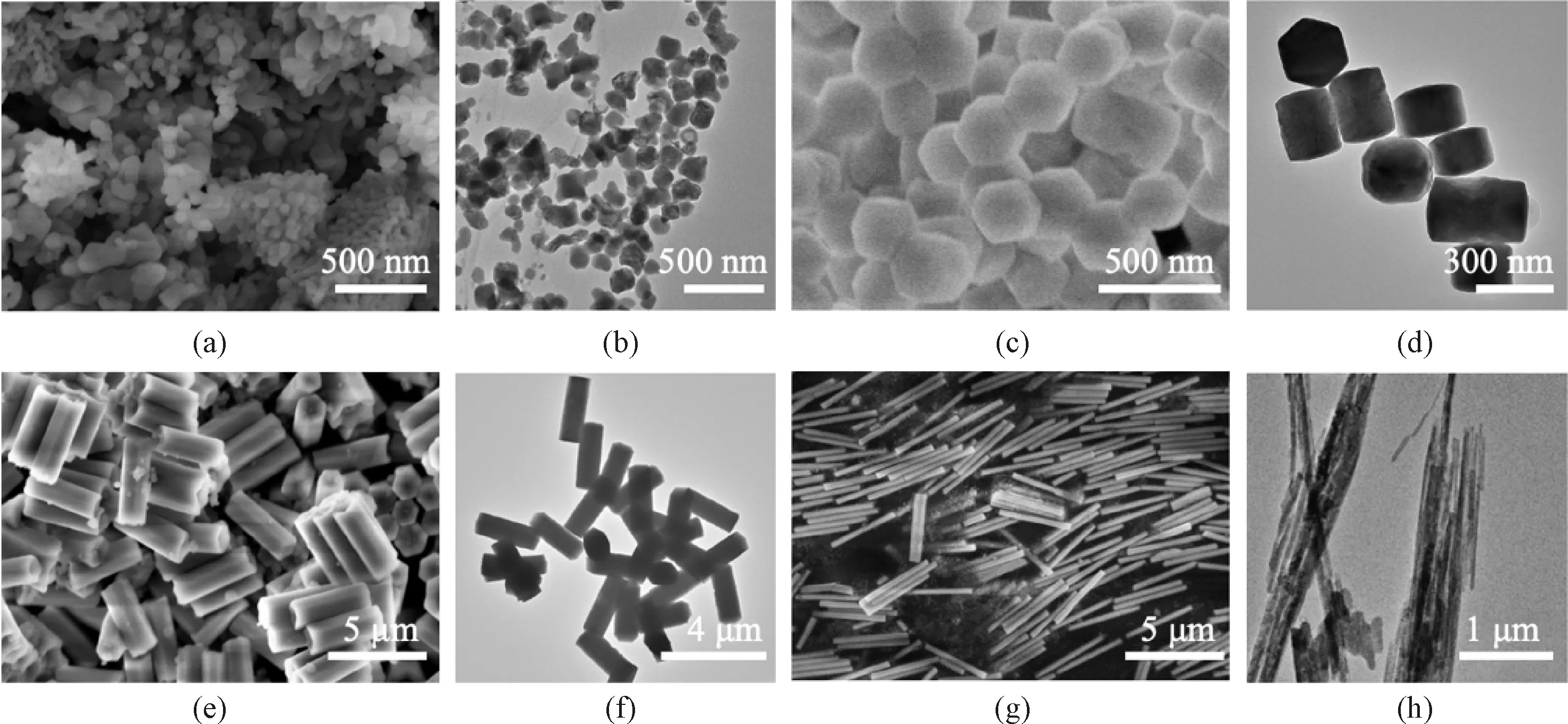
Fig. 1 FE-SEM and HR-TEM images of NaYF4∶Yb/Er nanophosphors fabricated with different surfactants: (a) and (b) PVP; (c) and (d) CIT; (e) and (f) EDTA; (g) and (h) SDBS
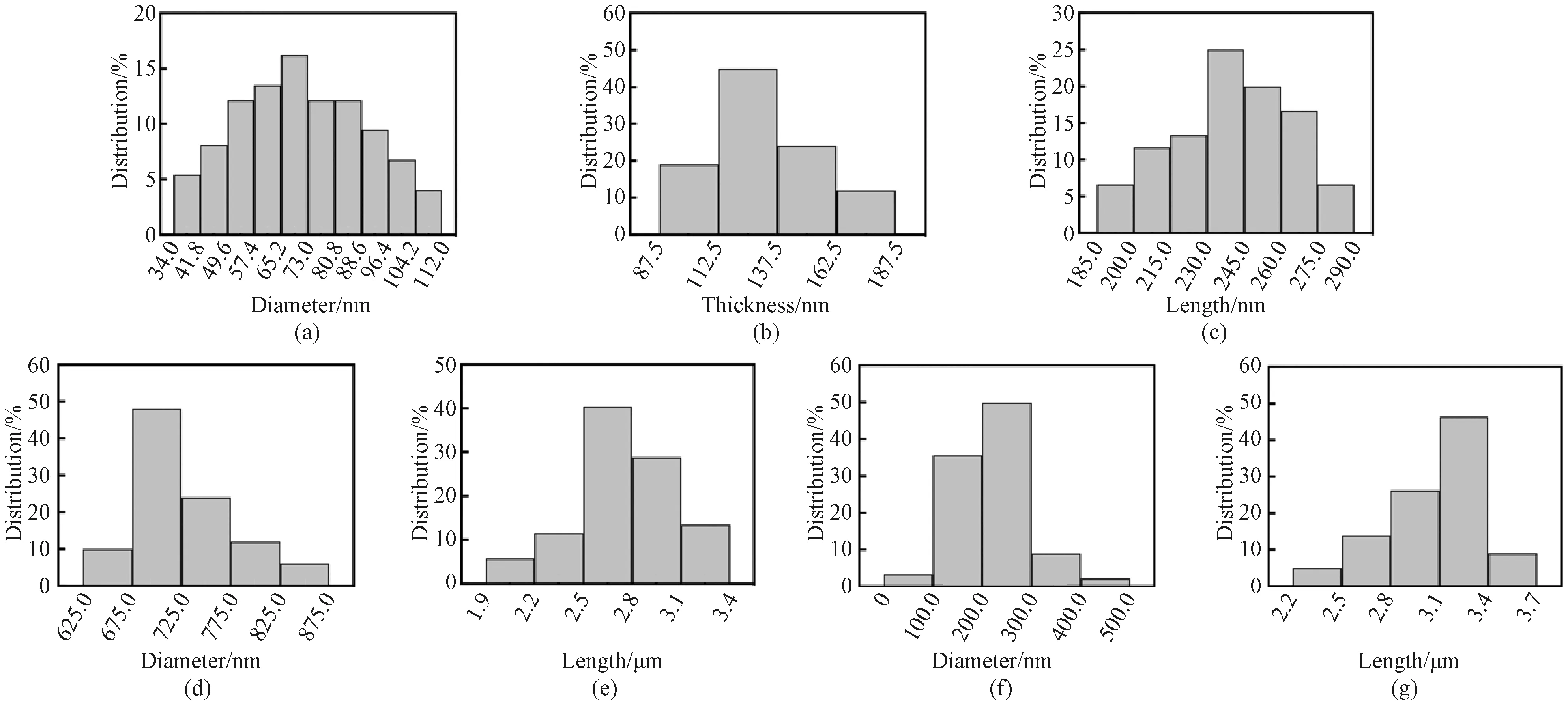
Fig. 2 Size distribution of NaYF4∶Yb/Er nanophosphors fabricated with different surfactants: (a) PVP; (b) and (c) CIT; (d) and (e) EDTA; (f) and (g) SDBS
Subsequently, the phases of NaYF4∶Yb/Er nanophosphors were characterized by XRD patterns (Fig.3). When CIT or EDTA is used as the surfactant, the nanophosphors exhibit six distinct peaks with 2θvalues of 17.20°, 30.06°, 30.79°, 43.49°, 53.28° and 53.75°, which respectively correspond to (100), (110), (101), (201), (300) and (211) crystal planes ofβ-NaYF4(JCPDS file No.16-0334). Interestingly, when PVP or SDBS is used as the surfactant, there are three additional diffraction peaks at 28.23°, 46.94° and 55.69° that can be respectively assigned to (111), (220) and (311) planes ofα-NaYF4(JCPDS file No. 77-2042)[26]. Thus, these NaYF4∶Yb/Er nanophosphors capped with PVP or SDBS are the mixture of cubic and hexagonal phases. The above results verify that the surfactant can regulate the phase of NaYF4∶Yb/Er nanophosphors.

Fig. 3 XRD patterns of NaYF4∶Yb/Er nanophosphors fabricated with different surfactants (PVP, CIT, EDTA and SDBS)


Fig. 4 FTIR spectra of NaYF4∶Yb/Er nanophosphors fabricated with different surfactants (PVP, CIT, EDTA and SDBS)
Owing to the presence of these surfactants, NaYF4∶Yb/Er nanophosphors can be easily dispersed in water. The up-conversion luminescence spectra of their aqueous dispersions (1 mg/mL) were recorded under laser irradiation at the wavelength of 980 nm(Fig.5). All the nanophosphors show three different Er3+emission bands that are consistent with the previous reports[29]. Two green emissions ranging from 514 nm to 534 nm and from 534 nm to 560 nm are observed, which result from2H11/2→4I15/2and4S3/2→4I15/2transitions, respectively. There is a red emission at 635-680 nm, which should be attributed to the transition from4F9/2to4I15/2. Importantly, these surfactants have strong effects on the up-conversion luminescence intensity. Obviously, PVP-capped NaYF4∶Yb/Er nanoparticles demonstrate the strongest up-conversion luminescence intensity, as vividly shown in the luminescence photo (the inset in Fig. 5). CIT-capped NaYF4∶Yb/Er nanoblocks demonstrate the second strongest up-conversion luminescence intensity which is almost 80% as strong as that of PVP-capped NaYF4∶Yb/Er nanoparticles. However, both EDTA-capped NaYF4∶Yb/Er nanorods and SDBS-capped NaYF4∶Yb/Er nanowires exhibit a weak up-conversion luminescence intensity(Fig.5). These results reveal the successful control of the up-conversion luminescence intensity of NaYF4∶Yb/Er nanophosphors by different surfactants.
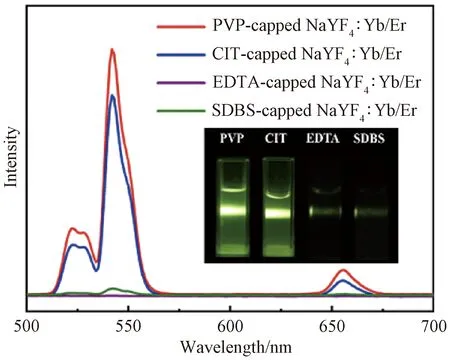
Fig. 5 Up-conversion luminescence spectra of aqueous dispersions containing NaYF4∶Yb/Er nanophosphors(1 mg/mL) fabricated with different surfactants (PVP, CIT, EDTA and SDBS) with luminescence photos in inset
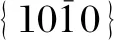
2.2 Applications as luminescent biological labels
Because of their smallest size and strongest up-conversion luminescence intensity, PVP-capped NaYF4∶Yb/Er nanoparticles should have a great potential as biological labels for cancer cell imaging. To evaluate their cytotoxicity, HeLa cells were incubated with PVP-capped NaYF4∶Yb/Er dispersions (0-0.25 mg/mL) for 24 h. MTT assay results reveal that there is no obvious difference in the cell viability (Fig. 7), and the cell viability in 0.25 mg/mL NaYF4∶Yb/Er dispersions is higher than 87%, suggesting a high biocompatibility.
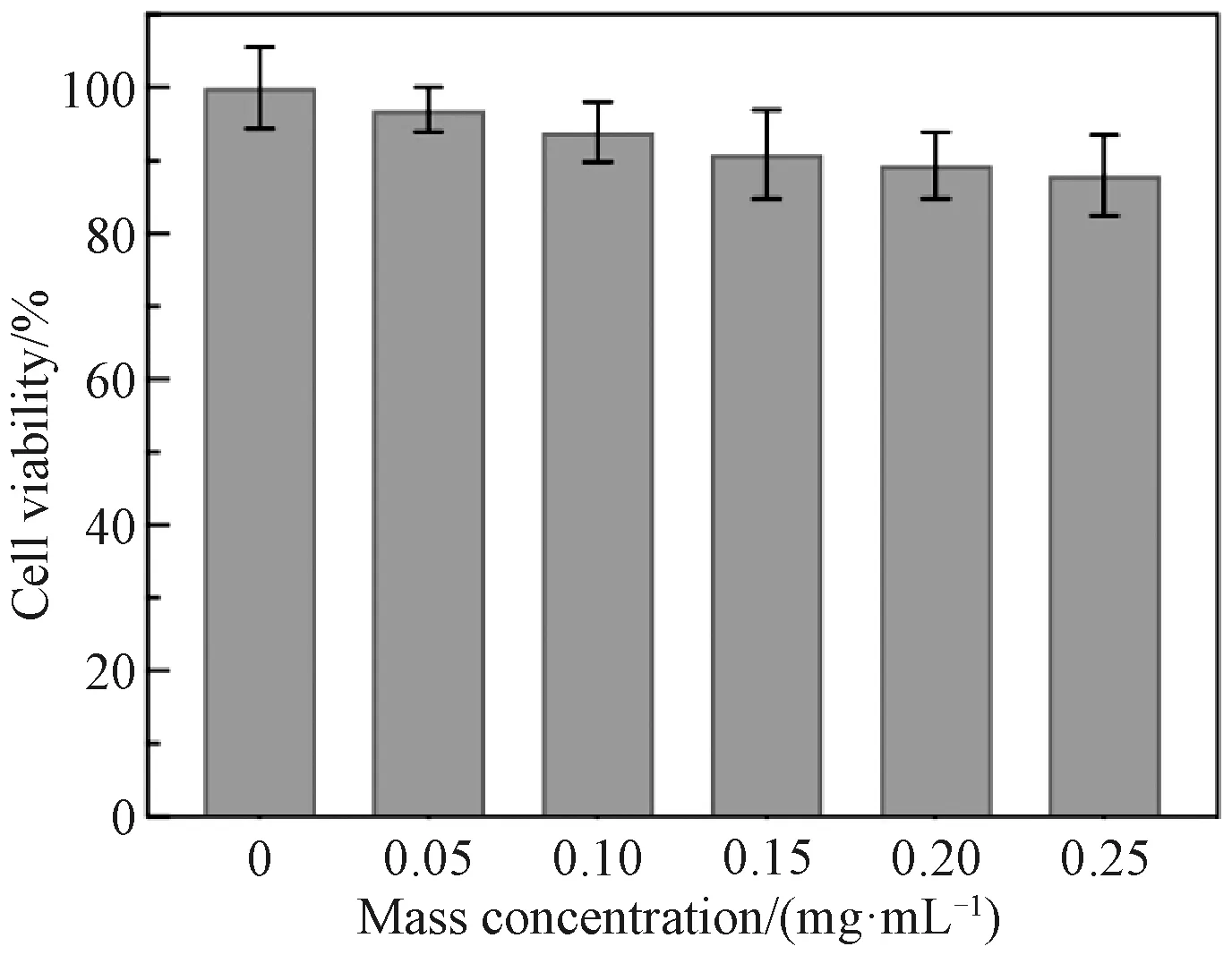
Fig. 7 Cell viability estimated by MTT assay versus mass concentrations (0, 0.05, 0.10, 0.15, 0.20 and 0.25 mg/mL) of PVP-capped NaYF4∶Yb/Er nanoparticles
To investigate the cell labeling ability, HeLa cells were incubated with PVP-capped NaYF4∶Yb/Er dispersions (0.20 mg/mL) at 37 ℃ for 3 h and then their nuclei were stained with DAPI, washed with PBS and then imaged by a microscope. Under laser irradiation at a wavelength of 980 nm, the typical HeLa cells exhibit strong up-conversion luminescence signals at 510-570 nm (green shown in Fig. 8(a)) and at 630-690 nm (red shown in Fig. 8(b)). In addition, the cell nucleus region is also displayed by DAPI (blue shown in Fig. 8(c)), and the brightfield image is also measured (Fig. 8(d)). The overlay images (Fig. 8(e)) reveal that up-conversion luminescence signals are located in the cytoplasm region but not in the DAPI-stained region. This fact suggests that PVP-capped NaYF4∶Yb/Er nanoparticles can be endocytosed by HeLa cells but cannot enter the nuclei of HeLa cells. Simultaneously, there is no obvious autofluorescence signal in the confocal images (Figs. 8(a) and 8(b)). To further quantify the signal, the up-conversion luminescence intensity across the line is recorded (Fig. 8(f)). Obviously, the up-conversion luminescence signal region has a very high intensity (counts in region 1 and region 3 are more than 4 095) and background fluorescence is zero (the count in region 2 is 0), which demonstrates a perfect signal-to-noise ratio and is similar to the previous report[31]. Therefore, such biocompatible PVP-capped NaYF4∶Yb/Er nanoparticles can be used as an efficient luminescence nanoagent for cancer cell imaging.
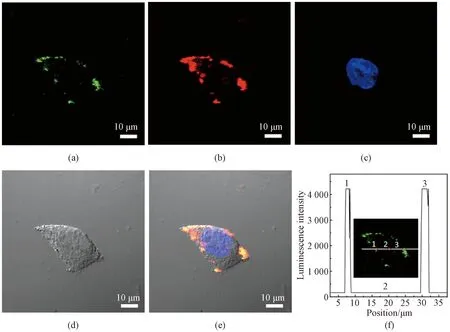
Fig. 8 Confocal images of cells incubated with PVP-capped NaYF4∶Yb/Er nanoparticles: up-conversion luminescence collected (a) at 510-570 nm and (b) at 630-690 nm; (c) fluorescent image of DAPI; (d) brightfield image; (e) overlay of (a)-(d); (f) luminescence intensity across the line shown in inset
3 Conclusions
The simultaneous control of morphologies and up-conversion luminescence intensities of NaYF4∶Yb/Er nanophosphors has been realized by adjusting surfactants. PVP-capped NaYF4∶Yb/Er nanoparticles show the smallest size and the strongest up-conversion luminescence intensity. Especially, PVP-capped NaYF4∶Yb/Er nanoparticles exhibit a low cytotoxicity and can act as an efficient luminescence nanoagent for imaging of cancer cells. Therefore, surfactant-dependent synthesis of NaYF4∶Yb/Er nanophosphors may bring new perspectives for bioimaging.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Porous Graphene-Based Electrodes for Fiber-Shaped Supercapacitors with Good Electrical Conductivity
- Fabrication of High-Efficiency Polyvinyl Alcohol Nanofiber Membranes for Air Filtration Based on Principle of Stable Electrospinning
- Construction of Oriented Structure in Inner Surface of Small-Diameter Artificial Blood Vessels: A Review
- Auto-Generation Method of Child Basic Block Structure
- Small Amplicons Mutation Library for Vaccine Screening by Error-Prone Polymerase Chain Reaction
- Proportion Integration Differentiation (PID) Control Strategy of Belt Sander Based on Fuzzy Algorithm
