Strong interaction and confine effect of manganese oxide-cobalt embedded in self-templated nitrogen-doped carbon enable ultra-stable zinc-air batteries
2023-03-30ZhongceHuToyiShenXingChenZhenhuYnTindiPnHouyongYu
Zhongce Hu, Toyi Shen, Xing Chen,**, Zhenhu Yn, Tindi Pn, Houyong Yu,***
a College of Textile Science and Engineering (International Institute of Silk), Zhejiang Sci-Tech University, Hangzhou, 310018, China
b Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin, 300071, China
Keywords:Zinc-air batteries Electrocatalyst Bifunctional Hybrid
A B S T R A C T Developing efficient bifunctional oxygen electrocatalysts with high stability is of great significance for the implementation of rechargeable Zn-air batteries.Herein, MOC-NC bifunctional electrocatalyst with active MnO-Co components embedded in the N-doped carbon sphere is prepared by facile hydrothermal conversion and post-pyrolysis process.The strong interaction between MnO and Co, and confine effect induced by the microstructure endow the fabricated MOC-NC composite with modified electronic configuration, improved conductivity,and significantly reinforced stability.Consequently,the MOC-NC electrocatalyst exhibits good activity and ultrahigh stability for oxygen electrocatalysis.Moreover, when the catalyst was applied to the cathode electrode for the zinc-air battery,it shows excellent performance(specific capacity of 718 Wh kgZn-1,power density of 152.3 mW cm-2) and extraordinary long-term stability (after 200 h of charge and discharge cycling, the round-trip efficiency still maintains at 44%).The present work demonstrates a strategy for synthesizing ultrastable bifunctional non-precious metal electrocatalysts for oxygen electrocatalysis.
1.Introduction
There is an increasing demand for sustainable and efficient energy storage and conversion technologies to achieve the goal of global carbon neutrality [1-6].Among these technologies, Zn-air battery (ZAB) have drawn intensive attention due to the advantages of the low-cost benefit from the zinc reserves, environmental friendliness, and safety derived from the aqueous electrolyte,and the high theoretical energy density of the reaction between zinc and oxygen [7-10].The reversible cathodic oxygen electrocatalysis reactions have a decisive effect on the performance of ZAB [11-15].However, the poor activity and stability of the cathodic materials for oxygen electrocatalysis remain the major obstacles.Currently, Pt, and Ru or Ir-based electrocatalysts are commercially chosen for oxygen reduction reaction (ORR) and oxygen evolution reaction (OER).Unfortunately, these noble electrocatalysts' limited reserve, high cost, single functionality, and poor stability restrict their large-scale implementation [16-20].Thus, developing highly efficient yet stable bifunctional non-noble electrocatalysts is significant for the development of Zn-air batteries[21-24].
Transition metal oxides, MnO especially, have advantages as competitive oxygen electrocatalysts such as earth abundance, facile synthesis, tunable morphologies, multiple phases and electron configurations,and decent activity.However,the intrinsically low conductivity restricts its catalytic activity.Hopefully, compositing with metal or carbonaceous materials proves to be an efficacious way to strengthen the conductivity[25-27].Specifically,metallic cobalt can not only improve the conductivity of the metal oxides but also greatly enhance the OER activity [28-31].Moreover, hybrid electrocatalysts always exhibit enhanced catalytic activity and stability[32].This enhancement can be attributed to the rapid charge transfer from the chemical interaction between metals,metal oxides,and carbon support[33-36].For example,Zhang et al.reported a bifunctional electrocatalyst of carbon nanotube-loaded MnO-Co that uses multi-walled carbon nanotube as support [37], the obtained composite shows excellent performance for rechargeable Zn-air batteries and overall water splitting.More recently,Zhou et al.demonstrate the Co/MnO nanoparticles in-situ fixed onto N,S doped carbon nanofiber through an electrospun-pyrolysis approach[38].However,it is still meeting the shortages of high reagents cost, and low yielding of the method in these reports.
On the other hand, self-accumulation of nanoscale electrocatalysts can lead to the degradation of catalyst stability under harsh electrochemical conditions, resulting in inferior competitiveness in electrochemical applications [39,40].Embedding electroactive components in carbon structures has been highlighted in literature to achieve highly stable electrocatalysts [41,42].Zhu et al.developed a method for synthesizing an efficient electrocatalyst by depositing Fe, N, P, and C elements onto the surface of silica particles using a polymerization approach, followed by high-temperature carbonization and chemical removal of the silica template.The resulting catalyst consisted of Fe,N,P,and other elements embedded in hollow carbon spheres,which not only increased the catalyst's specific surface area but also improved its stability [43].Ji et al.utilized decavanadate anions [H3V10O28]3= ({V10})as molecular vanadium oxide precursor and ZIF-67 as MOF model to synthesize CoVO/C by a chemical and pyrolytic approach.Due to the encapsulation of the catalytic components inside the carbon spheres,the resulting composite exhibited good stability[44].
Herein,based on the above considerations,we fabricate a manganese oxide/cobalt supported on nitrogen-doped carbon microspheres(MOC-NC-2)hybrid with active MnO-Co components embedded in the N-doped carbon spheres via a facile hydrothermal conversion and postpyrolysis strategy utilizing the inexpensive D-Glucose as the self templated carbon resource.MOC-NC-2 electrocatalyst features strong electronic interaction between MnO and Co due to the electron transfer indicated by the shifts in Mn 2p and Co 2p orbitals, which results in comparable OER and ORR activity, and superior stability compared to single-component manganese oxide supported on nitrogen-doped carbon microspheres (MO-NC) or cobalt supported on nitrogen-doped carbon microspheres (C-NC).Consequently, the ZAB based on MOC-NC-2 has higher energy density and cycle stability than that of RuO2+Pt/C.The versatile synthetic strategy presented here can not only be applied to synthesize other transition metal-based catalysts but also holds promising prospects for strengthening the stability of other bifunctional oxygen electrocatalysts.
2.Experimental section
2.1.Chemicals
Urea(CO(NH2)2),D-Glucose(C6H12O6),Potassium hydroxide(KOH),Manganese sulfate (MnSO4), cobalt nitrate hexahydrate (Co(NO3)2·6H2O)and Platinum on carbon(Pt/C,Pt 20%)were purchased from Macklin Biochemical Co.,Ltd,Ruthenium oxide(RuO2)were purchased from Aladdin biochemical Polytron Technologies Inc,conductive carbon paper was stocked from Suzhou Sinero Technology Co., Ltd, no further processing has been done on any of the materials.
2.2.Synthesis
The MOC-NC spherical catalyst follows the two-step route: carbon spheres containing Mn, Co, and N are synthesized hydrothermally, and then carbonized at high temperatures.In a regular synthesis method,2.7 g of CO(NH2)2, 0.19 g MnSO4, 0.74 g of Co(NO3)2·6H2O, and 4.9 g of C6H12O6were dissolved in a volume of 70 mL of deionized water.Then,the resulting aqueous solution was moved to a 100 mL Teflon-lined stainless steel autoclave and kept at 160°C for 20 h.Once the solution had cooled to ambient temperature naturally, the resulting brown precipitate was obtained through centrifugation.The precipitate was then washed with DI water and ethanol, and further dried at 60°C for 10 h.Subsequently,the sample was subjected to a temperature of 600°C under an Ar atmosphere for 2 h to yield MOC-NC microspheres.
2.3.Characterizations
The characterization of the specimens involved several techniques.Field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), and high-resolution transmission electron microscopy(HRTEM)were performed using JSM-5610(JEOL,Japan)for FE-SEM with an acceleration voltage of 3.0 kV,JEM-2010 F(JEOL,200 kV) for TEM and HRTEM.The degree of crystal structure and phase homogeneity of the samples were assessed using X-ray powder diffraction(XRD)analysis conducted on a Rigaku MiniFlex 600 I diffractometer with Cu Kα radiation(λ=0.15406 nm).Chemical characterization of the surface was implemented using X-ray photoelectron spectroscopy (XPS)with an Al Kα light source on a Thermo VG Scientific ESCA photoelectron spectrometer.Thermogravimetric analysis(TGA)was conducted using a thermogravimetric analyzer (TG209 F1, Netzsch, Germany) under a dynamic nitrogen atmosphere.The sample was heated at a rate of 20°C/min within a temperature range of 20-800°C.
2.4.Electrochemical test
The electrochemical measurements were conducted using the CHI 660e electrochemical workstation.The experimental setup included a three-electrode system comprising a platinum foil counter electrode, a working electrode loaded with electrocatalysts on carbon paper, and a Hg/HgO reference electrode.The potentials were calibrated with respect to the reversible hydrogen electrode (RHE) scale by applying the following equation:ERHE=EHg/HgO+ 0.0592 pH+0.095.
For the preparation of the working electrode, a 1 mL solution containing isopropanol and Nafion (5%) was mixed with 10 mg of the electrocatalyst.The resulting mixture was sonicated for 30 min to obtain a catalyst ink.Then,the working electrode was coated with 100 μL of the catalyst ink.
Linear Sweep Voltammetry (LSV) was performed in an oxygensaturated 1 M KOH solution to evaluate the activity of the Oxygen Evolution Reaction (OER).The potential was scanned from 1 V to 2 V (vs.RHE).Similarly, for evaluating the Oxygen Reduction Reaction (ORR)activity, an O2-saturated 0.1 M KOH solution was used for conducting LSV and was scanned from 0.2 V to 1 V (vs.RHE).Electrochemical impedance spectroscopy (EIS)measurements were conducted under the conditions of a potential of 1.62V(RHE)and a range of frequencies from 100 kHz to 0.01 Hz.
3.Results and discussions
3.1.Morphological and structural characterization
The synthesis method for MOC-NC is described in Section 2.2, as illustrated schematically in Scheme 1.According to previous reports,sugars can serve as precursors to produce hard carbon spheres through hydrothermal and high-temperature carbonization processes [45].In a high-temperature environment, sugar solutions undergo dehydration reactions, resulting in the formation of dehydrated sugar spheres [46].During the preparation process in this work,metal ions will be adsorbed on hydroxyl groups of glucose.Then,glucose microspheres were formed with the temperature increase.Meantime,urea can undergo hydrolysis at high temperatures to form NH3, NH4+, and HCO3-[47].HCO3-further decomposes to form CO32-and then combines with metal ions to form metal carbonates.At the end of the hydrothermal process, the intermediate manganese and cobalt carbonates encapsulated in the carbon spheres are generated,as evidenced in Fig.S1.Finally,the precursors are pyrolyzed to form MOC-NC during further heat treatment in the Ar atmosphere.
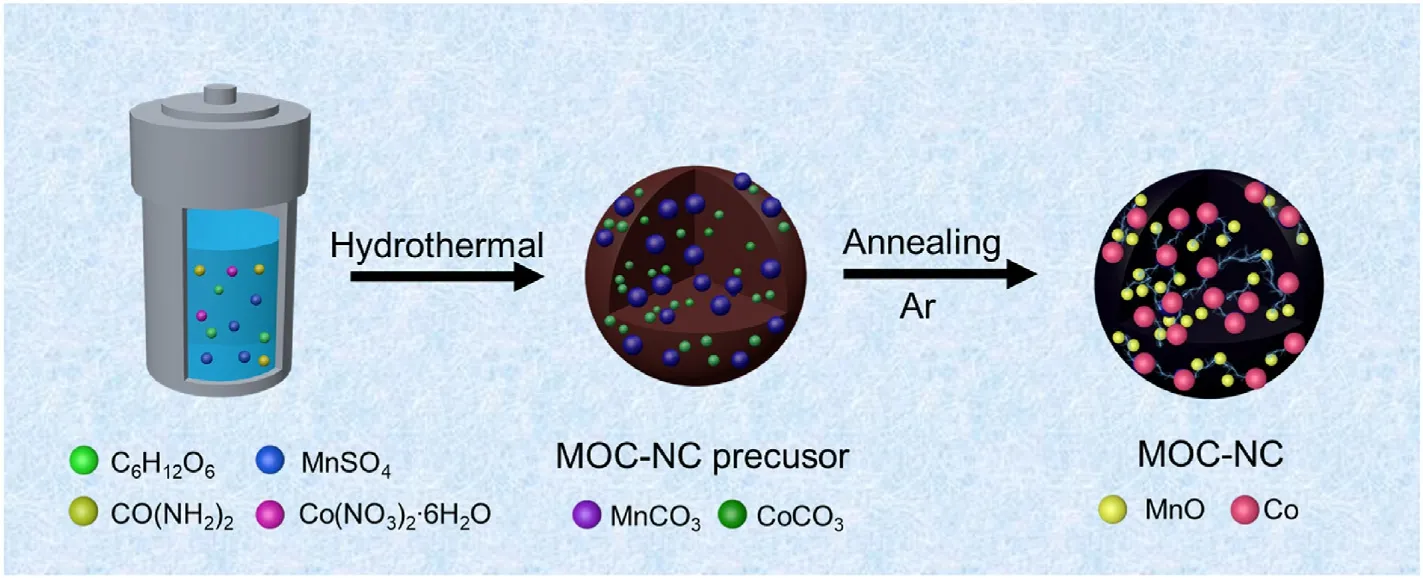
Scheme 1.Synthetic schematic illustration of MOC-NC.
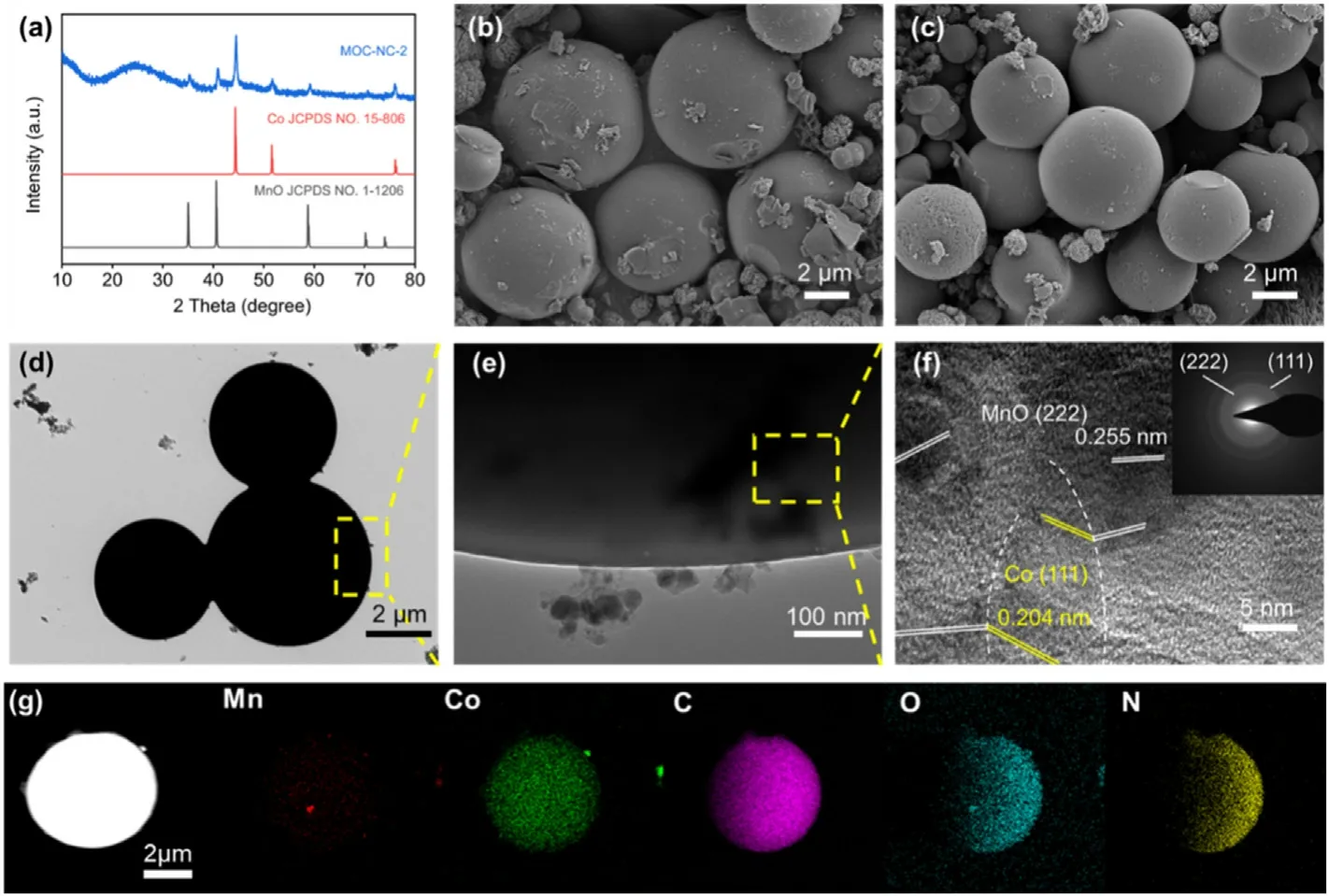
Fig.1.Microscopy characterizations and composition of MOC-NC.(a) XRD patterns, (b) SEM of MOC-NC precursor.(c) SEM of MOC-NC.(d)-(e) TEM images.(f)HRTEM image.(g) Elemental mapping images.
The crystalline structures of the obtained materials were studied by XRD technique.The XRD pattern of MOC-NC-2 is depicted in Fig.1a.The presence of graphitic carbon is evidenced by the broad peak observed around 25°.The additional diffraction peaks closely match the crystallographic patterns of MnO (JCPDS Card No.1-1206) and Co(JCPDS Card No.15-806),indicating the absence of detectable diffraction peaks from other phases.Fig.1b and c presents the SEM images of the asprepared precursor and products after calcination, respectively.The resulting products exhibit a spherical morphology with an average diameter of close to 8 μm,as depicted in Fig.S3.Based on the TEM image(Fig.1d and e), this indicates that the MOC-NC-2 microspheres have a solid structure.The corresponding high-resolution TEM image in Fig.1f show interplanar distance of 0.25 and 0.20 nm which separately refers to the d-spacing of the(222)and(111)plane of MnO and Co.EDX(energydispersive X-ray spectroscopy) elemental mapping (Fig.1g) of MOC-NC-2 microspheres demonstrates a homogeneous distribution of Mn,Co,N,O,and C elements throughout the entire microsphere.
To optimize the catalytic performance of MOC-NC, we also synthesized MOC-NC with different carbon contents denoted as MOC-NC-1 and MOC-NC-3(Figrue S5)by modifying the ratio of inorganic constituents in the precursor.The intensity of the peak decreases following the order of MOC-NC-1,MOC-NC-2,and MOC-NC-3,implying the various mass loading of MnO-Co in the composites (Figrue S2).Both the parallel sample share the same spherical morphology with MOC-NC-2,and with a diameter of about 5 μm and 8 μm,respectively(Fig.S4).Based on the thermogravimetric analysis (TGA) curve (Fig.S6), the mass loading of MnO-Co in MOC-NC samples is calculated to be 37.1 wt %, 22.1 wt%,18.9 wt%, individually.As for MOC-NC-2, the XRD results (Fig.S7)showed that the sample had been converted to MnCo2O4after the TGA characterization, and the mass loadings of MnO and Co in MOC-NC-2 are calculated to be 8.3%and 13.8%according to the reaction equation:MnO+2Co+3/2O2→MnCo2O4, respectively.Moreover, MO-NC, C-NC,and pure nitrogen-doped carbon microspheres (NC) were prepared for comparison,which were evidenced by XRD characterization(Fig.S8 a,c,e).All the samples except C-NC share an analogous morphology and microstructure as shown in Fig.S8 b,d,f.In addition,to investigate the effect of the confinement effect, we also synthesized neat manganese oxide/cobalt (MOC).MOC was obtained by annealing the precursor of MOC-NC-2 in air at 500°C to remove the carbon component,followed by a heat treatment at 600°C in a hydrogen-argon mixed gas atmosphere(Fig.S9a).SEM images showed that the overall structure of MOC remained spherical with many grooves and gaps, resembling a cauliflower-like morphology(Fig.S9b).
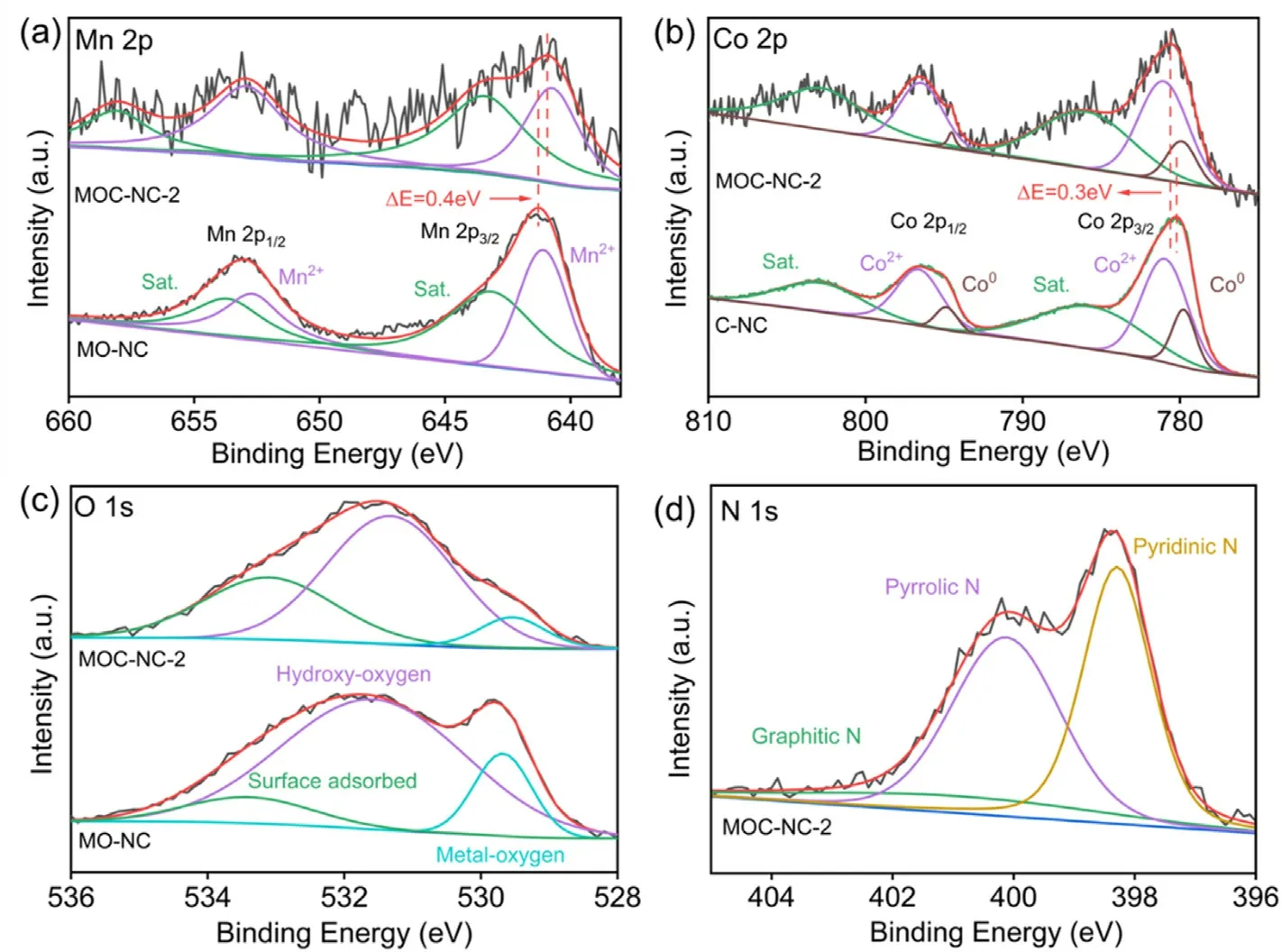
Fig.2.Compositional, textural, and surface chemistry analyses of the fabricated MOC-NC.(a) Mn 2p spectrum, (b) Co 2p spectrum, (c) O 1s spectrum, (d) N 1s spectrum.
XPS was employed to analyze the elemental constituents and bonding patterns of MOC-NC-2 at the surface.The XPS survey-scan spectra revealed the presence of Mn,Co,O,N,and C elements in the as-prepared samples.The high-resolution spectrum of Mn 2p in MOC-NC-2(Fig.2a)shows two prominent peaks at 640.8 eV (Mn 2p3/2) and 653.3 eV (Mn 2p1/2), accompanied by a smaller peak detected at 643.5 eV (Mn 2p3/2secondary peak) and 658.1 eV (Mn 2p1/2secondary peak).The spectra peaks of Co 2p at 781.1 and 796.5 eV were analyzed and deconvoluted into Co 2p3/2and Co 2p1/2components(Fig.2b),the fitting data of Co 2p indicate the presence of a pair of peaks assigned to metallic Co at 779.9 eV for 2p3/2and 794.5 eV for 2p1/2.Additionally,there is another pair of peaks observed at higher binding energy,which is attributed to Co2+and may be a result of surface Co oxidation.Compared with singlecomponent loading samples, the binding energy of Mn 2p and Co 2p core level spectra demonstrate a positive and negative shift for MOC-NC,respectively.The observed spectral shifts in the Mn 2p and Co 2p spectra indicate the significant interactions between MnO and Co, these interactions may enhance the sorption/desorption of intermediate species during the oxygen evolution process[26,30,48,49].
The O 1s spectra(Fig.2c)allow for the analysis and identification of three primary peaks: metal-oxygen (529.5 eV), hydroxy-oxygen (531.3 eV), and surface-anchored oxygen (533.1 eV).In comparison to the metal-oxygen peak observed in MO-NC,the reduced peak area of metaloxygen in MOC-NC provides additional evidence of the strong intermolecular coupling between MnO and Co.Furthermore, the C 1s XPS spectrum with high resolution (Fig.S10b) displays three distinct peaks centered at 284.6,285.7,and 288.2 eV,which align with the C=C,C-N/C-O, and O-C=O species, separately.As for N 1s spectrum (Fig.2d), it can be effectively deconvoluted into three distinct peaks, representing the presence of pyridinic nitrogen (398.3 eV), pyrrolic nitrogen (400.1 eV), and graphitic nitrogen (402.2 eV) (Fig.2d).The attainment of nitrogen integration into the carbon matrix is verified.
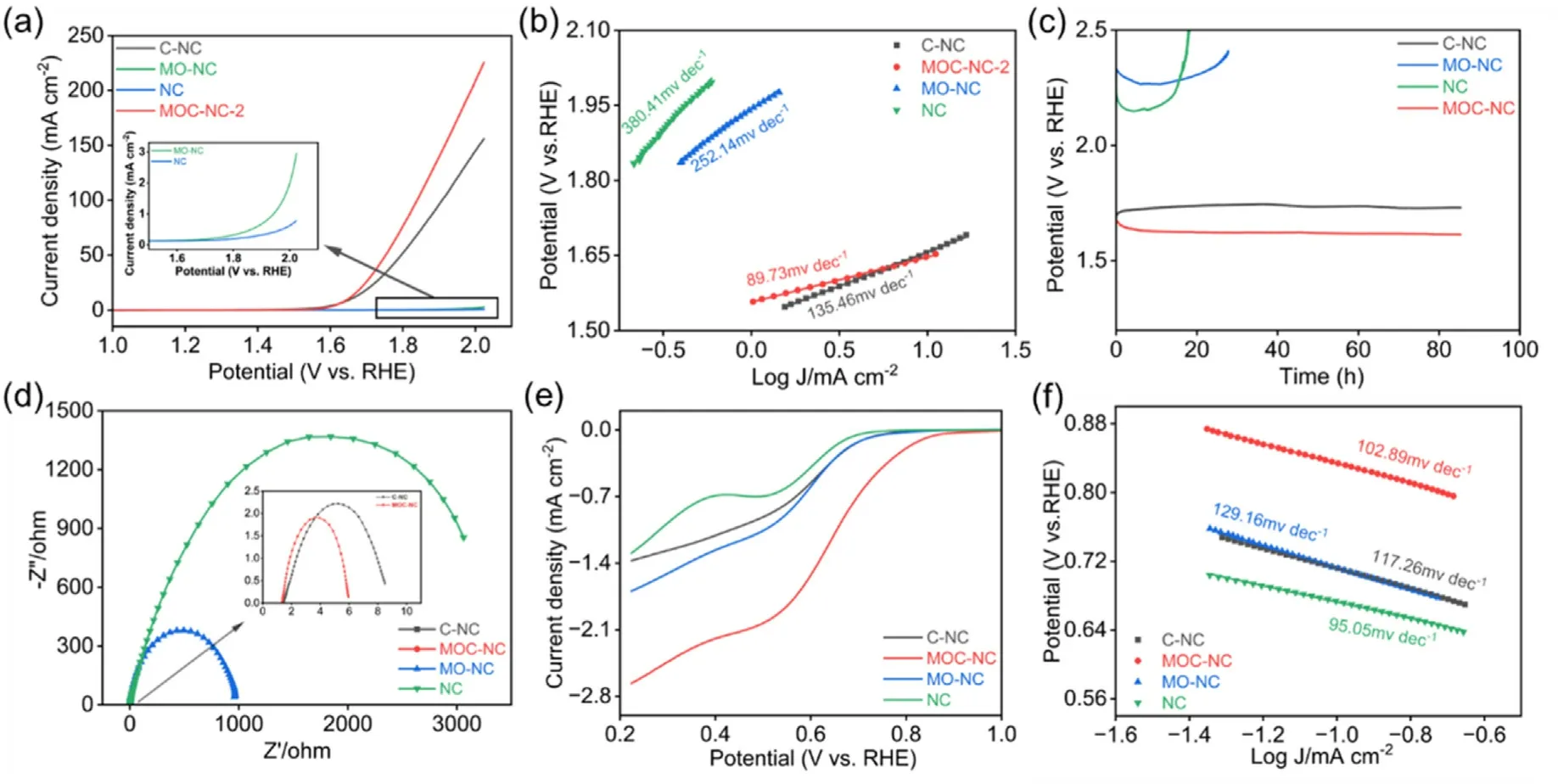
Fig.3.Electrochemical performance characterization of MOC-NC for OER and ORR.(a)LSV curves of MOC-NC-2,C-NC,MO-NC,and NC for OER in O2-saturated 1.0 M KOH electrolyte at 5 mV s-1.(b)Tafel slopes derived from(a),(c)E-t chronopotentiometry stability measurement for samples at a constant current of 10 mA,(d)EIS spectra of samples under 1.62V (RHE), (e) LSV curves of samples for ORR in O2-saturated 0.1 M KOH electrolyte at 1600 rpm, (f)Tafel plots derived from (e).
3.2.The electrocatalytic performance of the MOC-NC
The OER properties of the synthesized electrocatalysts were assessed in 1.0 M KOH,utilizing a conventional three-electrode configuration.The potentials were adjusted to the reversible hydrogen electrode (RHE)scale.In Fig.3a, the LSV profiles of MOC-NC-2,MO-NC,C-NC,and NC are shown.Among the samples, the MOC-NC-2 electrode exhibits an ampacity of 10 mA cm-2at an overpotential (η10) of 417.8 mV, surpassing those of MO-NC, C-NC (426.8 mV), and NC.The overpotential difference at 10 mA cm-2is not significant between MOC-NC-2 and CNC.Whereas, the current density of MOC-NC-2 rises rapidly at higher potential, which can be ascribed to the lower diffusion impedance of MOC-NC-2(Fig.3a and Fig.S12a).The significantly higher OER activity of MOC-NC-2 indicates that the strong interaction between Co and MnO augments the catalytic performance in facilitating the formation of molecular oxygen.
Tafel plots were generated from the polarization curves to analyze the kinetics of the OER.The observations depicted in Fig.3b and Fig.S11b indicate that the MOC-NC-2 exemplifies the lowest Tafel slope of 89.73 mV dec-1, this suggests that the MOC-NC-2 sample demonstrates superior OER kinetics compared to the other samples.The results of EIS are depicted in Fig.3d and 11c.The Nyquist plot shows that MOC-NC-2 exhibits the smallest semicircle diameter, suggesting the samples'superior charge-transfer conductivity.This can be ascribed to the pronounced interparticle interaction between MnO and Co in MOC-NC-2.
A chronoamperometry test Fig.3c at a fixed current density of 10 mA cm-2was adopted to test the stability of electrocatalysts.The activity of MOC-NC-2 tended to increase during the continuous chronoamperometry test of 85h, which indicated its good catalytic stability and further activation during the catalytic process.The ECSA (electrochemically active surface area) of the electrocatalyst was determined through the evaluation of the double-layer capacitance from the CV(cyclic voltammetry) curves, as depicted in Fig.S12 and Fig.S13.MOC-NC-2 exhibits an elevated Cdl of 11.14 mF cm-2compared to MONC (0.15 mF cm-2), C-NC (3.74 mF cm-2), and NC (0.14 mF cm-2),implying greater accessibility of reactive sites.
The ORR performance was additionally assessed through the utilization of a rotating disk electrode (RDE) loaded with the synthesized electrocatalysts.A linear scanning voltammetry (LSV) curve was recorded at 1600 rpm in an O2-saturated electrolyte employing a threeelectrode system.According to Fig.3e, MOC-NC-2 demonstrates exceptional ORR performance,demonstrating a remarkable Eonset(onset potential)of 0.83 V and a notable E1/2(half-wave potential)of 0.62 V vs.RHE.The Tafel plots also unveil insights into the ORR catalytic reaction dynamics.As depicted in Fig.3f, MOC-NC-2 demonstrates the most reduced Tafel slope of 102.89 mV dec-1, indicating its superior ORR reaction dynamics contrasted with MO-NC and C-NC.
3.3.Performance evaluation of Zn-air batterie in liquid electrolyte
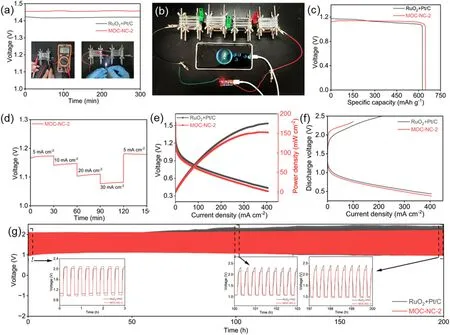
Fig.4.Performance comparisons of liquid MOC-NC-2 ZAB and RuO2 + Pt/C.(a) Open circuit voltage, (b) A photograph of a phone powered by four MOC-NC-2-based batteries in series.(c)Specific capacity curves,(d)Discharge curves at different current densities,(e)Discharge polarization curves and power density curves,(f)Discharge and charge polarization curves, (g) Galvanostatic discharge-charge cycling curves at a current density of 7.8 mA cm-2.
The promising bifunctional OER/ORR characteristics of MOC-NC-2 prompted us to conduct further investigations into its performance in Znair batterie.We fabricated an aqueous rechargeable Zn-air battery,featuring a zinc plate as the anode, a carbon fiber paper-based MOC-NC-2 electrode as the air cathode, and a 6.0 M KOH +0.2 M ZnCl2electrolytic solution.To facilitate comparison, a ZAB based on RuO2+ Pt/C was also assembled and tested with consistent operating conditions.In Fig.4a, it is evident that the MOC-NC-2-driven ZAB demonstrates a stable OCV (open circuit voltage) of 1.45 V, which is higher than the OCV of the RuO2+ Pt/C-driven ZAB (1.42 V).As a demonstration, a LED (light-emitting diode) can be powered by two MOC-NC-2 ZABs connected in series, providing an output voltage of approximately 3 V (inset in Fig.4a).Furthermore, the charging of a mobile phone is achievable by connecting four ZABs in series,resulting in an output voltage of approximately 5 V (Fig.4b).These results validate the promising potential of MOC-NC-2 in practical sustainable energy devices.The discharge curves of the ZAB at a current density of 10 mA cm-2are presented in Fig.4c.While the MOC-NC-2 electrode initially shows a lower operating voltage compared to Pt/C + RuO2, it demonstrates a more stable discharge voltage plateau over time.As a result,the MOC-NC-2 electrode achieves a high specific capacity of 647 mAh gZn-1at a current density of 10 mA cm-2based on the utilized Zn weight.This corresponds to an impressive energy density of 718 Wh kgZn-1,surpassing the performance of the RuO2+Pt/C electrode (energy density: 658 Wh kgZn-1; specific capacity: 621 mAh gZn-1).Fig.4d illustrates the rate performance of the MOC-NC-2-based ZAB at diverse current densities (5,10, 20, and 30 mA cm-2).Remarkably, the discharge levels of the MOC-NC-2 electrode exhibit a notable recovery without noticeable voltage decay upon returning the current densities to their initial states.This remarkable behavior indicates the superior rate capability and excellent cycling stability of the MOC-NC-2-based ZAB.Fig.4e presents the discharge voltage profiles and their corresponding power density performance curves.Impressively, the MOC-NC-2-driven ZAB demonstrates a remarkable peak power density of 152.3 mW cm-2, which closely approaches the performance of ZABs driven by RuO2+Pt/C(175.5 mW cm-2).This result showcases the highly competitive power generation capability of MOC-NC-2 in ZAB applications,reaffirming its potential for practical and efficient energy conversion.The ZABs underwent repeated galvanostatic charging-discharging cycles,maintaining a constant current density of 7.8 mA cm-2for a duration of 20 min in each cycle on the Neware Battery Testing System which has a current limitation of 10 mA.This thorough investigation aimed to evaluate the ZAB’s ability to withstand extended cycling periods and maintain consistent performance throughout the cycles.After the 100h test, the energy utilization efficiency (discharge terminal voltage divided by charge terminal voltage) of RuO2+Pt/C-based and MOC-NC-2-based battery becomes from 51.1% to 46.3% at the first cycle to 46.3% and 50%,respectively.Furthermore,in the comparison between MOC-NC-2 and neat MOC,it can be observed that the energy utilization efficiency of the zinc-air battery based on MOC decreased from 46.2%to 37.5%after the first cycle.The energy utilization efficiency of the MOC-based battery started to decline from the 14th hour.This indicates the positive impact of the embedded structure on performance enhancement and stability(Fig.S11d).Notably, the round-trip efficiency of the MOC-NC-2-based cell was improved after the first 100 h of long cycle testing, demonstrating that the catalytic properties of MOC-NC can be activated during the catalytic process.When the long-cycle test reached 150 h,the energy efficiency of Pt-based cells and MOC-NC-2-based cells dropped to 41.3%and 47.2%,respectively.Remarkably,the MOC-NC-2-based battery can still retain 44%energy efficiency after a 200 h long cycles test,surpassing that of the RuO2+Pt/C-based battery.The obtained results provide strong evidence to support the exceptional activity and stability of MOC-NC-2 when employed as the air-cathode in aqueous rechargeable ZAB.These findings confirm that MOC-NC-2 exhibits outstanding performance and long-term stability,making it a highly promising candidate for efficient and reliable ZAB applications.
4.Conclusions
In summary,we have successfully synthesized a bimetallic composite microspheres electrocatalyst(MOC-NC-2)through a facile solvothermal method followed by annealing, utilizing glucose as the self-templated carbon source.The obtained electrocatalyst features embedded microstructure and strong electronic interaction between MnO and Co.The strong interaction and confine effect synergistically enhance the catalytic activity and stability.As a result,the MOC-NC catalyst shows remarkable bifunctional activity to ORR and OER.Moreover, when applied as the cathode electrode in a zinc-air battery, the catalyst demonstrates excellent performance and exceptional long-term stability.The proposed versatile strategy offers significant advantages in the development of efficient and stable catalysts for various energy conversion processes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was financially supported by Zhejiang Provincial Natural Science Key Foundation of China (LZ20E030003; LGG22E030005),Outstanding Youth Project of Zhejiang Provincial Natural Science Foundation (LR22E030002), the National Natural Science Key Foundation of China(52273095),the Fundamental Research Funds of Zhejiang Sci-Tech University (22202289-Y) and the Key Research and Development Program of Zhejiang Province (2022C01049).Zhejiang Provincial Natural Science Foundation of China under Grant No.LGG22E030008.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.pnsc.2023.08.013.
杂志排行
Progress in Natural Science:Materials International的其它文章
- Research progress of composite cathode materials for Solid oxide fuel cells
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
- The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
- Towards high-efficiency of hydrogen purification in metal hydride