Research progress of composite cathode materials for Solid oxide fuel cells
2023-03-30QunyngLongRuiShRuohngWngBoXuHichoMenQiWngJieHou
Qunyng Long, Rui Sh, Ruohng Wng, Bo Xu, Hicho Men, Qi Wng,*, Jie Hou
a Engineering Research Center of Ministry of Education for Geological Carbon Storage and Low Carbon Utilization of Resources, Beijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing, 100083, China
b School of Resource Environment and Safety Engineering, University of South China, Hengyang, 421001, China
Keywords:SOFC Composite cathode materials Composite methods Mechanical-physical composite Infiltration composite In-situ composite
A B S T R A C T Solid oxide fuel cell (SOFC) is a device that converts the chemical energy of hydrogen or hydrocarbons and oxygen into electric energy through electrochemical reaction, with high efficiency and low emissions.Cathode materials with high electrochemical catalytic activity,stability and medium-low temperature durability continue to play a crucial role in SOFC.However,single-phase cathode materials suffer from low conductivity,low activity and poor thermal matching.Therefore, various composite technologies have been employed to enhance the performance of SOFC cathodes.In this review, various composite methods have been illustrated including mechanical-physical composite, infiltration composite and in-situ composite.Besides, this article provides an overview of the fundamentals, recent advances, advantages, disadvantages, challenges, and prospects of these composite methods.Furthermore, this article provides valuable guidance and potential directions for the development of composite cathode materials.
1.Introduction
1.1.Solid oxide fuel cell
New energy technologies have paid much attention due to the depletion of energy sources and the increase in environmental pollution.SOFC has received much attention owing to high efficiency and nonpollution characteristics [1-5].SOFC work on a same principle as batteries,but in contrast to batteries,SOFC does not need to be charged and will not run out.It only needs to supply fuels and oxidizers to the electrode for normal operation[3].SOFC is the third generation of fuel cells following phosphate fuel cell and fused carbonate fuel cell.It inherits various advantages of fuel cells,which are as follows[2,3,5-9]:
(1) SOFC has high conversion efficiency due to its electrochemistry is not limited by the Carnot cycle.
(2) SOFC is flexible in the choice of fuels, for instance carbon-based fuels, hydrocarbon and even solid carbon.
(3) SOFC has the peculiarity of low pollution and conform to the requirements of new energy.
(4) SOFC is modular and solid-state without moving parts,hence it is voiceless to be installed indoors and free of electrode corrosion and electrolyte leakage.
(5) SOFC has a potential life of 40,000-80,000 h.
(6) The performance of SOFC is greatly influenced by the oxygen reduction reaction(ORR)in the cathode part.
The basic working principle of SOFC is demonstrated in Fig.1.SOFC consists of anode, cathode and electrolyte.SOFC electrolyte characteristics only allow protons or oxygen ions through.Therefore according to the different kinds of conductive ions and electrolyte materials,the SOFC can be divided into two categories: oxygen ion conductive SOFC and proton conductive SOFC, whose schematic diagrams are manifested in Fig.1[1,2,7,8].The anodes not only serve as electrochemical oxidation sites for fuel,but also transfer charge to conductive contacts.The cathode is the sites of ORR,reduction of adsorption oxygen and then generation oxygen ions on the cathode.The oxygen ions are driven by the concentration and potential difference through the oxygen vacancy channel to the anode, where the fuel is electrochemically oxidized.The released electrons flow through the external circuit to the cathode to complete the circuit and do work as follows[7,8,10-12]:
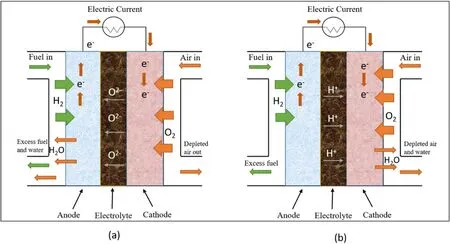
Fig.1.Schematic diagram of SOFC showing: (a) oxide-ion conducting electrolyte, (b) proton conducting electrolyte during its operation.

As shown above,SOFC produce mostly CO2and H2O,which explains their low emissions.Consequently,SOFC is one of the main directions of energy development in the future.
1.2.Development of SOFC cathode materials
As shown in Fig.2, based on the conducting mechanism and the pathways for the ORR, cathode materials can be categorized into two groups: (a) pure electronic conducting materials, and (b) mixed ionicelectronic conductors (MIECs) [13].Until now, single perovskite oxide,double perovskite oxide, perovskite-like oxide, spinel oxide and composite cathode materials are universally used as SOFC cathode materials[14-19].Pd, Pt and other precious metals are served as the earliest cathode materials of SOFC.However, its further promotion is restricted by the high-cost, and the adoption of low-cost materials become the development goal.La0.8Sr0.2MnO3-δ(LSM)with perovskite structure was discovered as a SOFC cathode material with excellent performance at 800°C-1000°C.Nevertheless, ORR only occurs at the three-phase interface due to its extremely low ionic conductance, which usually requires mixing with electrolyte materials or another cathode material to prepare an auxiliary cathode to improve the effective reaction area[20-22].When it is operated at high temperatures,SOFC has suffered a range of challenges such as shortened material life,difficultly in selecting connecting and sealing materials and long battery start-up and shutdown times.A large number of studies have shown that compared to high temperature SOFC(800-1000°C),low temperature SOFC(300-500°C)expand the choice of materials and stack geometries,reduce system costs and reduce the corrosion rate of stacks and system components in principle [23-26].The composite cathode materials can meet the current development requirements of SOFC with high efficiency and low operating temperature.Zhang et al.proposed combining 50% of yttrium oxide zirconia(YSZ)in LSM cathode material,and the resulting LSM-YSZ composite cathode material not only has better performance in terms of electronic and ionic conductivity, but also has higher efficiency than a single LSM cathode material while ensuring lower operating temperature[20].Eman et al.use inkjet printing to deposit nanostructured particles of LSM on Y-doped BaZrO3(BZY) backbones as cathodes for proton-conducting SOFC,which allows the battery to have a high output power density even at 600°C [27].Ai et al.synthesized a 40 wt%Er0.4Bi1.6O3decorated LSM electrode through a new gelation method,electrocatalytic activity of LSM electrodes for ORR was enhanced by the addition of ESB [28].All these examples have prepared excellent composite cathodes based on LSM at low to medium temperatures.It is evident that the potential exists for composite cathodes.Therefore, we summarize some potentially excellent composite cathodes in the low and medium temperature range.As shown in Fig.3,we can focus on excellent and medium composite cathodes for future research,and their composite strategies and methods are worth to be studied thoughtfully.
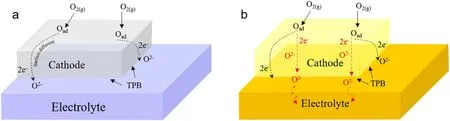
Fig.2.Schematic of the possible pathways for the ORR in (a) pure electronic conductor and (b) mixed-ionic electronic conductor (MIEC).

Fig.3.Potential composite cathodes in a temperature range (550-800 °C).
1.3.Research status of SOFC composite cathode materials
Composite cathode materials are a new type of cathode materials consisting of combination of two or more single-phase cathode materials,or cathode materials and electrolytes,which combine the advantages of different phases to a certain extent and overcome the deficiencies of one single phase.As shown in Table 1, composite cathode materials are superior to single-phase cathode materials in terms of polarization resistance (Rp) and maximum power density (MPD).As shown in Fig.4, the research articles on composite cathode materials have increased significantly from 2003 to the present.Although the research on composite cathode materials has encountered some bottlenecks in recent years,the composite cathode materials with better performance and more comprehensive performance are still the keen direction for most researchers.Traditional composite cathode materials are prepared by simple mechanical-physical methods with a process of grinding the cathode powder and then mixing simply.Xu et al.prepared Pr2NiO4-Pr0.2Ce0.8O1.9composite cathode by mechanical-physical mixing approach [29].The Pr2NiO4and Pr0.2Ce0.8O1.9powders were prepared by the glycine-nitrate combustion method and the modified sol-gel method, respectively, and then the two powders were mixed by the solid-phase synthesis method for several hours to finally produce the Pr2NiO4-Pr0.2Ce0.8O1.9composite cathode material.With the development of composite cathode materials, infiltration composite and in-situ composite have gradually received attention from researchers, and an army of researchers have proposed their brand-new understandings and thoughts in the field of composite cathode materials,providing resultful strategies for the subsequent development of composite cathode materials.Here are quite a few examples, Zhang et al.impregnated the LSM homologous ion nitrate solution on the cathode LSM-YSZ prepared by conventional mechanical-physical, and the impregnation reduced the initial non-ohmic impedance of the LSM-YSZ cathode at 600°C-800°C[20].Geng et al.used a microwave water bath to heat up the ion-electron hybrid conductor SrFe0.9Nb0.1O3-δreacted in-situ with a uniformly coated SrO layer and partially transformed into the three-phase conductor Sr3Fe1.8Nb0.2O7-δ, which improved the oxygen reduction reactivity [30].Apparently, different composite methods affect the preparation, structure and properties of composite cathodes.Thus, we summarize the advantages and disadvantages of different composite methods in Fig.5, the challenges of different methods are various.Therefore,how to utilize the advantages of different composite methods and weaken its deficiencies is an issue worth considering and discussing.
During the past,although there are many publications on composite cathode materials, most of these reviews focus on the types and properties of composite cathode materials and therefore there is a lack of relevant reviews to summarize the research progress on the composite methods of composite cathode materials[1,8,31-33].In this review,we specifically summarize recent advances in the development of newcomposite cathodes and innovative composite methods.Finally, the current challenges and future development trends are provided.
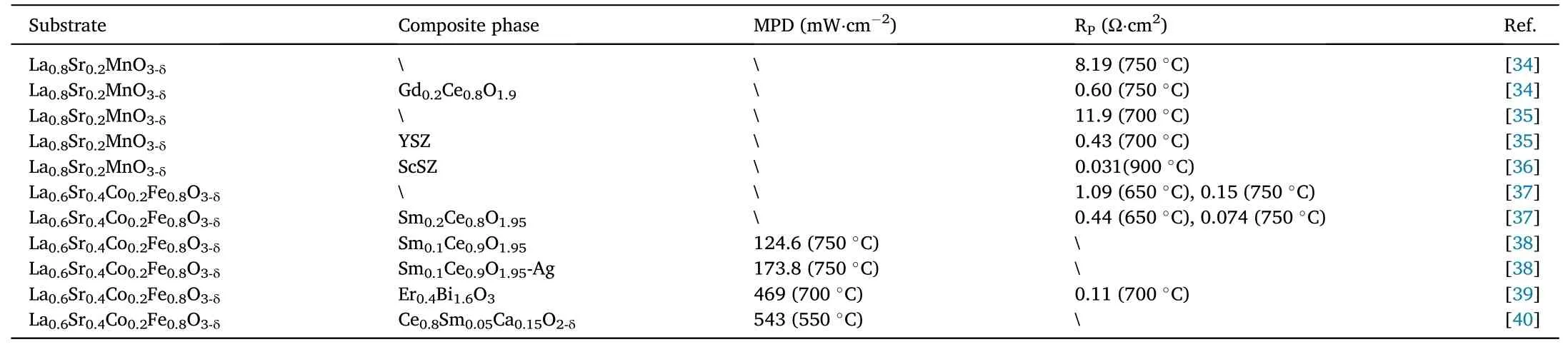
Table 1 Performance comparison between single-phase cathode and composite cathode materials.
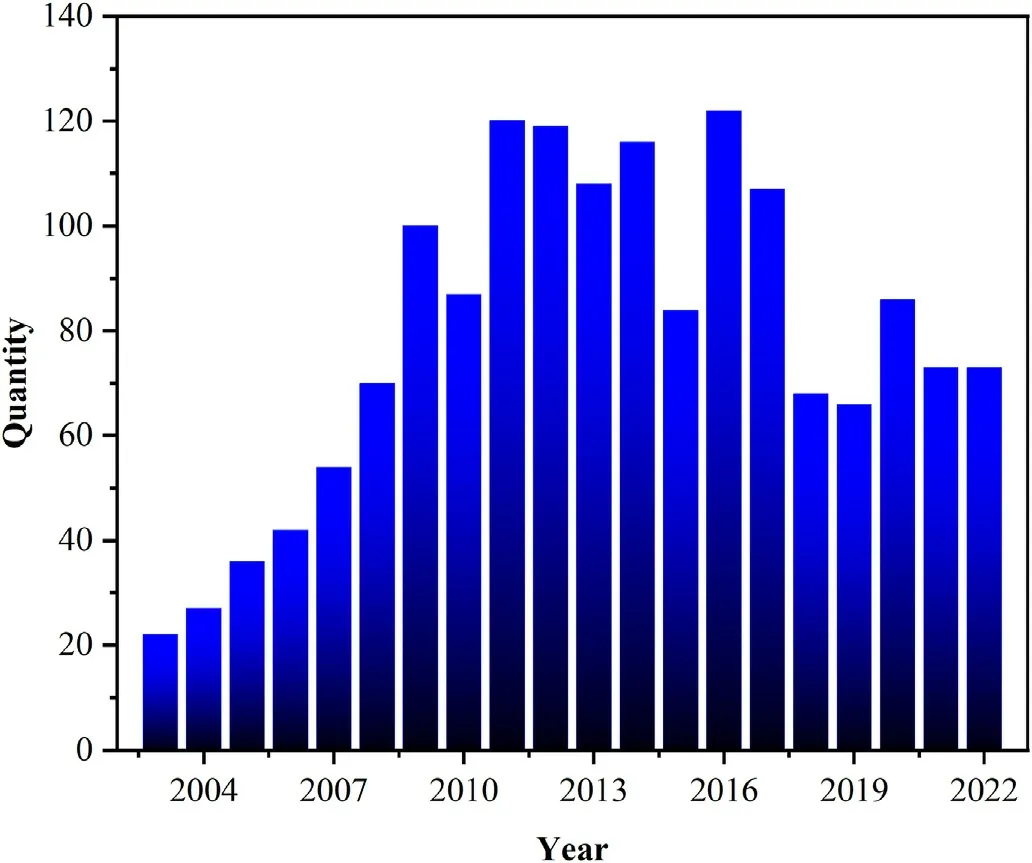
Fig.4.Number of research articles published on SOFC composite cathode materials (Source Web of Science.Statistics based on the current number of articles on SOFCs from 2003 to 2022).
2.Mechanical-physical composite
As the most shared composite method for traditional composite cathodes, mechanical-physical composite is simple and short timeconsuming.Under normal conditions, the cathode materials served for mechanical-physical composite must be complementary to each other.What's more,one phase has a strong oxygen ion conductivity,while the other phase has a strong electronic conductivity or a high oxygen catalytic activity.Min et al.mixed LaNi0.6Fe0.4O3-δwith Sm0.5Sr0.5CoO3-δpowder as a composite cathode, the former one has high conductivity,positive thermal and chemical stability at low temperature,and the latter one has relatively excellent oxygen catalytic activity.The composite cathode has MPD of 1427 mW cm-2and RPof 0.039 Ω cm2at 700°C which indicates that the ORR in the composite cathode is significantly accelerated [41].The simple and basic process of mechanical-physical composite is to prepare precursor materials by solid-phase method,precipitation method and sol-gel method, grind the prepared precursor materials and mix them in a certain proportion, and then obtain composite cathode with positive performance after drying and sintering.The schematic diagram is shown in Fig.6.For instance, Jeong et al.mixed LSM and ScSZ electrolytes with organic binders and ground them at high energy for 15 min to produce a sticky electrode paste, which was then made into half-cells by screen printing and high-temperature sintering methods [42].Besides, depending on the composite phase, the mechanical-physical composite is divided into two types: the composite of substrate cathode materials with electrolyte and the composite of substrate cathode materials with other components cathode materials.
2.1.Composite with electrolyte
Cathode materials with perovskite structure have received much attention owing to its typical mixed ion-electron conductivity,outstanding electrochemical properties, low Rpand high ORR at lowmedium temperature.However, the mismatch thermal expansion coefficient between the cathode and the electrolyte led to high contact resistance and unsatisfactory overall performance of SOFC when employing the single-phase perovskite materials as cathodes.As a result,the cathode materials of perovskite structure with shared electrolytes were mechanically mixed by some researchers to prepare composite cathode materials with excellent compatibility and positive performance.Marshenya et al.mixed perovskite cobaltite Pr0.9Y0.1BaCo2O6-δand samarium-doped cerium dioxide Ce0.8Sm0.2O1.9to obtain a composite cathode material that matches the thermal expansion coefficient of Ce0.8Sm0.2O1.9and high performance [43].Chen et al.used a citrate combustion method to prepare cobalt-free double perovskite oxide PrBa0.5Sr0.5Cu2O6-δpowder and compounded it with the proton conductor electrolyte BaZr0.1Ce0.7Y0.2O3-δto form a composite cathode material,which corresponds to a single cell with a MPD of up to 669 mW cm-2at 750°C[44].Nevertheless,it is not one electrolyte combination fixed with one type of perovskite electrode that yields a high-performance composite cathode.In addition, the same electrolyte can be mechanically mixed with different perovskite cathodes.Shimada et al.combined three electron-conducting perovskite oxides as composite materials with proton-conducting BaZr0.1Ce0.7Y0.1Yb0.1O3-δand found that BaZr0.1Ce0.7Y0.1Yb0.1O3-δwith La0.6Sr0.4Co0.2Fe0.8O3-δ,La0.6Sr0.4CoO3-δand La0.6Ba0.4CoO3-δhave excellent compatibility and possess positive electrochemical properties[45].As can be seen from the above, the composite method of mechanically mixing the electrolyte in the cathode material can effectively improve the match between the cathode and the electrolyte.This is due to mixing the electrolyte in the cathode material can effectively expand the active region of the cathode electrochemical reaction,increase the ionic conductivity of the cathode,reduce the polarization resistance, and increase the compatibility between the cathode and the electrolyte, thereby improving the cathode electrochemical performance.In addition,as shown in Table 2, conventional perovskite cathodes composite with different electrolytes can also prepare cathode materials with positive properties besides new type cathodes.Thus, it is a significant way to reinforce the performance of cathode materials by means of the mechanical-physical composite of cathode materials with electrolytes.
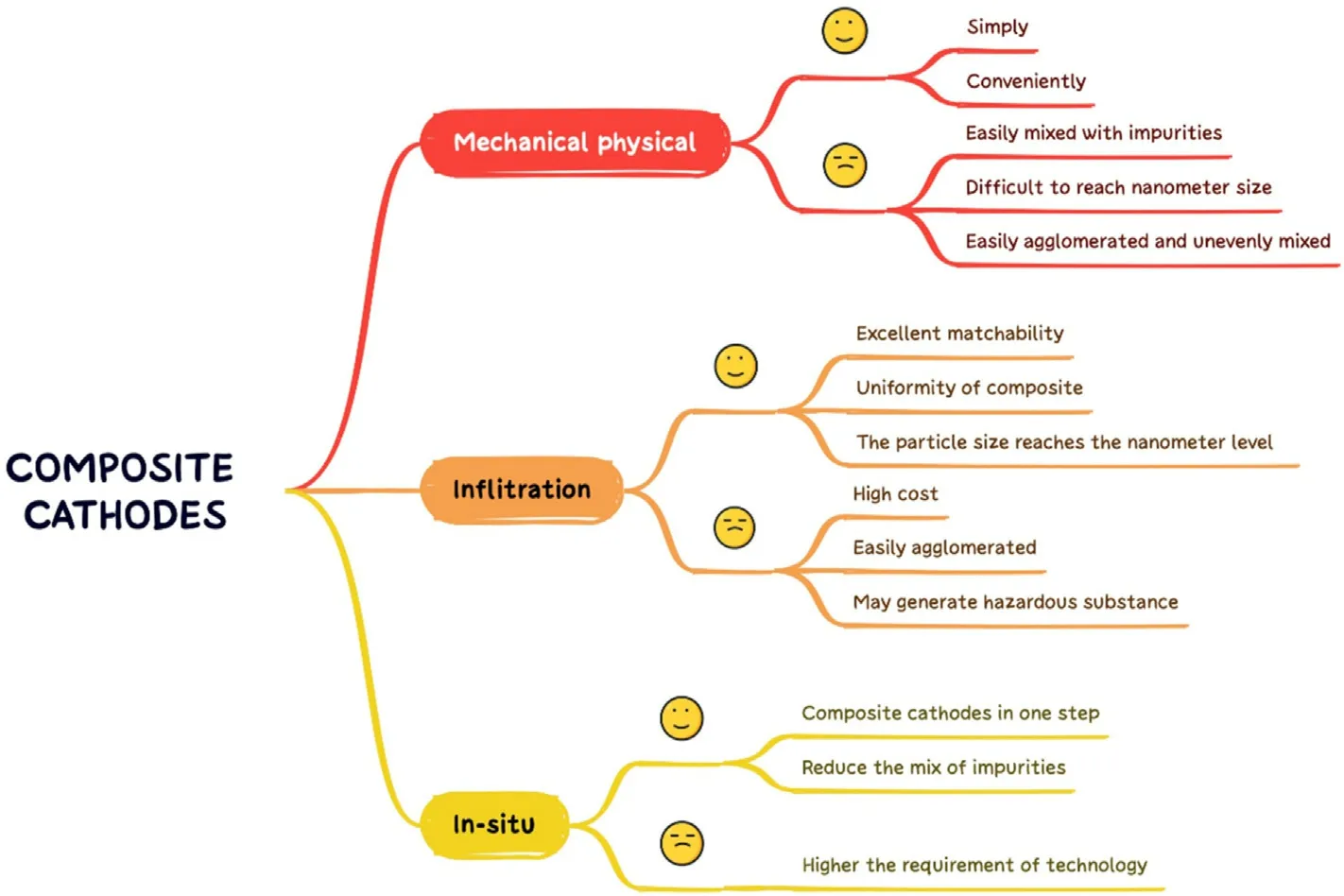
Fig.5.Advantages and disadvantages of different composite methods.
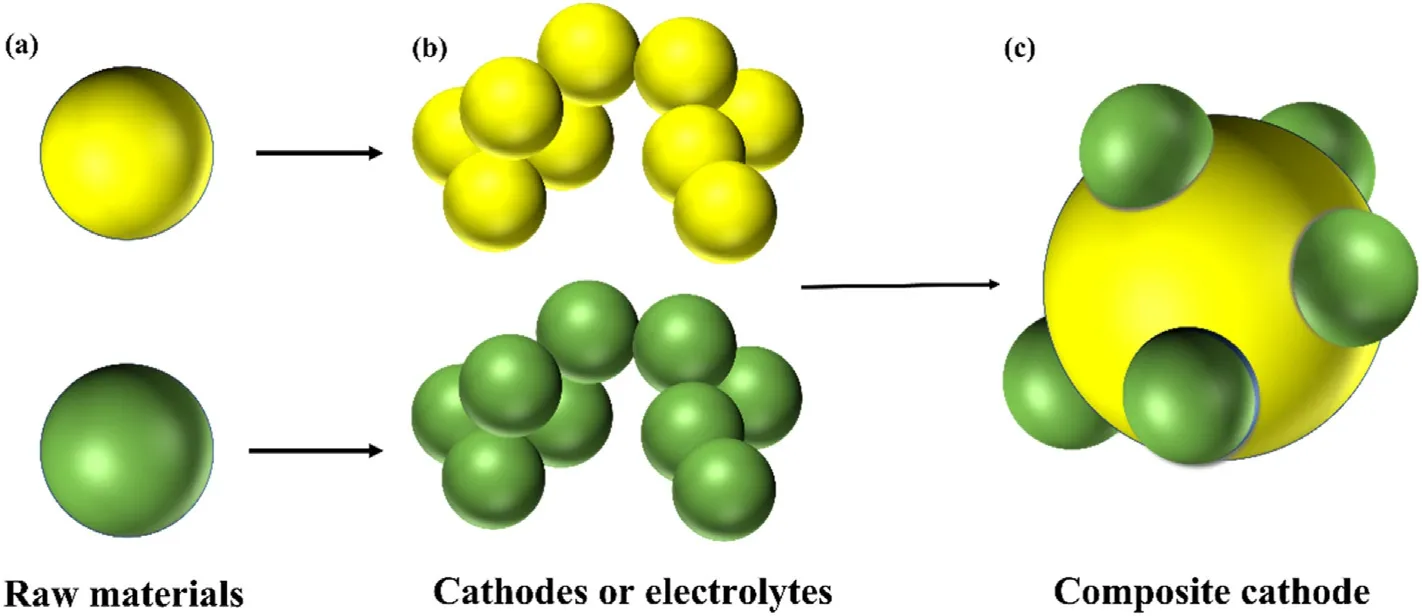
Fig.6.Schematic diagram model of the process of mechanical-physical composite:(a)Raw materials for preparing cathodes or electrolytes;(b)Preparation of cathode precursors or electrolytes by sol-gel method,etc.(c)The composite cathode material is prepared by grinding and mixing the cathode precursor and electrolyte or other cathode materials, and then sintering them.
2.2.Composite with other cathode materials
In recent years,a mechanical-physical composite of two or more highperformance perovskite materials has been proposed to obtain a stable and high-performance composite electrode.From the data in Table 3,themechanical-physical composite of multiple cathode materials is a crucial way to enhance the performance of cathode.Alice et al.obtained higher performance composite electrodes by combining two high-performance perovskite materials, La0.6Sr0.4Co0.2Fe0.8O3-δand Ba0.5Sr0.5Co0.8Fe0.2O3-δ[46].Besides,cobalt-based materials among perovskite materials have high-cost, high thermal expansion coefficient and poor chemical stability, although they have excellent ORR catalytic activity.Therefore, the composite of two and more high-performance perovskite cathode materials can be accompanied by negative cathode stability.However, the high-temperature treatment promotes the positive results of the interdiffusion of ions at the interface of the composite cathode.In addition,heterostructure cathode materials can synergize the advantages of two-phase materials with abundant heterogeneous interfaces to improve the contact sites with oxygen, from improving the electrochemical performance of composite cathode materials.Meng et al.prepared a heterogeneous composite cathode by mixing Pr0.8Sr0.2Fe0.7Ni0.3O3-δand Pr1.2Sr0.8Ni0.6Fe0.4O4+δin a solid state with a mass ratio of 3:7.The results show that the oxygen reduction catalytic activity of the composite cathode is significantly enhanced due to the presence of a heterogeneous interface that promotes the cathodic ORR [47].The cathode materials include spinel structure and perovskite-like structure in addition to perovskite structure, which all possess different characteristics and positive performance.Cathodes powders with different structures can be mixed to prepare higher electrochemical performance by mechanical-physical composite.Besides,it faces the challenge of how to balance the stability and activity for high performance cathode materials.
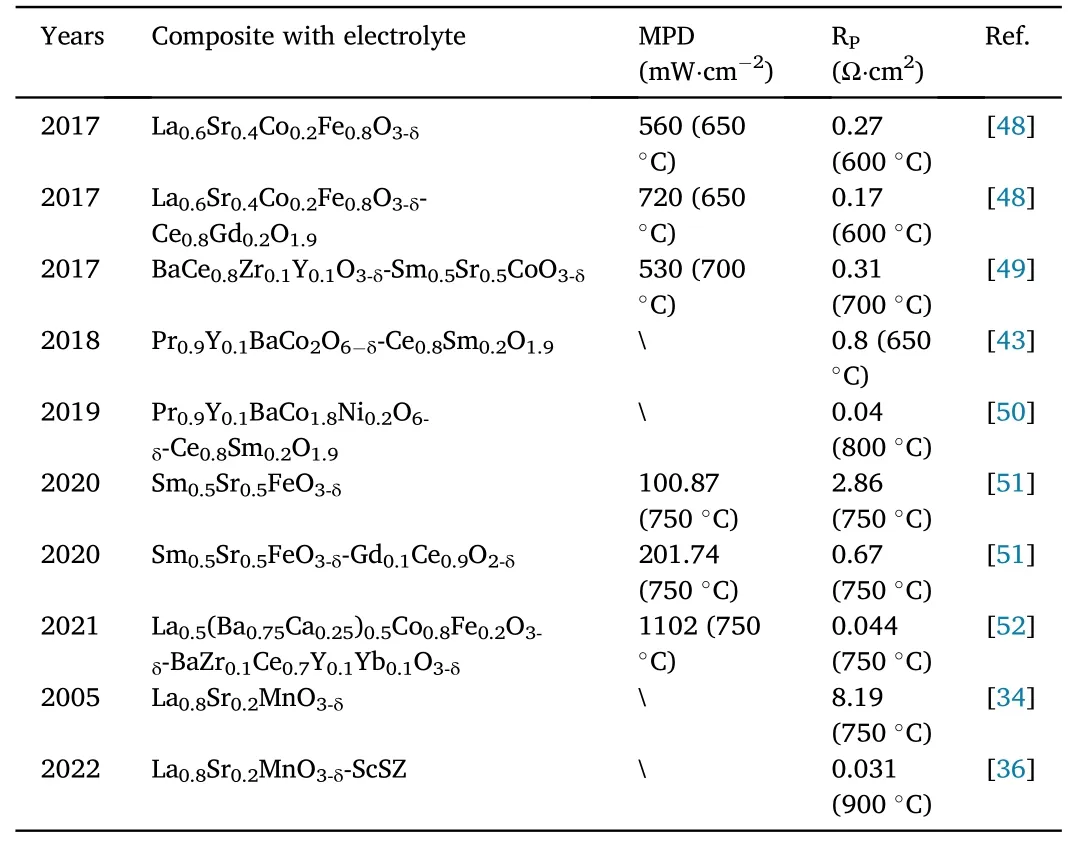
Table 2 Performance of composite cathode materials with electrolyte made by mechanical-physical composite and performance of some single-phase cathodes in recent years.

Table 3 The performance of composite cathode materials with other cathode materials made by mechanical-physical composite and the performance of some singlephase cathodes in recent years.
To conclude, mechanical-physical composite as a more traditional composite method, it has been more widely served on composite cathode.For different structures of cathode materials, it can effectively and promptly composite them.Composite cathode materials that have different high performance and excellent compatibility or cathode materials with electrolytes can achieve the performance to complement each other.In the future,the idea of mechanical-physical composite not only can choose a wider range of materials to be compounded, but also optimize its synthesis process steps to reduce the problems and impurities that exist in the materials after compounding.In the mechanical-physical composite preparation of composite cathode materials,it is necessary to fully grind and mix the composite materials, to control sintering conditions of temperature and time and ratio of the mixing material to prepare a composite cathode with high-performance.The superior synthesis steps can effectively reduce the mixing of impurities as well as improve the stability of the cathode,which is a direction that needs to be investigated for future mechanical-physical composites.
3.Infiltration composite
The specific surface area of the cathode and the catalytic reaction active zone can be improved by infiltration composite,which is a shared and effective method to enhance cathode performance.The schematic diagram of the impregnation composite is shown in Fig.7, liquid solutions or sols containing stoichiometric metal salt precursors and certain favorable surfactants and complexes are imported into the pre-filtered backbone, which is then subjected to the relevant heat treatment, and two typical morphologies can be observed after impregnation: discrete particles as shown in Fig.7(c),and a continuous protective thin layer as shown in Fig.7(d) [54,55].An ocean of works has been devoted to improving the microstructure of the cathode materials by impregnation composite to enhance the comprehensive performance of the cathode materials, and to develop SOFC single cells with excellent electrochemical performance[32,54-57].
3.1.Impregnate the electrolyte skeleton
Impregnating the electrolyte skeleton is frequently used impregnation method,which has the advantages that the cathode porous structure can be an electrolyte material, the heat treatment temperature is not high,and the impregnated particle size is ordinary controlled at the nanometer level, so that many reactive zones can be generated to improve the electrode performance, also to avoid the thermal expansion coefficient matching between cathode with the electrolyte.Shao et al.prepared CuCo2O4-SSZ composite cathode by coating spinel-structured CuCo2O4nanoparticles on porous stable zirconia (SSZ) frameworks through a solution impregnation process.The CuCo2O4nano-particles are uniformly distributed on the surface of the porous SSZ backbones, thus increasing the length of the triple phase boundaries and obtain low Rpand high peak power density (PPD) [58].The specific process of impregnating the electrolyte skeleton is roughly divided into two processes: fixing the electrolyte skeleton and impregnating the metal nitrate solution.The commonly used impregnation methods are solution impregnation, wet impregnation and ionic impregnation.Zhou et al.used the solution impregnation method to prepare La0.6Sr0.4Fe0.9Sc0.1O3-δ-YSZ composite cathodes [59].Baijnath et al.used the wet impregnation method to prepare La0.5Sr0.5CoO3-YSZ composite cathodes[60].Xu et al.used ionic impregnation to make LSM-SDC composite cathodes[61].The results of the three impregnation methods demonstrate that the preparation of composite cathodes by impregnating the electrolyte skeleton not only reduces the Rpof the cathodes, but also forms the cathode phase structures such as perovskite and spinel within the electrolyte skeleton,which improves the electrochemical performance of the whole battery.However, the performance of the composite cathodes prepared by impregnating different electrolyte skeletons with the same cathode materials will demonstrate various performance(Table 4).Küngas et al.researched the performance of composite cathodes after LSF impregnation in ScSZ,YSZ and yttrium-aluminum co-stabilized zirconium (YAZ) porous skeletons and found that the Rpof the corresponding symmetrical electrodes were 0.09 Ω cm2,0.15 Ω cm2and 0.75 Ω cm2at 700°C[62].As can be seen through Table 4 that the YSZ composite cathode impregnated by LSM and then impregnated with palladium can obtain a higher performance cathode material.From this, impregnation of electrolyte skeleton is a widely adaptable impregnation method to obtain higher performance composite cathodes.

Fig.7.A schematic of a typical infiltration process: (a) an as-fired electrode backbone; (b) a process of solution drops entering the electrode backbone; two typical morphologies of infiltrated electrode after thermal treatment: (c) particle deposition and (d) thin film coating [54].

Table 4 Performance of composite cathode materials with impregnated electrolyte skeleton.
3.2.Impregnate the cathode frame
Impregnation of nanoscale catalysts and ionic conductors into the cathode framework increases the triple-phase boundary (TPB) and reactivity of the cathode, to reduce the Rpof the cathode materials and giving it a higher MPD.Gao et al.impregnated nanoscale SrFe0.75-Mo0.25O3-δcatalysts into porous La0.9Sr0.1Ga0.8Mg0.2O3supports to obtain composite cathodes with lower Rp[63].Zhou et al.impregnated tri conducting NdBa0.5Sr0.5Co1.5Fe0.5O5+δon the La0.6Sr0.4Co0.2Fe0.8O3-δsurface to obtain a composite cathode with a Rpof 0.14 Ω cm2at 700°C due to the impregnation of tri-conducting materials promotes oxygen adsorption and ionic migration [64].Although high performance perovskite materials can also be composited by impregnation to form higher performance composite cathodes,the impregnation volume must be controlled.Liu et al.prepared high-performance composite cathodes with double perovskite PrBaCo2O5+δ(PBCO)and La0.6Sr0.4Co0.2Fe0.8O3-δ(LSCF) skeletons by solution impregnation, and found that a moderate amount of PBCO impregnation can significantly improve the performance of the composite cathode, while excessive impregnation, on the contrary, increases the Rpof the composite cathode [65].As shown in Table 5, in addition to the impregnation of perovskite materials,electrolyte-impregnated cathode frames can also be effective in enhancing the performance of cathodes.Jiang et al.impregnated yttria-stabilized bismuth oxide (YSB) in the framework of conventional LSM of perovskite materials, and the electrochemical performance was greatly improved compared with the conventional LSM[66].However,it was found that the performance of the conventional high-performance composite cathode could be further enhanced by re-impregnation.Zhang et al.used the impregnation composite to impregnate nano-sized Ce0.9Mn0.1O2-δin the (La0.75Sr0.25)0.95(Cr0.5Mn0.5)O3-δ-Ce0.8Gd0.2O1.9composite cathode to improve the stability and electrocatalytic activity during electrolysis of CO2[67].Therefore,it can be said that impregnation on the cathode framework is a strategy to re-enhance the performance of cathodes.
3.3.Metal impregnated cathode materials
At the beginning of the development of SOFC cathode materials,metals such as Pt were used as cathode materials.However, due to the development of materials such as perovskite and spinel, these metal cathodes gradually step down from the stage.However, it has been recently found that impregnation of Co,Ag,Pt,Ni,and other metals into the cathode materials by means of impregnation can improve the electronic conductivity of the material in the reducing atmosphere, thus improving the performance of cathodes [68,69].Fan et al.composited Pr2NiO4with Ag to improve the electrode activity toward ORR through three various methods of impregnation, solid-state mixing and freeze-drying, respectively [69].Compared to solid-state mixing and freeze-drying, the impregnation method provides a moderate particle size and isolated distribution of the metal, resulting in more positive performance.Not only that but re-impregnating the metal on the composite cathodes will also improve its performance.For instance, the performance of Pd impregnated LSM-YSZ [70] and LSCF-GDC [71]composite cathodes was improved.Therefore, metal impregnation is an effective way to enhance cathode performance.
In summary, the impregnated composite with certain functional materials can also improve the catalytic activity,electronic conductivity,ionic conductivity, and thermal stability of the cathodes.However, to prepare composite cathodes with excellent performance, the impregnation number of times, temperature, and volume must be controlled.Although the chemical impregnation method can improve the performance of cathodes,there are still the following problems:
(1) How to reduce the extra cost caused by the impregnation process.
(2) The particle size of the chemical impregnation reaches the nanometer level,and the particle size and agglomeration are simple to occur at the working temperature.
(3) The impregnated material and the cathode skeleton material may react at a higher working temperature to deactivate the material or generate harmful substances.
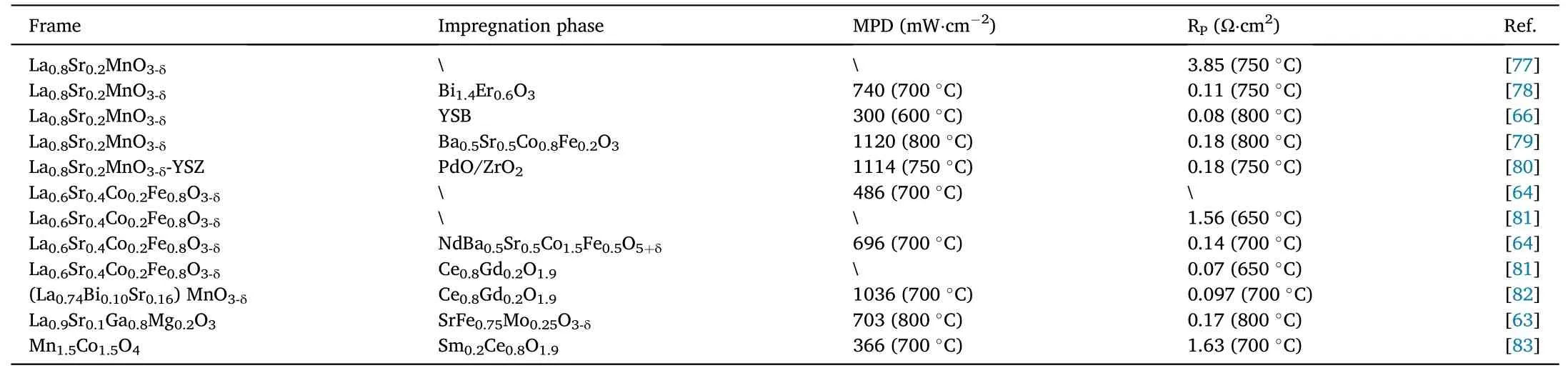
Table 5 Performance of composite cathode materials with impregnated cathode frame.
4.In-situ composite
In-situ composite is different from mechanical-physical composite and impregnation composite.It is not to prepare precursor materials separately and then make composite phase by mechanical mixing or skeleton impregnation, but directly generate composite phase by onestep method.The composite principle is shown in Fig.8.The composite phase generated in-situ not only has a homogeneous phase, but also has no impurity contamination at the interface with the matrix.The conventional preparation method of composite cathode materials is to prepare more kinds of powders respectively and then mix them by mechanical method.It is simple to introduce impurities in the mixing process,or the more materials are not uniform when mixing.
In-situ composite is a relatively new type of compounding, and its one-step method is even more novel.Self-assembled of in-situ composite has received much attention owing to its unique and efficient preparation of high-performance composite cathode materials in recent years.Two or more phase is introduced into certain cathode materials through in-situ one step with improved performance (Table 6).Song et al.reported an oxygen ion-proton-electron-conducting nanocomposite that selfassembly during high-temperature calcinations results in the formation of a nanocomposite consisting of a mixed H+/e-conductor BaCex-YyCozO3-δphase and mixed O2/e-conductor BaCoxCeyYzO3-δand BaCoO3-δphases,with maintaining a robust operational stability of 812 h at 550°C[84].The in-situ composite can partially convert the first phase into the other phases simultaneously to obtain a composite cathode material.Jie et al.proposed a new strategy of using K2NiF4type lamellar La0.5Sr1.5MnO4+δ(LSMO4)as the conductive phase of LSM based cathode ions, and obtained LSMO4-LSM powder by one-step in situ co-assembly and calcined at 1000°C for 3 h [85].The composite cathodes made by in-situ composite not only simplifies the process of compounding, but also improves the performance of the cathodes to a certain extent,which provides a new idea and strategy for the development of composite cathodes.
In conclusion, in-situ composite jumps from the preparation of raw materials to the synthesis of composite cathodes in one step, which breaks the barrier of first preparation and then composite,and avoids the influence brought by the preparation of cathode materials.This one-step composite method is more direct, reduces the incorporation of impurities, and further improves the performance of the composite cathode.In-situ composite have higher the requirement of technology and materials,with a certain contingency.Therefore,the research of its composite technology is extremely promising direction.
5.Other composite structure cathodes
We classify the methods other than the three composite methods in the article as other composites.It improves the performance of composite cathodes ordinarily by combining modern technology pretreatment,improving traditional composite methods or by combining multiple composite methods.As shown in Table 7, through new composite methods, not only can the performance of traditional cathode materials be improved, but also provide a new strategy for the development of composite cathode materials.
Zhu et al.have developed a novel heterostructure cathode with silver nanoparticles decorated on an active perovskite backbone, which was fabricated using a single-step exsolving process, with achieving a very low area specific resistance of 0.214 Ω cm2at 500°C[92].Compared to infiltration, this method allows for more robust decoration of silver nanoparticles on an active perovskite backbone with higher activity and lower expense.Shao et al.reports a method to prepare one-dimensional nanostructured CuCo2O4-Sm0.2Ce0.8O1.9nanofiber composite cathode materials by electrospinning and one-step sintering, and the electrochemical properties of CuCo2O4-Sm0.2Ce0.8O1.9showed significant improvement,reaching a Rpof 0.061 Ω cm2and a MPD of 976 mW cm-2at 750°C [93].The combination of electrostatic spinning and one-step sintering puts the CuCo2O4-Sm0.2Ce0.8O1.9in nano-sized fibers and forms a porous structure,which enhances the ORR activity of the cathode and reduces its Rp.This is a combination and innovation of traditional methods.Francisco et al.synthesized nanoscale La0.6Sr0.4Co0.2-Fe0.8O3-δ-Ce0.8Sm0.2O1.9composite cathode materials by one-step microwave self-assisted combustion.As shown in the data in Table 7, the composite cathode La0.6Sr0.4Co0.2Fe0.8O3-δ-Ce0.8Sm0.2O1.9prepared by the one-step microwave combustion route exhibited a lower overall Rpcompared to similar cathodes produced by other synthetic routes reported in the literature[56].From the above analysis,the new composite methods are not only the innovation of modern technology,but also the combination and innovation of traditional methods.Through the characteristics of the material, combining the existing technology with the traditional composite method to develop excellent composite cathode is the future development direction and challenge of the composite cathode industry.
To conclusion,other composite methods have excellent development potential in the future and are also crucial ways to enhance the performance of traditional cathode materials, develop new materials and optimize traditional composite methods.
6.Conclusions and future prospective
SOFC is very promising in meeting the growing demand for renewable and clean energy sources and in solving problems related to the energy crisis and environmental issues.To commercialize SOFC, the operating temperature must be reduced, which has considerable technical and economic benefits.The development of medium and low temperature SOFC (IT-SOFC) is dependent on various aspects such as environment, energy and resources, and the research of composite cathode materials is a key step in the development of IT-SOFC.In this context, high-performance composite cathodes prepared by various composite methods have become an effective way for this goal.Therefore,a comprehensive review of the development of composite cathodes is presented.The preparation of higher performance composite cathodes by multiple composite methods, as an example mechanical physical composite,impregnation composite,in-situ composite,etc.;and multiple types of composite cathodes, such as perovskite composite cathodes,spinel composite cathodes,metal oxide composite cathodes,etc.;as well as carefully selected and unique examples from the literature are described in detail.
Composite cathodes prepared by mechanical-physical composite,which combine the advantages of two-phase cathode materials and weaken the deficiencies of single-phase cathodes.By simple mechanical mixing, two materials with different structures and properties can be compounded to obtain higher performance composites.However, to prepare high performance composite cathodes by mechanical mixing, a few points need to be considered:
(1) The sintering temperature and time.It determines the phase
structure of the composite cathode.
(2) Whether the mixing is sufficient.The mechanical-physical composite is the secondary particle mixing, the insufficient mixing always leads to particle agglomeration and form uneven phase formation, affecting the performance of the cathode.
(3) The stability of the cathode.Generally, the activity and stability are trade-offs for high performance cathodes.The higher activity for cathode, the more unstable it is.Cathode stability can be improved greatly through high temperature sintering and other methods.(4) The difference in thermal expansion coefficient.It leads to negative cathode/electrolyte interface structure.The thermal expansion coefficient of composite cathode can be reduced by the mechanical mixing of cathode material and electrolyte, thus forming better cathode/electrolyte interface structure.

Fig.8.Schematic diagram model of the process of in-situ composite.

Table 6 Performance of composite cathode materials made by in-situ composite and some single-phase cathodes in recent years.
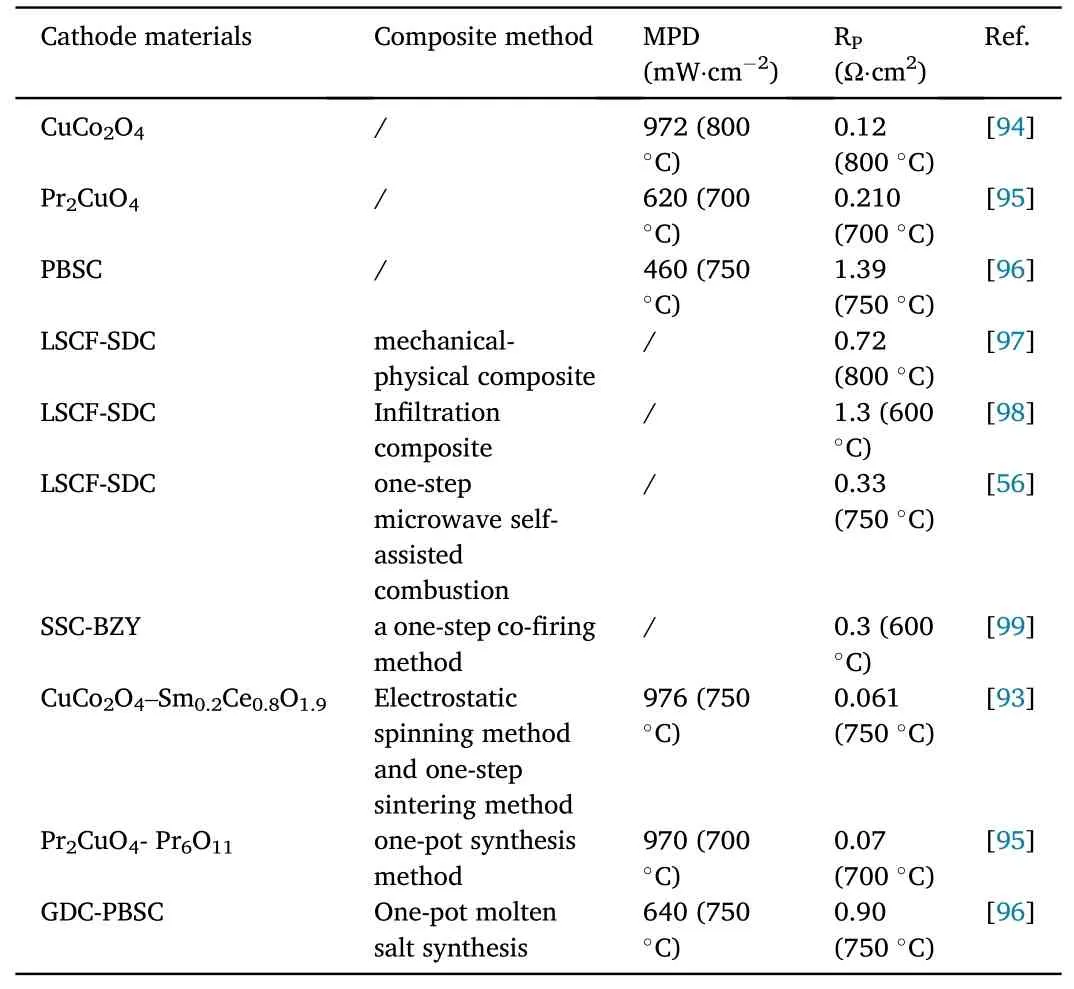
Table 7 Performance of composite cathodes made by other composites compared with single-phase cathodes and cathodes made by conventional composites.
Therefore, as the most traditional type of composite, mechanicalphysical composite must optimize the process of preparation and incorporate modern technology to further develop more excellent composite cathodes.
The preparation of composite cathodes by means of impregnation composite can lead to composite cathodes with better compatibility and more stable performance.The impregnation can make the other-phase materials better attached to the electrolyte skeleton or cathode framework, which can effectively improve the specific surface area of the cathode skeleton, extend the length of the three-phase interface, and increase the number of reaction activation sites, thus enhancing the electrochemical performance of the cathode materials.However, the impregnation technique still faces some challenges in preparing high performance composite cathodes:
(1) Impregnation number of times and temperature.It is an essential factor that affecting the activity of impregnated particles and the degree of impregnation uniformity.Proper number of times and temperature can effectively improve the performance of composite cathodes.
(2) Chemical stability between the impregnated particles and the skeleton.At high temperatures,the chemical reaction between the impregnated particles and the skeleton greatly affects the cathode performance.Therefore,it is extremely significant in the selection of materials.
(3) High expense of technology.The impregnation technology is a high-cost composite method, how to reduce the cost is a major challenge.
(4) Environmental issues.The chemical pollution generated by chemical impregnation is more serious, so environmentally friendly composite method is a must.
As technology advances and develops,new impregnation methods or methods like impregnation are emerging, but the challenges remain,which need to be addressed in the future.
The use of in-situ composite is somewhat limited compared to the other two types of composites.Because of the special conditionality of insitu generation,the method is not particularly widely used.However,due to the in-situ nature of this method,it is possible to skip the preparation of cathode materials from the preparation of raw materials to the synthesis of composite cathodes, which breaks the barrier of preparation before compounding and avoids the impact in the preparation of cathode materials.In-situ composite provides a new way of thinking and strategy for composite cathodes and is a promising composite method.Difficulties in in-situ composite technology are both challenges and opportunities.
In addition,not only the composites,but also the morphology of the composite cathode affects its electrochemical properties.The morphology of composite cathodes prepared by different composite methods are various.Simple mechanical mixing is the mixing of secondary particles with no special situation, and mixing uniformity needs to be considered mostly.Impregnation composite is the composite between the particles and the skeleton.The porosity of the composite cathode is affected by the skeleton and impregnation volume, thus affecting its performance.In-situ composite is a composite on the scale of crystal structure.It may form heterogeneous junction affecting the properties of composite cathode.Therefore, the morphology of the composite cathode also needs to be concerned in preparing highperformance composites apart from the steps and processes of the composite method.By controlling the composite time, temperature and the proportion of materials,the morphology and structure of the composites are controlled.
In summary, this paper presents the progress and potential of composite cathode materials and composite methods for preparing composite cathodes,a scientific understanding of current SOFC technology and for fuel cell processes, and points to opportunities and directions for continued efforts to develop potentially fruitful pathways and breakthrough areas.Nevertheless,our investigation in this direction is still in its infancy.Before composite cathode materials are applied to SOFC,we should consider multiple challenges, such as composite methods for preparing composite cathodes to scale up, cost effectiveness, mass production processes and stability aspects.
Finally,this paper focuses on the development of different composite methods to prepare high-performance composite cathodes, which can effectively improve the application and development of cathode materials in the field of SOFC.In the future development of SOFC, the development of composite cathodes is expected to improve the efficiency and electrochemical performance of SOFC as well as expand the application scale of SOFC, which eventually brings about environmentally friendly and sustainable development.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors wish to acknowledge the financial supports from National Natural Science Foundation of China, No.52204368 and Fundamental Research Funds for Central Universities of the China University of Geosciences Beijing,No.590121038.Moreover,we would also thank the project supported by the College Student Innovation and Entrepreneurship Training Program of the China University of Geosciences Beijing,No.202211415025.
杂志排行
Progress in Natural Science:Materials International的其它文章
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
- The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
- Towards high-efficiency of hydrogen purification in metal hydride
- Research advances on electrode materials for solid oxide electrolysis cells