Polyvinyl alcohol-potassium iodide gel probe to monitor the distribution of reactive species generation around atmosphericpressure plasma jet
2023-03-15TrungNguyenTRANMinHUTomohiroOGASAWARAYukiIWATAHarukaSUZUKIJinSAKAMOTOMasafumiAKIYOSHIHirotakaTOYODAandHirotoMATSUURA
Trung Nguyen TRAN, Min HU, Tomohiro OGASAWARA, Yuki IWATA,Haruka SUZUKI, Jin SAKAMOTO, Masafumi AKIYOSHI,Hirotaka TOYODA and Hiroto MATSUURA
1 Department of Quantum and Radiation Engineering,Osaka Prefecture University,Osaka 599-8570,Japan
2 Department of Electronics, Nagoya University, Nagoya 464-8603, Japan
3Faculty of Chemistry, Materials and Bioengineering, Kansai University, Osaka 564-8680, Japan
4 Department of Quantum and Radiation Engineering, Osaka Metropolitan University, Osaka 599-8570,Japan
Abstract This study proposes polyvinyl alcohol-potassium iodide (PVA-KI) as a novel gel chemical probe.The probe uses the reactions among PVA, KI, water, borax, and oxidative species to visualize the distribution of reactive species.This method provides information regarding the distribution of reactive species by coloration on the gel surface.The effects of the surrounding gas phase on the distribution and diffusion of the reactive species are also investigated using the PVA-KI gel probe.Further,the relationship between the irradiation distance and reactive species diffusion is determined on the surface of the PVA-KI probe with and without plastic shielding.Adjusting the irradiation distance appropriately leads to an increase in the modified area as detected by the PVA-KI gel probe analysis.The relative concentration distributions of the reactive species are also obtained from visualized color distributions measured using a colorimeter.Furthermore,reactive species generation by long-scale line plasma is confirmed by the color reaction on the PVA-KI gel surface, with a greater area being covered by an atmospheric-pressure pulsed microwave line plasma source.
Keywords: atmospheric-pressure plasma jet (APPJ), PVA-KI gel, chemical probe, argon plasma jets
1.Introduction
Atmospheric pressure plasma jets (APPJs) have exhibited great potential for a wide variety of applications, ranging from the treatment of materials [1-3] to agricultural applications [4-6]and plasma medicine [7-10].These APPJs operating in argon(Ar),or helium gas are capable of generating plasma-containing electrons, photons, ions, and a complex mixture of reactive species in ambient air [11].Reactive species transported to the target along the gas flow also play an important role in many practical fields [12-16].Multiple different APPJ designs [17]have been reported with various electrode configurations,plasma jet length ranging from small-scale jets [18] to 50 mm [19].
Atmospheric-pressure plasma jets have numerous advantages as a tool for supplying excited particles, such as reactive oxygen (ROS) and nitrogen species, to the target.There have been many related reports on the gas- and plasma-phase chemistry,and several processes are already understood[20,21].Researchers have analyzed the electron and reactive species densities diffusing in the gas phase using several measurement methods, such as laser-induced fluorescence [22, 23], optical emission spectroscopy [24], Fourier transform infrared spectroscopy [25], and Thomson scattering [26].
However, in plasma jet treatment, it is very difficult to control the concentration, composition, and distribution of reactive species to the target surface owing to issues when operating the plasma source under atmospheric conditions.This is partly a result of the complex mixture of reactive species produced by APPJs.Furthermore, APPJs have the disadvantage of being limited in the treatment of large surfaces.To overcome this challenge, understanding the region of reactive species distribution,diffusion on the target surface,and reactant composition is crucial.
An extensive study on reactive species and transport was previously conducted using the potassium iodide (KI)-starch method [27-29].The KI-starch method enables easy visualization of the concentration distribution of reactive species on the target, even in water.Recently, in radiation chemistry and radiation measurement, polyvinyl alcohol (PVA)-KI has been gathering attention as a dosemeter to visualize radiation distribution based on its color-change reaction [30-32].Sunagawa et al reported the red colorization of PVA-KI complexes in applied x-ray and proton beam experiments [33].Generally,PVA-KI has a complex reaction mechanism[34,35].Up to this point, it has not been applied in atmospheric plasma research.
In a previous paper, this research group reported the development and biomedical application of PVA-KI liquid as a novel chemical probe to study free radicals[36].The PVAKI color reaction depends on the sample conditions, particularly the temperature.Although the detailed mechanism requires further study, an experimental protocol for applying PVA-KI was established in the paper.In particular, liquidtype PVA-KI was used to observe the liquid flow induced by plasma irradiation.Then,the absorbance was measured using an ultraviolet-visible (UV-vis) spectrometer to obtain the relative concentration distributions of the reactive species from the obtained color reactions.At the same time, the gel form of PVA-KI appears to be well suited to detecting reactive species transportation.However, it is difficult to measure the absorption spectrum of gel-type PVA-KI with a UV-vis spectrometer.Therefore,this study uses a colorimeter to determine the color distribution on the surfaces of the PVA-KI gel samples after plasma irradiation.
In this work, a PVA-KI gel reaction is proposed as a novel chemical probe to detect and visualize plasma-generated reactive species transportation to the target surface.Color-change reactions are generally induced where the PVA-KI gel surface comes into contact with the reactive species, and many irradiation conditions have been reported to affect the PVA-KI color-changing area.This study focuses on the impact of a plasma jet and the surrounding gas-phase area on the PVA-KI gel surface with different irradiation conditions.Plastic shielding is also used to prevent exposure between the plasma jet and the gel sample as well as to evaluate the effects of reactive species diffusion resulting from the gas flow to the target surface.This paper also reports the effects of irradiation distance on the ability of the jet to supply reactive species over a wide range.The ability to achieve color-changing propagation of the PVA-KI gel after contact with the reactive species is also investigated.The relative concentrations and distributions of reactive species are obtained by measuring the different color spaces using a colorimeter, as the color of the PVA-KI gel turns from translucent to red and dark red when impacted by the plasma jet.
In this work,the reactive species distribution of a long-scale line microwave plasma source is easily detected using the PVAKI gel probe.At the same time, the area of reactive species distribution varies depending on the irradiation distance.It is expected that PVA-KI gel will be useful as a new chemical probe standard in low-temperature plasma applications.
2.Experimental setup
Figure 1 shows a schematic of the experimental setup used to perform visual measurements of the reactive species distribution on the PVA-KI gel surface during Ar plasma jet exposure with and without plastic shielding.The plasma source consisted of a glass tube, a needle power electrode,and a ring ground electrode.The glass tube had a length of 180 mm,an outlet nozzle diameter of 1 mm,and an inner tube diameter of 8 mm.The needle power electrode was a tungsten wire with a length of 200 mm and a diameter of 0.2 mm.A glass tube was wrapped with 5 mm wide copper tape as a ground electrode.The copper tape has a thickness of 0.5 mm.The Ar gas was supplied through the glass tube and controlled constantly by a mass flow meter (Kofloc, Model RK1710).
Electric power was supplied to the power needle electrode by a commercial power source (LHV-13AC, Loggy Electronics Ltd, Japan).The input power supply was connected to a transformer (12 A, Variac, 110 V) with a voltage range of 0-15 kV.A high-voltage probe(10 ft cable,75 MHz,Tektronix P6015A) was used to measure the voltage across the electrodes.A digital oscilloscope (Tektronix, TDS 2014)was used to record the output signals.
The experiment was designed to analyze the roles of various irradiation parameters,including time and distance,in reactive species distribution on the PVA-KI gel surface, as shown in figure 1(a).For the experiment on the effect of exposure time,the irradiation time was varied from 0 to 180 s with an Ar gas flow rate of 3 l min-1and a supplied voltage of 3 kV.The exposure distance between the nozzle of the glass tube and the target surface was fixed at 15 mm.To evaluate the effects of the irradiation distance on the reactive species distribution, the irradiation distance from the nozzle of the glass tube to the target surface was varied from 5 to 30 mm.The PVA-KI gel samples were irradiated for 120 s.
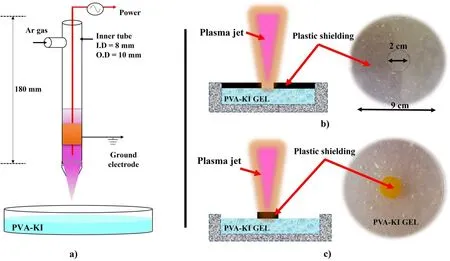
Figure 1.(a)Schematic of the experimental setup used to perform measurements of reactive species distribution on the surface of the PVAKI gel during Ar plasma jet exposure with and without plastic shielding.Schematic and photographs of the experimental setup in the case of(b) direct plasma exposure to PVA-KI gel at the central area, with plastic shielding covering the surrounding area, and (c) yellow plastic shielding used to prevent direct plasma jet exposure to the PVA-KI surface in the central area.
Figure 1(b) shows the PVA-KI gel surface covered by the plastic shielding except in its center area.The PVA-KI gel was placed in a Petri dish with a diameter of 90 mm.The plastic shielding was placed onto the gel sample to prevent the supply of reactive species to the gel surface.The shielding had a thickness of 0.3 mm and a hole with a diameter of 20 mm at its center.This experiment was performed to evaluate the supply of reactive species to the gel surface from direct plasma jet exposure and the color propagation ability of PVA-KI gel in areas covered by the plastic shielding.The PVA-KI gel surface was directly exposed to the plasma jet at its center.The plasma exposure time was varied among 60,180 and 300 s.The plasma jet was generated at a voltage supply of 3 kV with an Ar gas flow rate of 3 l min-1and an irradiation distance of 1.5 cm.
Figure 1(c) shows a schematic of the experiment setup used to confirm the reactive species supply by the gas phase surrounding the plasma jet on the gel surface.The 20 mm diameter yellow plastic shielding was placed at the center of the PVA-KI gel sample to prevent direct exposure between the plasma jet and the gel surface at a small irradiation distance.In this experiment,the plasma jet was directly exposed to the yellow plastic shielding surface.The plasma irradiation distance was varied from 10 to 20 mm,and the plasma jet was generated at a voltage supply of 3 kV with an Ar gas flow rate of 3 l min-1.The irradiation time was 120 s.
Figure 2 shows a schematic view of the atmosphericpressure microwave long-scale line plasma source.This plasma source was introduced by Toyoda et al the plasma line had a maximum length of 300 mm and a gap width of 0.12 mm based on the inner diameter of the slot.The Ar/oxygen (28/0.84 l min-1) gas mixture flowed in through a waveguide.The input microwave power was 2.45 GHz,derived from a magnetron with a peak power of 3.5 kW, a pulse frequency of 15 kHz,and a duty cycle of 30%[37-39].The Petri dish containing PVA-KI gel was placed in a slot below the plasma source.
According to Maria Tatar, Perrault s Puss in Boots has been seen as a creature of his time, a cat who models the kind of behavior required to succeed in grand society under Louis XIV in seventeenth-century France (Tatar 2002, 235).Return to place in story.
The PVA-KI gel used to study the concentration distribution of reactive species was prepared in a Petri dish with a diameter of 90 mm.The reactive species generated by the plasma source were transported to the PVA-KI gel surface.Finally, color reactions were induced on the PVA-KI gel surface via chemical reactions with the transported reactive species.The PVA-KI gel contained PVA, borax, KI, and water.The KI and borax were commercially supplied by Fujifilm Wako Pure Chemical Corporation Ltd, Japan.The PVA was commercially supplied by Osaka Glue Honpo Co.,Ltd.The PVA-KI mixture was composed of 100 ml of PVA and 100 ml of distilled water mixed with 25 g of KI and 10 g of borax.The mixture was heated using a microwave for 15 s three times.Then, 400 ml of distilled water was added to 20 ml of the PVA mixture.Finally,water was added to obtain a total mixture volume of 500 ml via stirring.
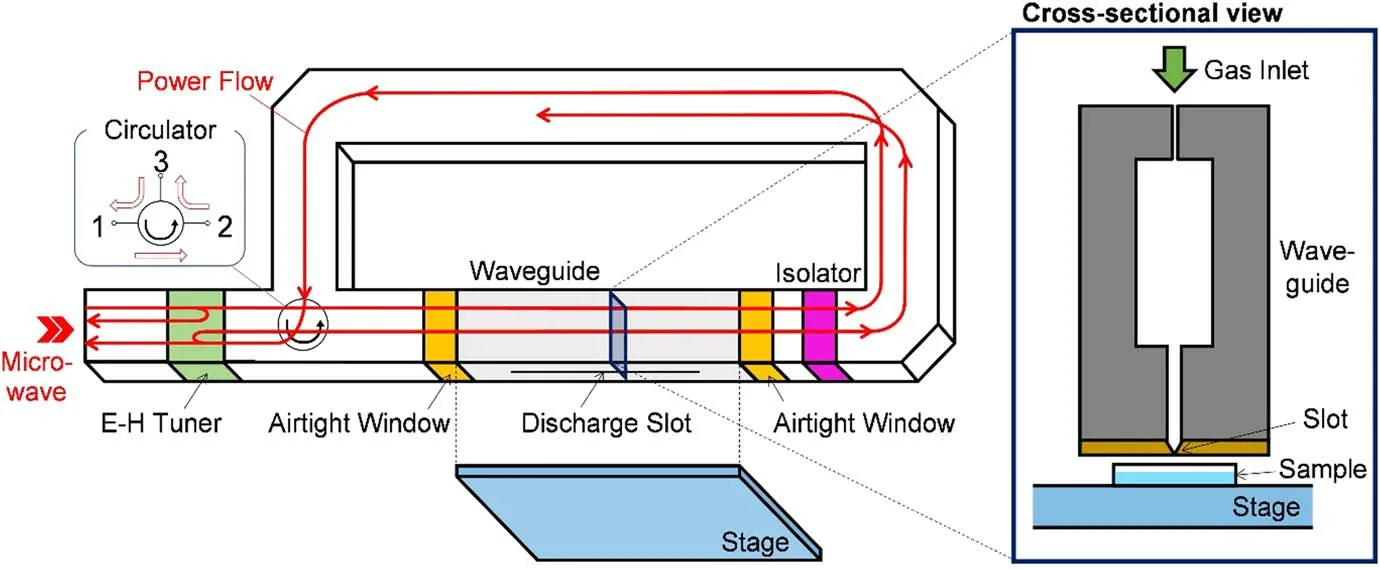
Figure 2.The long-scale line microwave plasma apparatus with exhaust from a slot (length: 30 cm, gap width: 0.12 mm).
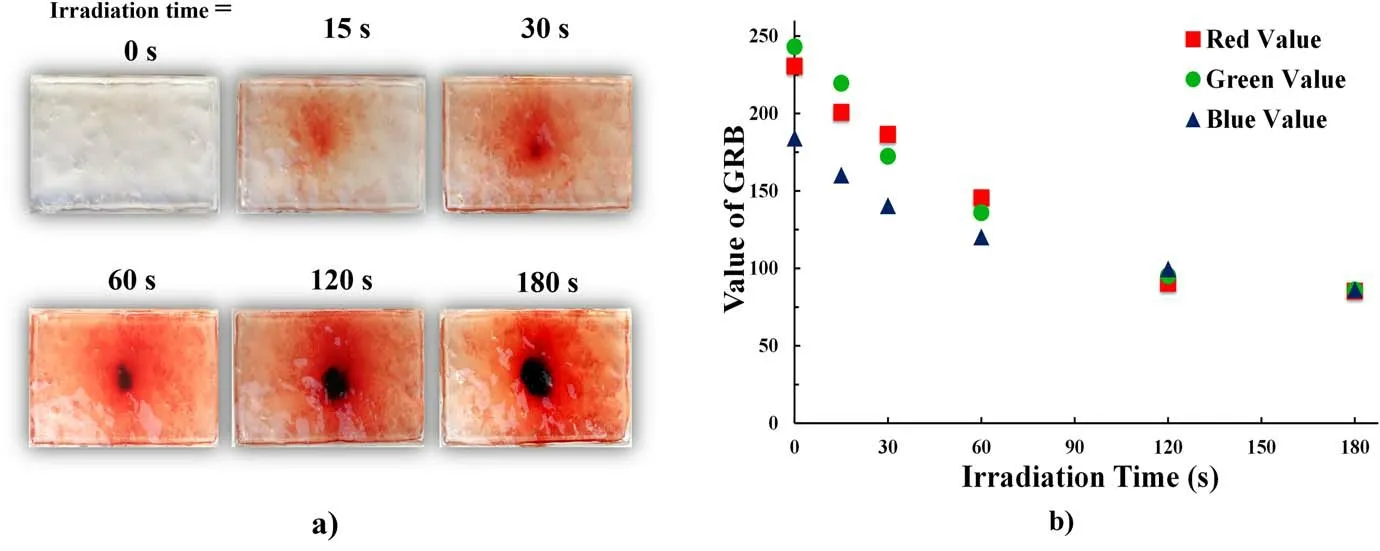
Figure 3.(a)Effects of irradiation time on the reactive species distribution,as visualized by the PVA-KI gel.(b)Relative concentrations of the reactive species corresponding to the RGB values at the irradiation point obtained using the colorimeter.
The appearance of radical production as judged by PVAKI gel color can be used to determine the presence and number of reactive species.The color-change area on the PVA-KI gel surface was analyzed using an extremely compact tristimulus colorimeter (TES-135A) to determine the color difference on the target surface.The values of red,green, and blue (RGB) needed to form any color is called its tristimulus value.The meter was designed to measure the color of non-luminescent and non-fluorescent samples.Combining RGB light is the standard method of producing color images.The color can be expressed as an RGB triplet(R, G, B), with each component able to vary from 0 to a defined maximum value of 255.If all the components are 0,the result is black; if all are 255, the result is the brightest representable white.The higher the RGB values, the lighter the color.The lower the RGB values, the darker the color.
3.Results and discussion
3.1.Plasma jet: effects of irradiation time and distance on polyvinyl alcohol-potassium iodide gel surface
In this study, Ar gas was used to generate the APPJ.Various reactive species were generated in the gas-phase region owing to ambient air mixing into the Ar plasma jet.These reactive species generated in the gas-phase region were the major agent responsible for changing the color of the PVA-KI gel.Figure 3(a) shows the distribution of reactive species on the PVA-KI gel surface, represented by the red and dark red regions in the photographs.The reactive species distribution was obtained by direct plasma exposure to the gel surface with different irradiation times.The PVA-KI gel sample irradiation times were 0, 15, 30, 60, 120 and 180 s, with a supplied voltage of 3 kV and an Ar gas flow rate of 3 l min-1.The distance from the outlet to the surface of the PVA-KI gel was 15 mm.The plasma jet was directly exposed to the PVAKI gel surface.Based on the results shown in figure 3(a), the color-change area expanded when increasing the plasma treatment time.
The PVA-KI surface gradually turned red and dark red at the irradiation point and light red in the surrounding area.The reactive species distribution area also expanded with the irradiation time.The relationship between the reactive species distribution and the irradiation time could be easily clarified using the PVA-KI gel probe.
Figure 3(b) shows the relationship between the RGB values at the irradiation point and the irradiation time.The RGB values were measured in the central area of the PVA-KI gel samples.In an RGB value analysis,the color will become lighter when the RGB values increase.Similarly, decreasing the RGB values will lead to a darker color.In the case of the unirradiated PVA-KI gel sample,the R,G,and B values were 230.5, 243 and 184.1, respectively.Among the irradiated samples, the minimum RGB values (65.04, 65.92, 64.79,respectively) were recorded in the dark red color area at an irradiation time of 180 s.As these results demonstrate, the RGB values decreased as the plasma treatment time increased,and the color of the PVA-KI gel gradually turned a darker red.The difference in color corresponded to different concentrations of reactive species distributed on the PVA-KI gel surface.Overall, the results indicated that the concentration distribution of the reactive species could be increased by increasing the plasma treatment time.Figures 4(a) and (b)show the plasma jet irradiation to the PVA-KI gel surface with and without direct contact, respectively.Figure 4(c)shows photos of the PVA-KI gel samples taken immediately after plasma treatment with different distances between the nozzle and the sample surface(5,10,15,20,25 and 30 mm).The red and dark red regions correspond to the treated areas reached by reactive species on the PVA-KI gel surface.The PVA-KI gel samples were set underneath the plasma jet in a perpendicular position and treated for 120 s with an Ar gas flow rate of 3.0 l min-1and a supplied voltage of 3 kV.The plasma jets were fully in contact with the PVA-KI surface(figure 4(a))at the irradiation distances from 5 to 20 mm.The plasma jet did not contact the PVA-KI surface(figure 4(b))at the irradiation distance of 25 and 30 mm.The color modification on the PVA-KI gel surfaces was observed accordingly.Reactive species were directly supplied from the plasma jet and the gas-phase region surrounding the jet.In general, the reactive species were able to reach the target surface faster with shorter distances.It can be seen that in almost all cases, the color-change area covered nearly the entire PVA-KI gel surface(except the case with an irradiation distance of 5 mm).At the center area where the plasma jet was in close contact with the PVA-KI gel surface, an indentation and change to a dark red color appeared.The color-change area then spread from the center radially outwards.It is important to note that the PVA-KI gel has a very soft and damp surface.Therefore, the Ar gas flow deformed the surface, creating an indentation at a shorter irradiation distance.Then,it was able to perturb the admixture of Ar gas and ambient air and spread reactive species.Figure 4(c)shows that in all cases, the PVA-KI gel surface was divided into two areas:a central area with a strong dark red color and a surrounding area with a lighter red color.
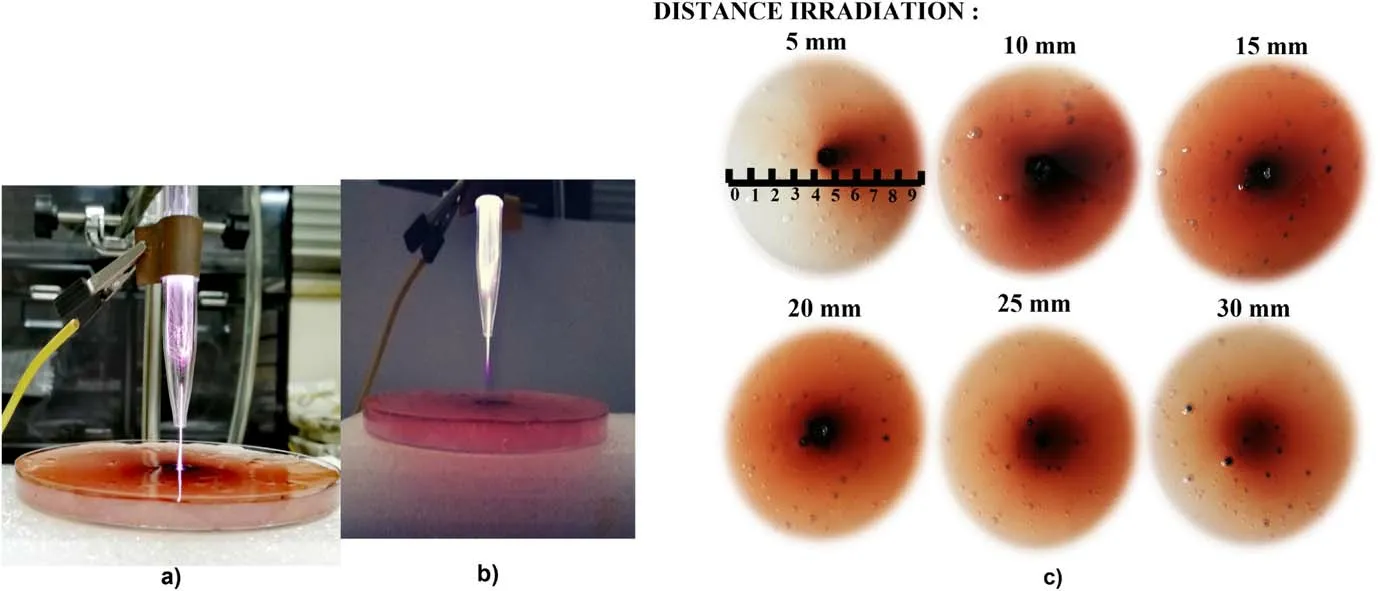
Figure 4.(a) Plasma jet directly contact with PVA-KI gel surface.(b) The plasma jet did not contact the PVA-KI surface.(c) Effect of irradiation distance on the reactive species distribution, as visualized by the PVA-KI gel.
For the visualization at a distance of 5 mm (figure 4(c)),the indentation appeared to be larger and deeper at the irradiation point relative to the other cases.The dark red color area was also restricted to the central area with direct exposure to the plasma jet, and the red color in the surrounding area gradually became weaker.Further, the PVA-KI gel sample turned red in the right-hand half of the sample plate relative to the irradiation point.A portion of the radical products was diffused outside along the gas stream owing to the short irradiation distance.Therefore, the other half of the PVA-KI gel sample was almost unchanged in color.
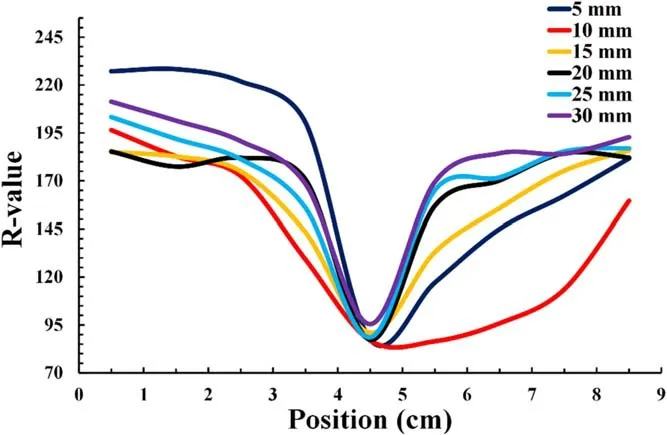
Figure 5.Reactive species concentration distributions corresponding to R-values at different irradiation distances along the ruler.
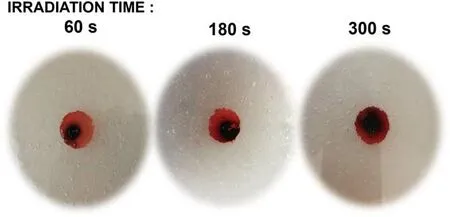
Figure 6.Observation of the color conversion and spreading reaction of PVA-KI gel using plastic shielding at different irradiation times.
An increase in the affected area was observed when increasing the irradiation distance for the tested distances between 5 and 15 mm.Larger reaction color areas were expected when the reactive species could easily spread on the surface.For the distances from 20 to 30 mm, the affected areas were larger than those at 5 mm but smaller than those at 10 and 15 mm owing to the lack of contact between the plasma jet and the surface.Depending on the irradiation distance and gas flow rates, the reactive species appeared to spread further away from the plasma plume, especially for longer distances.The spreading area of radical species increased when the plasma jet was placed further away from the surface within the range of 5-15 mm.In figure 4(c), at 30 mm irradiation distance, the chemical probe detected reactive species which are probably long-lived species such as H2O2.As for the 10 mm case,the effect of short-lived species might be significant.
The R-values of the color reaction areas were measured to evaluate the relative concentration distributions of the reactive species supplied to the PVA-KI gel surface.Figure 5 shows the concentration distributions of the reactive species along the ruler shown in figure 4(c).A high reactive species concentration was obtained at the center area and right-hand half of the pattern, corresponding to areas with lower Rvalues.In all cases,the highest concentration was obtained at the center area, where the exposure distance between the plasma jet and PVA-KI gel surface was the smallest.The maximum concentration distribution of reactive species was obtained at a distance of 10 mm.The concentrations of the reactive species obtained at distances of 15,20,25 and 30 mm were smaller than those at 10 mm, though the distribution areas were similar.These results reveal that the optimal distance for the treatment target in this study was 10 mm.The results also indicate that the concentration distributions of the reactive species can be controlled by irradiation distance.
3.2.Plastic shielding: effective treatment area
To investigate the color conversion and spreading reaction of the PVA-KI gel in detail, plastic shielding with a hole at its center was utilized to cover the surface of the PVA-KI gel surrounding the center area.The PVA-KI gel was thus directly exposed to the plasma jet at the center area.
The effect of the reactive species distribution exposure on the PVA-KI gel sample (plasma jet exposure) at the irradiation point was studied at different times.Figure 6 shows the reactive species distribution after plasma irradiation with a supplied voltage of 3 kV, an Ar gas flow rates of 3 l min-1,and an irradiation distance of 15 mm, appearing as red-black color regions in the PVA-KI gel photographs.The irradiation times were 60, 180 and 300 s.At the irradiation point, the red-black color area, which corresponded to the reactive species distribution area,increased in the radial direction with the increase of irradiation time (figure 6).This indicates that the reactive species were distributed in the radial direction from the center area by the gas flow along the PVA-KI gel surface with an increase in the irradiation time.As can be seen in the photographs,the gel surfaces were indented because of the force of the gas stream, which increased as the gas exposure time increased.The indentation diameter was also expanded over time when the irradiation time increased.
In the regions where the target surface was covered by plastic shielding,the color of the PVA-KI gel did not change owing to the inability of the reactive species to reach the gel surface.The plastic shielding clearly prevented the plasma jet exposure from supplying reactive species to the PVA-KI gel surface.The visualized reactive species-supplied area at the surface corresponded exactly to the hole without plastic shielding at the center.The reactive species were only supplied to the PVA-KI gel surface in the area without plastic shielding.In addition,no propagation reaction occurred inside the PVA-KI gel after the surface changed color.
The corresponding relative concentrations of the reactive species were obtained from the R-value measurements at the center along the horizontal from 2.3 to 6.3 cm, as shown in figure 7.The maximum reactive species concentration value corresponded to the minimum R-value obtained using the colorimeter.This result indicates that the reactive species were mostly concentrated at the irradiation point.Figure 7 also shows the position of the minimum R-value expanding with irradiation time in the central area.The lowest R-value position expanded from 3 to 9 mm with irradiation times ranging from 60 to 300 s.The lowest R-value position corresponded to the dark red area and the width of the indentation appearing on the PVA-KI gel surface.
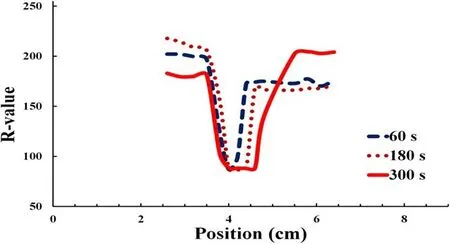
Figure 7.Reactive species concentration distributions corresponding to R-values along the horizontal shown in figure 6.
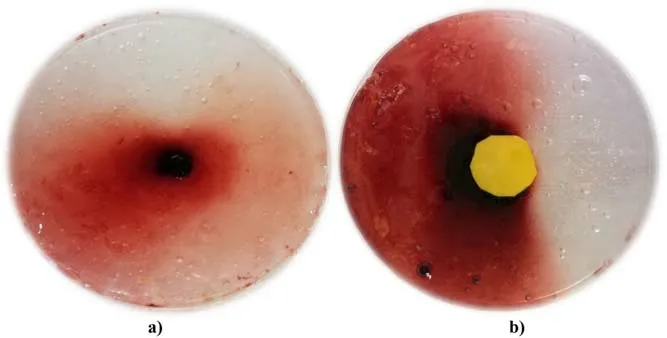
Figure 8.Comparison of reactive species distribution on the PVAKI gel without (a) and with (b) yellow plastic shielding.
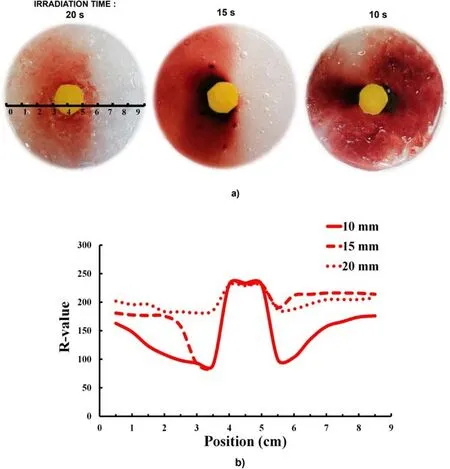
Figure 9.(a) Effects of irradiation distance on the reactive species distribution using yellow plastic shielding, as visualized by the PVA-KI gel.(b) Relative concentrations of the reactive species obtained by the colorimeter.
To obtain a better understanding of the effect of the gas flow on the reactive species delivery and the differences in reactive species transportation between direct and indirect plasma contact with the PVA-KI gel surface at small irradiation distances, plastic shielding was used to prevent direct plasma jet-gel contact.Figure 8 shows a comparison of the reactive species distribution on the PVA-KI gel with and without plastic shielding.In this experiment, the plasma jet was generated at a voltage supply of 3 kV with an Ar gas flow rate of 3 l min-1.The distance from the nozzle of the plasma source to the PVA-KI gel surface was fixed at 15 mm.The plasma jet was directly exposed to the surface of the plastic shielding or the PVA-KI gel.Figure 8(a) shows a visualization of the reactive species distribution resulting from direct plasma exposure to the PVA-KI gel surface.Here, a black indentation with a diameter of 5 mm was observed at the irradiation point.The areas around the irradiation point changed to a light red color, indicating that the reactive species distribution was concentrated in the area where the plasma jet was directly exposed to the PVA-KI gel sample.The surrounding area then turned a light red color because of reactive species transported by the gas phase.To understand the role of the gas phase around the plasma jet in the reactive species transportation, a 20 mm diameter plastic sheet was placed on the surface of the PVA-KI gel.Figure 8(b) shows the yellow plastic shielding placed at the center of the PVAKI gel sample to prevent direct contact between the plasma and the target surface.Based on the results, the color of the surrounding area turned darker than in the case without the plastic shield.The plastic shield thus enhanced the gas flow diffusion, thereby allowing the reactive species to spread further into the surrounding areas.
The effect of reactive species distribution exposure on the PVA-KI gel sample (without plasma jet exposure) using plastic shielding was studied at various distances.Figure 9(a)shows the distribution of reactive species surrounding the plastic shielding area after 2 min of plasma irradiation.In this experiment, a plasma jet was generated using a 3 kV voltage supply and an Ar gas flow rate of 3 l min-1, and the plasma jet was directly exposed to the plastic shielding.The colorimeter was used to analyze the different colors distributed along the horizontal axis at the center of the Petri dish.According to the results, the PVA-KI gel color turned light red on the left-hand side of the dish.However, for the experiment, the plasma jet was not exactly perpendicular to the surface of PVA-KI gel; instead, the angle between the surface of the PVA-KI gel and the plasma jet was slightly inclined.Therefore, the reactive species distribution was almost entirely concentrated on the left-hand side of the sample area.The color of the PVA-KI gel was light red at 20 mm but turned dark red as the irradiation distance decreased owing to the strong gas diffusion.The treated area of the PVA-KI gel was extensible to the entire PVA-KI gel surface when the irradiation distance was reduced from 20 to 10 mm.At a 10 mm irradiation distance, black coloring was also observed on the left- and right-hand sides of the plastic shield.This indicated that the gas flow carrying reactive species diffused to both sides of the shield.
Figure 9(b)shows the R-values along the horizontal axis at the center of the PVA-KI gel samples.The R-values were the highest at the center,corresponding to the area covered by the yellow plastic shielding.This area did not change color because the reactive species did not reach the surface of the PVA-KI gel.The R-values decreased at the left- and righthand sides of the central area, corresponding to the colorchange areas.On the right-hand side of the sample, the reactive species were not transported by the distribution of the gas flow either.Thus, the gas flow played a highly important role in the distribution of reactive species to the target,changing the concentration of ROS and the effective range.Altering the inclination angle also changed the distribution of gas flow and affected the effective range of the ROS, despite Ar gas being heavier than air,though the reason for this is not understood.
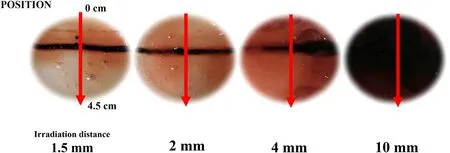
Figure 10.Effects of irradiation distance on the reactive species distribution using an atmospheric-pressure pulsed microwave line plasma source, as visualized by the PVA-KI gel.
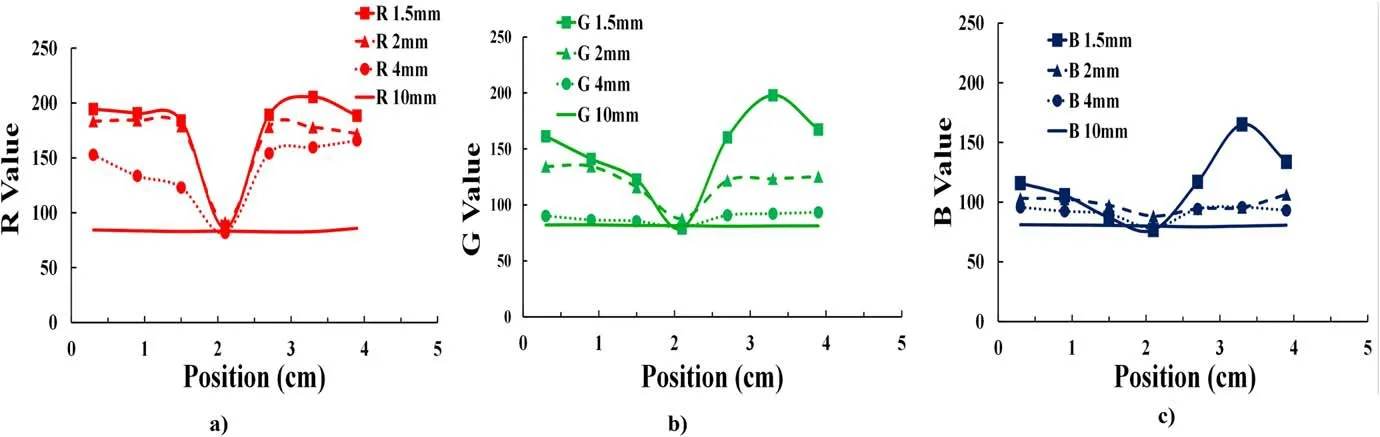
Figure 11.Relative concentrations of the reactive species obtained using the colorimeter,corresponding to the RGB values along the arrow shown in figure 10 (length=4.5 mm).
3.3.Long-scale line plasma: reactive species distribution
An atmospheric-pressure pulsed microwave line plasma source is being developed for the large-area treatment of surfaces in plasma medicine and surface modification applications.Further, a long-scale line Ar plasma source using one-directional microwave power flow through a waveguide was proposed by Toyoda et al.This concept is highly effective for generating plasma over large surface areas,and it is applicable to the production of atmospheric-pressure plasma using rare-gas discharges in the meter-scale slot.Thus,this research group suggested visualizing the reactive species generated by the atmospheric-pressure pulsed microwave line plasma source using the PVA-KI gel probe through a cooperation program between our two research groups.
In this research,PVA-KI gel was applied to visualize the reactive species transportation and test the expanded radical distribution.Figure 10 shows the distribution of the reactive species on the surface of the PVA-KI gel after Ar/O2plasma treatment.The irradiation time was fixed at 30 s.The irradiation distance was varied from 1.5 to 10 mm.This change in the plasma irradiation conditions led to changes in the region of reactive species distribution.When the distance between the slot and the PVA-KI surface was 1.5 mm,a dark red line was observed on the surface of the PVA-KI gel after plasma irradiation.The dark red line corresponded to the plasma slot longitudinal.The uniformity of reactive species generation distribution in the longitudinal direction was confirmed by the PVA-KI gel probe.As the irradiation distance was increased from 1.5 to 10 mm, the dark red line gradually disappeared, and the color-change area expanded over the entire surface of the PVA-KI gel.The surrounding color-change area also turned from light to dark red when increasing the irradiation distance from 1.5 to 10 mm.Relatively low-concentration areas were observed in the region surrounding the dark red line, represented by the light red coloring.The distribution of the reactive species could thus be expanded to a large area by appropriately increasing the irradiation distance.
Figure 11 shows the relative concentration distributions of the reactive species obtained by the RGB value measurement along the arrow shown in figure 10(length=4.5 mm).The minimum RGB value was around 70 for all irradiation distances,corresponding to the dark red color.At irradiation distances of 1.5, 2 and 4 mm, the minimum RGB value was obtained at the position of 2 mm,representing a dark red area.At a 10 mm irradiation distance, the RGB values exhibited an almost uniform color change at all measurement positions along the arrow.Under the same irradiation condition as shown in figure 10,O atom density is measured below a slot by vacuum ultraviolet absorption spectroscopy (VUVAS) [40].O atom density is 1014cm-3at 10 mm irradiation distance.The VUVAS method will be continuously used to compare with the PVA-KI gel probe in the near future.
4.Conclusion
The color change of PVA-KI gel samples after plasma jet treatment was investigated under different conditions to detect the reactive species distribution on the surface of the PVA-KI gel.Color is one of the most important attributes allowing for easy visualization of reactive species diffusion and distribution behavior.Accordingly, the PVA-KI color measurement and analysis helped illuminate the distribution of reactive species and optimize the treatment for each application.This study identified two important alterations in the experimental setup configuration that led to a larger treatment area and enhanced treatment homogeneity.First, adjusting the irradiation distance was shown to promote the spreading of reactive species on the surface of the PVA-KI gel samples,leading to enhanced treatment for larger regions.Assessing the absolute color values of the surface also allowed for an evaluation of the values of reactive species distributed on the surface of a target.It was found that,according to the desired application, the choice of a particular irradiation time and distance can provide better results and a more efficient treatment.Second, the use of plastic shielding and the effect of different irradiation times and distances indicated that not only the plasma jet but also the gas phase surrounding it played an important role in the supply of reactive species to the target.
Acknowledgments
This work was partially supported by the ZE Research Program IAE (No.ZE2021B-27) and the joint usage/research program cLPS (No.21020).
杂志排行
Plasma Science and Technology的其它文章
- Relativistic toroidal light solitons in plasma
- Valley-dependent topological edge states in plasma photonic crystals
- Bulk moduli of two-dimensional Yukawa solids and liquids obtained from periodic compressions
- Modeling of magnetized collisional plasma sheath with nonextensive electron distribution and ionization source
- Observation of the poloidally asymmetrical density perturbation of sawtooth collapse on J-TEXT
- Alfvén continuum in the presence of a magnetic island in a cylinder configuration
