Embolization of brain arteriovenous malformations with squid co-polymer embolic material: Initial experience
2023-03-06LiShyanChngZulkifliZakiAhmadSoriMuda
Li Shyan Ch'ng, Zulkifli Zaki, Ahmad Sori Muda
a Radiology Department, Faculty of Medicine UiTM, Sungai Buloh, Malaysia
b Radiology Department, Hospital Sungai Buloh, Sungai Buloh, Malaysia
c Radiology Department, HSAAS, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Malaysia
Keywords:
ABSTRACT Objective:To analyze the safety and effectiveness of the ethylene-vinyl alcohol copolymer (EVOH)liquid embolic agent Squid (Emboflu, Switzerland) for the treatment of brain arteriovenous malformations.
1.Introduction
Endovascular embolization has traditionally been used as a supplement to surgery or gamma-knife therapy.Embolization of brain arteriovenous malformations (BAVM) using liquid embolic agents is an emerging treatment option.N-butyl cyanoacrylate and newer ethylenevinyl alcohol copolymer (EVOH) agents, such as Onyx (EV3, USA),Squid (Emboflu, Switzerland), and precipitating hydrophobic injectable liquids(PHIL)(Microvention,USA),are among the liquid embolic agents used.Onyx has gained popularity since its introduction to the market because of its slow polymerization and non-adhesive properties, which provide longer embolization times.Squid and PHIL are copolymers that have recently been used for the treatment of BAVM.Previous studies showed that Squid is less viscous than Onyx and provides better vascular penetration of the nidus.1In this report,we describe our experience using Squid for the embolization of BAVM.
2.Materials and methods
Between April 2015 and July 2017, we assessed 46 embolization procedures for BAVM performed in our facility using formulations of Squid-18 and Squid-12 in 1.5 ml vials.All embolization procedures were performed under general anesthesia, using a Siemens biplane angiography system.Patient data including age,sex,and presenting symptoms were collected.Spetzler-Martin(SM)grade,feeding artery,draining vein,and location of cerebral AVM were identified using radiological examination data.Intraprocedural information on endovascular embolization, including the type and volume of liquid embolic agents,microcatheters,and complications,was also documented.

Fig.1.Left temporoparietal AVM demonstrated on the anteroposterior (A) and lateral views (B).Left internal carotid arteriogram after embolization with Squid showing approximately 30% obliteration of the AVM on the anteroposterior (C) and lateral (D) views.
2.1.Embolization technique
The carotid and vertebral arteries were cannulated using a 6 French(Fr) guiding catheter into the common femoral artery under systemic heparinization.Selective cannulation of the target feeder artery was performed with a flow-directed microcatheter compatible with dimethyl sulfoxide (DMSO), either conventional (Magic, Balt; Ultraflow, Medtronic) or with a detachable tip microcatheter (Sonic, Balt; Apollo,Medtronic),facilitated by the physician's choice of a 0.007 or 0.008 inch microguidewire (Hybrid, Balt; Mirage, Medtronic).Once the microcatheter tip was positioned for safe injection, the microcatheter dead space was primed with DMSO.Squid vials were shaken for at least 20 min prior to use to prevent precipitation of the tantalum powder.Slow,controlled injections of Squid were administered under roadmap guidance.Slow injection was performed to avoid a sudden increase in the concentration of the solvent(DMSO),as it can irritate the soft tissue and vessel wall.Squid was aimed at maximal nidal occlusion and injected every few minutes,depending on penetration and reflux patterns,using the push-and-plug injection technique.1The arterial component of the BAVM was occluded first to avoid bleeding complications associated with early occlusion of venous drainage.The draining veins were occluded when the nidus was completely filled.2We ensured that the reflux was always kept below the permitted limit for conventional microcatheters(2 cm) and that the acceptable reflux for detachable tip microcatheters was up to 5 cm, depending on the fusion length.After satisfactory embolization was achieved, the microcatheter was removed using backward tugging (Fig.1).The goal of the procedure was complete obliteration of the nidus or staged embolization.The interval between each stage of embolization ranged from 3 months to 1 year depending on the residual nidus and patient preference.

Table 1 Demographics of patients included in the study.
3.Results
A total of 25 patients(6 female and 19 male patients)underwent 46 embolization procedures.Ages ranged from 9 to 62 years (mean, 34 years).The majority of the procedures were performed on SM grade III BAVMs,and none were grade I(Table 1).The AVMs were located in the temporal lobe in 5 patients,parietal lobe in 7,frontal lobe in 3,posterior fossa in 6, basal ganglia in 3, and the parasagittal in 1.The BAVM was right-sided in 11 patients (44%) and left-sided in 14 patients (56%).BAVMs drained into the deep draining veins in 13 patients (52%) and into the superficial venous system in 12 patients (48%).Among the 25 patients, 15 presented with hemorrhage, 5 with seizures, and 5 with headache and neurology.
We achieved nidal obliteration rates of AVMs between 10% and 100%, with a mean of 33%.Complete occlusion was observed in 2 patients (8%).Most of the patients were undergoing their first or second embolization procedure.The average volume of Squid injected was 1 ml per procedure.Thirteen procedures used the Squid-12 formulation and 29 procedures used the Squid-18 formulation.Three procedures were performed with a combination of Squid-12 and-18 formulations.
In our study, the incidence of hemorrhage after embolization was 16%.Of the four patients with intracranial hemorrhage, one patient is deceased.Partial venous embolization of Squid causes venous drainage stagnation, giving rise to venous thrombosis and resulting in venous bleeding in the tight posterior fossa.One patient had expansion of a preexisting intracranial bleed and 2 patients had punctate hemorrhages.A patient with a fractured catheter in the vertebral artery developed transient seizures without major complications.
Possible vessel perforation during catheter manipulation was observed in 7(28%)patients.The perforations were sealed with Squid or 16% histoacrylic glue with no sequelae postprocedure.Histoacryl glue was used because it polymerizes faster than Squid.However,no further evaluations (such as cone-beam CT) were performed during the procedure,and the majority did not undergo post-procedure CT if no obvious clinical deterioration was observed.All 7 patients with vessel perforation were extubated in the angiography suite post-procedure and discharged within 7 days.
4.Discussion
Onyx, Squid and PHIL are now the most widely utilized copolymers for the embolization of BAVMs.3Squid is available in 6 different formulations: Squid-12, Squid-12LD, Squid-18, Squid-18LD, Squid-34, and Squid-34LD, with the numbers indicating the viscosity in cps at 40°Celsius.The low-viscosity Squid formulations are formulated to penetrate more easily.Two experimental studies employing extra-low-viscosity Squid-12 and PHIL LV exhibited a significant extent of embolization and less reflux.4,5However, establishing a plug with a low-viscosity formulation is challenging.The LD versions contain 30% less tantalum,which may help with the fluoroscopic visualization of structures beneath dense embolic casts, allowing for better evaluation of the embolized BAVM vasculature and the injected volume.3Furthermore, because the LD versions contain less tantalum than the standard versions, they produce fewer artifacts.4The smaller sizes of the tantalum powder grains in Squid also improved visibility during longer injection times owing to a slower sedimentation rate.5Squid should be injected immediately after mixing as tantalum settling within the syringe may result in poor visualization during injection under fluoroscopy or microcatheter occlusion.Squid-18 was preferred for initial plug formation in the embolized feeder,and injection was then continued with Squid-12, as we observed that it penetrated the embolic cast more efficiently than Squid-18.A more viscous Squid-18 is preferred in faster-flow niduses, especially in those with a fistula.In our experience,the Squid-18 behavior was very similar to that of Onyx 18.Squid-34 is primarily used for peripheral purposes.
Intravascularly, the EVOH copolymer and the suspended tantalum precipitate created a spongy coherent embolus.Onyx and Squid solidify from the exterior to the interior,similar to lava,whereas PHIL solidifies and flows similar to a column.An opacified blood vessel embolized with Onyx or Squid could still be perfused centrally,suggesting that it was not completely embolized.An opacified artery is one that is completely blocked by PHIL.6
Because Squid is non-adhesive,the microcatheter can be left in place when slow controlled injections are performed.However,even with the use of detachable tip catheters,7premature obliteration of the microcatheter lumen,leading to catheter entrapment,has been reported during EVOH embolization.Excessive pressure applied to the catheter tip to clear the solidified Squid may cause the catheter to burst.
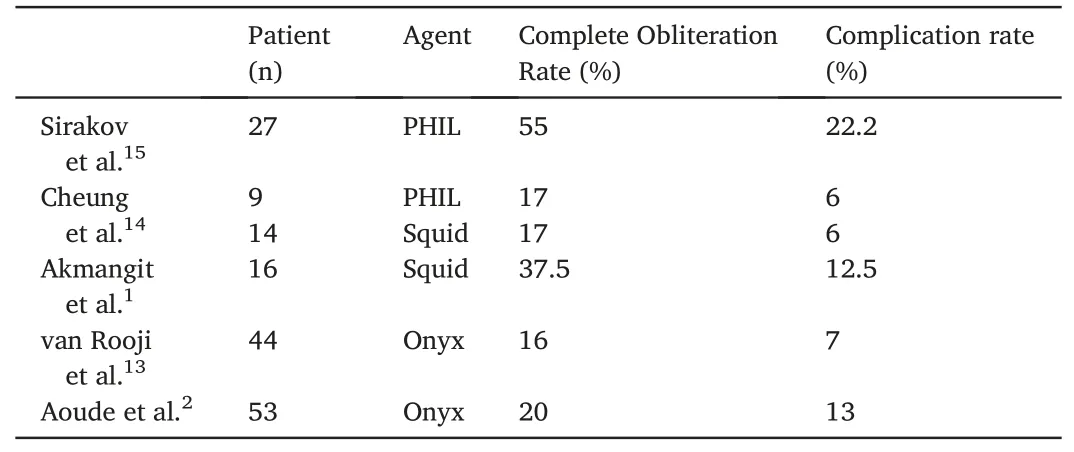
Table 2 Literature review of complete obliteration and complication rates of cerebral AVM embolization using PHIL, Squid, and Onyx liquid embolic agents.
Premature obliteration of the microcatheter lumen by Squid,leading to stranding of the tip of the microcatheter during withdrawal, is a dreaded complication.Two cases of fractured catheters occurred in our series (4%); however, no major complications were observed.Extravasation of Squid can occur during injection, possibly causing a subacute inflammatory response.In our experience, vessel perforations sealed with Squid did not have any post-procedural sequelae.DMSO may also cause local toxic effects such as vasospasms, inflammation of the vessel wall,or angionecrosis.8
Hemorrhage during or immediately after embolization is a serious consequence that may occur in up to 17% of cases, despite all precautions.9A previous study using Onyx reported an incidence rate of 13%for intracranial bleeding.10Jordan et al.recommended limiting the extent of devascularization during each session and inducing post-procedure hypotension to reduce the incidence of post embolization hemorrhage.11In our study, post procedural hemorrhage occurred in 16%of the cases.
According to their investigation in 2014, Akmangit et al.concluded that Squid is a safe and effective embolic agent after 16 AVMs and 9 DAVFs were treated, with 37.5% achieving complete closure.1Another study in 2020 reported a high rate of complete angiographic occlusion(17/19 patients)using Squid-12 for the embolization of DAVFs,with no major periprocedural adverse events.12Moreover, van Rooij et al.reported total occlusion with Onyx in 16% of 44 patients.13One study reported that PHIL could be an effective and safe alternative to Onyx and Squid.In that study,total occlusion was achieved in one session in 52%of patients,with complications observed in 22.2%of patients(see Table 2).Our study results showed total occlusion in 8% of patients.Previous studies indicated that Onyx, Squid, and PHIL are safe for use in intracranial AVMs and DAVFs.However, Onyx, Squid, and PHIL occlusion rates vary, likely due to different protocols, intent of embolization, and AVM morphology at different centers.14
Male sex and unruptured or right-sided BAVMs were associated with improved functional outcomes.14In our case series, 44% of the BAVMs were right-sided, and 40% were unruptured.Due to the retrospective nature of the study,the modified Rankin score was unavailable for most cases.
5.Conclusion
This study demonstrated that Squid is a safe and effective embolic agent for the treatment of cerebral AVMs.Nevertheless, larger multicenter trials are required to compare the safety,efficacy,and durability of different embolic agents.
Contributions
1) Ch'ng Li Shyan - conception and design, acquisition, analysis and interpretation of data,drafting the article,final approval of the version to be published.
2) Zaki Zulkifli - conception and design, revising it critically for important intellectual content, final approval of the version to be published.
3)Ahmad Sobri Muda-conception and design,interpretation of data,revising it critically for important intellectual content, final approval of the version to be published.
Ethical approval
The study was approved by the ethics committee of Hospital Sungai Buloh.All clinical practices and observations were conducted in accordance with the Declaration of Helsinki.Informed consent was obtained from each patient before the study was conducted.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interests
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
杂志排行
Journal of Interventional Medicine的其它文章
- Mechanisms and therapeutic strategies to combat the recurrence and progression of hepatocellular carcinoma after thermal ablation
- Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods
- A novel cerebrovascular drug-coated balloon catheter for treating symptomatic intracranial atherosclerotic stenosis lesions:Study protocol for a prospective, multicenter, single-arm, target-value clinical trial
- Combination of transarterial radioembolization with atezolizumab and bevacizumab for intermediate and advanced staged hepatocellular carcinoma: A preliminary report of safety and feasibility
- Argon-helium cryoablation treatment of undifferentiated pleomorphic sarcoma of the thyroid: A case report and literature review
- “Guidezilla” extension catheter combined with balloon technique for treating pulmonary artery stenosis caused by Takayasu arteritis
