Current status of yttrium-90 microspheres radioembolization in primary and metastatic liver cancer
2023-03-06YsmnAnriFloortjeVeermnGrceKeneArthurBrtMrtenSmitsRutgerBruijnenWenleTnYeLiFengDunMrnixLm
Ysmn Anri, Floortje E.Veermn, Grce Kene, Arthur J.A.T.Brt,Mrten L.J.Smits, Rutger C.G.Bruijnen, Wenle Tn, Ye Li, Feng Dun,Mrnix G.E.H.Lm
a University Medical Center Utrecht, Utrecht, the Netherlands
b Interventional Radiology Department, Chinese PLA General Hospital, Beijing, China
Keywords:
ABSTRACT Liver malignancy, including primary liver cancer and metastatic liver cancer, has become one of the most common causes of cancer-related death worldwide due to the high malignant degree and limited systematic treatment strategy.Radioembolization with yttrium-90 (90Y)-loaded microspheres is a relatively novel technology that has made significant progress in the local treatment of liver malignancy.The different steps in the extensive work-up of radioembolization for patients with an indication for treatment with 90Y microspheres,from patient selection to follow up,both technically and clinically,are discussed in this paper.It describes the application and development of 90Y microspheres in the treatment of liver cancer.
1.Introduction
Radioembolization, also known as selective internal radiation therapy (SIRT) or trans-arterial radioembolization (TARE), is a locoregional intervention for primary and metastatic liver malignancy.Radioactive microspheres are administered through a microcatheter placed in the hepatic arterial vasculature.Currently, two90Y microspheres are available, produced with different carrier materials.The options are90Y on the surface of a resin microsphere (SIR-Spheres, Sirtex Medical) or incorporated in a glass microsphere (TheraSphere, Boston Scientific)(Table 1).
Administration of either glass or resin microspheres is based on the principle that liver lesions are almost exclusively supplied by the arterial vasculature, while normal liver parenchyma is mainly supplied by the portal vein.After intra-arterial administration,90Y microspheres lodge in the peripheral blood vessels of the tumor and accumulate in the microvasculature of the tumor.This results in the emission of high-energy betaradiation in the liver lesions,inducing cell death,while relatively sparing the healthy liver parenchyma.Since the average tissue penetration of90Y in the liver is only 2.5 mm(maximum 11 mm),90Y causes little damage to normal tissue.Different from the traditional transcatheter arterial chemoembolization (TACE),90Y radioembolization mainly depends on the radiation effect of90Y microspheres, rather than relying on the hypoxia caused by embolism.1
2.Patient selection
According to the most recent guidelines for radioembolization of the European Association of Nuclear Medicine(EANM),radioembolization is indicated for unresectable liver tumors, both primary tumors and metastases.2Contraindications can be classified into absolute and relative contraindications.Absolute contraindications include life expectancy of less than three months,clinical liver failure, and pregnancy.
Relative contraindications include a Child-Pugh score higher than B7,extensive intrahepatic tumor burden (depending on the tumor type, a cut-off of 50–70% is often reported), extrahepatic tumor burden(depending on tumor type, more (in case prognosis depends on liver disease, e.g., more indolent neuroendocrine tumors) or less (e.g., more aggressive intrahepatic cholangiocarcinoma (ICC)) extrahepatic disease is acceptable),main portal vein tumor thrombosis(PVT),poor targeting of portal vein tumor thrombosis in the main trunk, contraindications to hepatic artery catheterization (unmanageable coagulation disturbance,renal failure, allergy to contrast media and vascular abnormalities) and lung shunting that leads to a lung dose >30 Gy or 50 Gy cumulatively after repeated treatment.Of note: technetium-99 m (99mTc)-labelled macro-aggregated albumin (99mTc-MAA) lung shunting generally leads to some overestimation.2
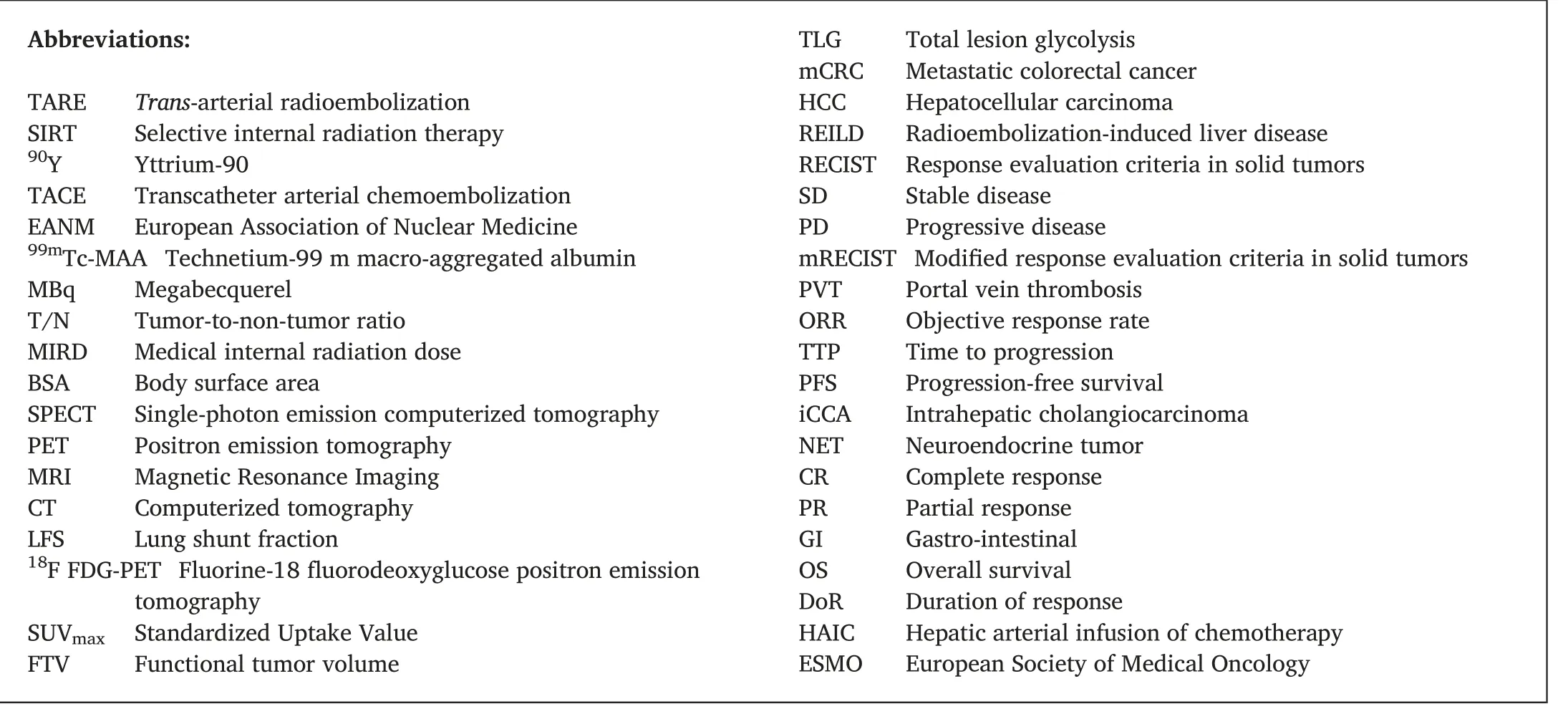
Abbreviations:TARE Trans-arterial radioembolization SIRT Selective internal radiation therapy 90Y Yttrium-90 TACE Transcatheter arterial chemoembolization EANM European Association of Nuclear Medicine 99mTc-MAA Technetium-99 m macro-aggregated albumin MBq Megabecquerel T/N Tumor-to-non-tumor ratio MIRD Medical internal radiation dose BSA Body surface area SPECT Single-photon emission computerized tomography PET Positron emission tomography MRI Magnetic Resonance Imaging CT Computerized tomography LFS Lung shunt fraction 18F FDG-PET Fluorine-18 fluorodeoxyglucose positron emission tomography SUVmax Standardized Uptake Value FTV Functional tumor volume TLG Total lesion glycolysis mCRC Metastatic colorectal cancer HCC Hepatocellular carcinoma REILD Radioembolization-induced liver disease RECIST Response evaluation criteria in solid tumors SD Stable disease PD Progressive disease mRECIST Modified response evaluation criteria in solid tumors PVT Portal vein thrombosis ORR Objective response rate TTP Time to progression PFS Progression-free survival iCCA Intrahepatic cholangiocarcinoma NET Neuroendocrine tumor CR Complete response PR Partial response GI Gastro-intestinal OS Overall survival DoR Duration of response HAIC Hepatic arterial infusion of chemotherapy ESMO European Society of Medical Oncology
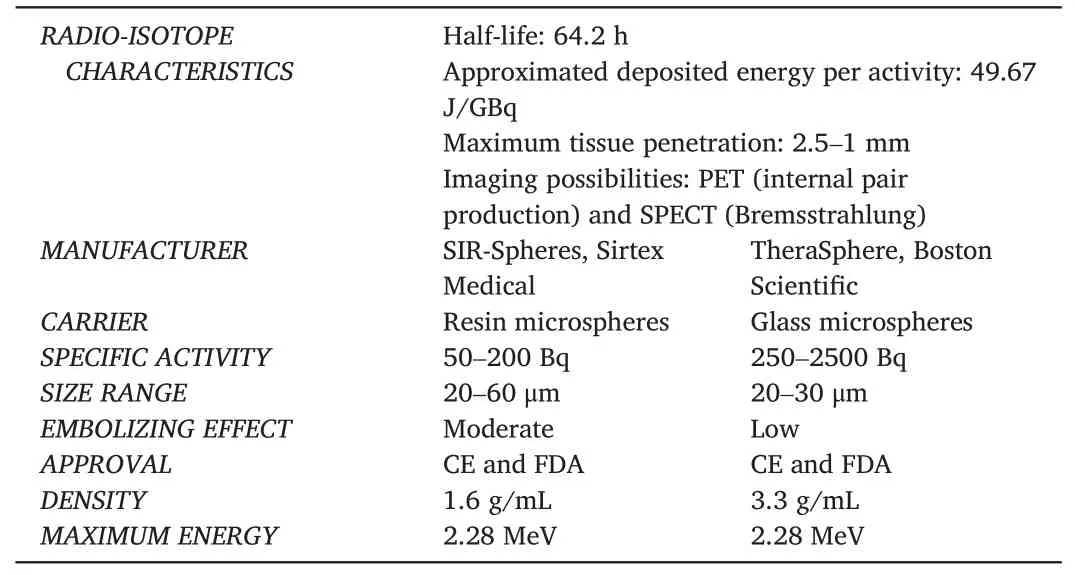
Table 1 90Y microspheres characteristics.
3.Baseline imaging
Imaging techniques include contrast-enhanced CT or MRI performed within 30 days of the procedure for the calculation of the tumor volume and for staging purposes.Also,(early)arterial CT may be performed prior to radioembolization to identify and evaluate the hepatic arterial anatomy (e.g., the origin of the right gastric artery, the origin of segment 4 arteries)and identify any variations or abnormalities in the vasculature.This step is important to establish the feasibility and objectives of treatment.2
4.Pre-treatment work-up
The actual treatment is preceded by a simulation angiography of the upper abdominal vessels in which a surrogate for microspheres is used,99mTc-MAA.During the angiography, vessels of the coeliac trunk and upper mesenteric artery are visualized,and the position of the catheter in the hepatic artery is determined, after which a test dose of99mTc-MAA(approximately 150 MBq2) is administered.Shortly after the test procedure, a SPECT/CT is obtained to assess the possible inadvertent distribution of99mTc-MAA in the lungs and abdominal extrahepatic tissue.Hepatico-enteric anastomoses may lead to extrahepatic deposition of activity in the lungs or in the gastro-intestinal (GI) tract.Microsphere deposition in the GI tract can cause radiation-induced tissue damage,including ulceration and inflammation.Lung shunting is seen in HCC more often than in other tumor types and can lead to radiation pneumonitis.Excessive lung shunting or extrahepatic deposition of activity are contraindications that are ruled out during this test procedure.
The dual vascularization principle implies that most intra-arterially administered microspheres will accumulate in and around the tumor.The tumor-to-non-tumor ratio (T/N) can be variable among patients.If the T/N is low,a relatively low tumor dose is achieved to ensure the nontumor dose is not too high.The99mTc-MAA distribution is used for the simulation of the distribution of the microspheres in the liver and in the healthy liver parenchyma.This simulation can be used for treatment planning(Fig.1).
5.Treatment planning
5.1.Pre-treatment activity measurements
The goal of radioembolization is to yield the maximum achievable tumor absorbed dose, inducing the maximum apoptosis in tumor cells,while maintaining minimum effect on the non-tumorous tissue.Therefore, the ideal implementation of this technique relies on dosimetric optimization and individualized treatment planning.Pre-treatment calculations for activity planning ensure an effective and safe administration of radioembolization, contributing to individualized treatment.
Pre-treatment activity measurement approaches have favorably evolved over time.Contemporarily, three approaches, including the socalled body surface area (BSA) method, medical internal radiation dose(MIRD) method, and partition method, are commonly applied among treatment centers.(Table 2).
6.The BSA method
The BSA method was the most used activity calculation method for resin microspheres.In a myriad of randomized clinical trials, the BSA method calculates a patient-specific prescribed activity by a theoretically estimated normal liver volume based on the body surface area.Additionally,this method takes the tumor load into account,but not the tumor absorbed dose and the non-tumor absorbed dose.
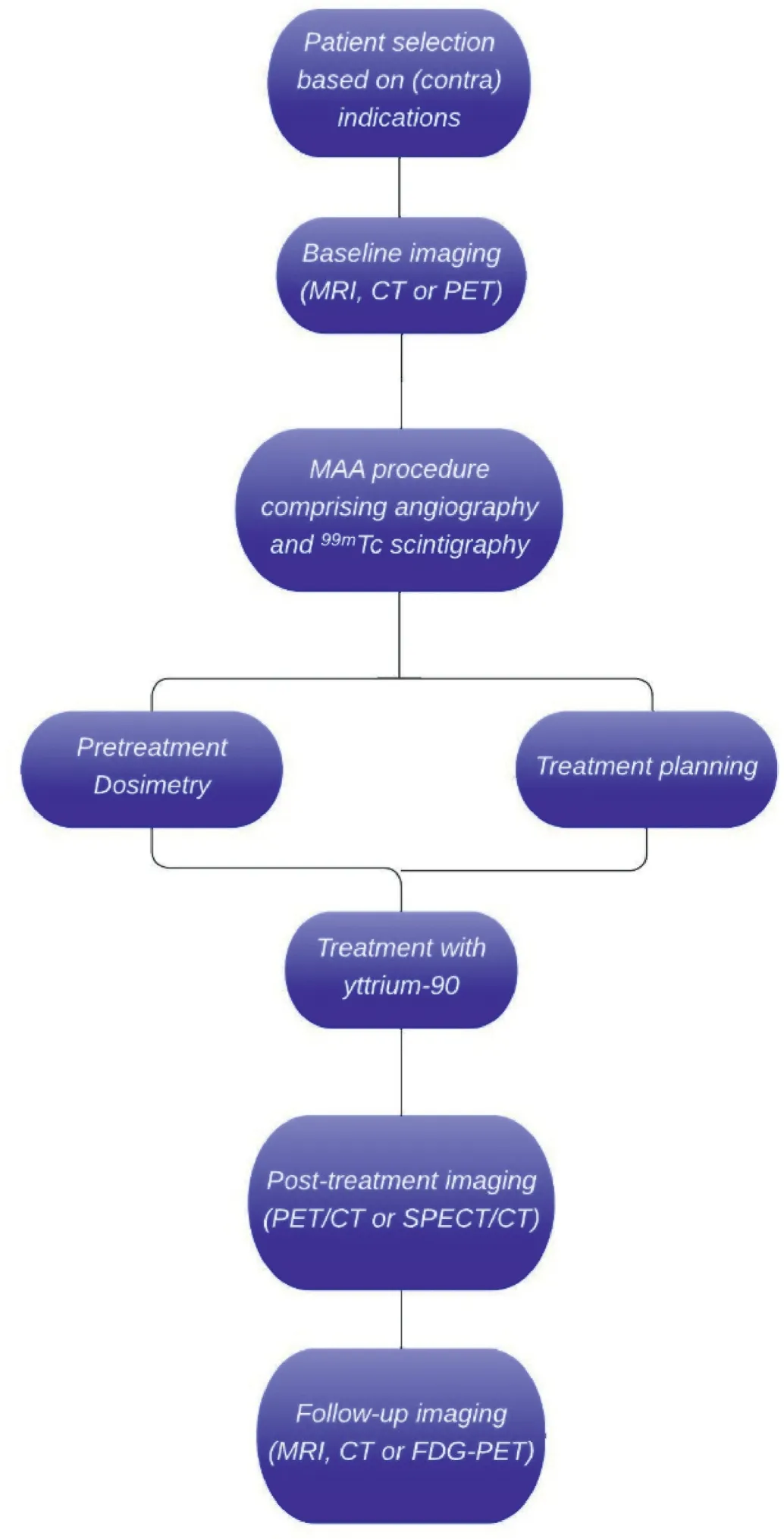
Fig.1.The workflow of radioembolization.
The BSA method may underestimate the optimal activity in small patients with a large liver or overestimate the optimal activity in large patients with a small liver.Plus, the data by which the BSA method estimates the liver volume is derived from a healthy cohort of patients,whose liver volume is not representative of patients in a disease state.The BSA method does not consider the intrahepatic distribution variations derived from the T/N ratio, leading to an inaccurate dose distribution in patients with hypo-or hypervascular cancer tissue.
7.The single-compartment MIRD method
The MIRD method is a mono-compartment activity calculation approach that is mainly used for glass microspheres.The MIRD methodconsiders the target average dose and the volume of the targeted hepatic tissue.Based on the clinical interpretation by the responsible physician,an average absorbed dose between 80 and 150 Gy can be considered for glass microspheres.The volumetric liver measurement may be achieved by CT,MRI,or PET/SPECT.Unfortunately,specific factors are not taken into consideration in this approach.The volume of the tumor and the normal liver tissue, the T/N ratio, and the heterogeneous dose distribution within compartments are among the disregarded attributes.3

Table 2 Comparing different pretreatment activity calculation equations.
8.Multi-compartment MIRD or partition method
Owing to the advances in dosimetry techniques, the most accurate and safe activity measurements have evolved.The partition model,also known as the multi-compartment method, is the most accurate and comprehensive activity planning approach in use clinically today.The basis of this MIRD-derived method is the theoretical determination of the radiation activity partitioned into the tumor, non-tumorous liver, and lungs.
Most of the patient-based factors overlooked by the BSA and the MIRD methods are taken into consideration in the partition method.This technique aims for a maximum absorbed dose to the cancer tissue and a minimum absorbed dose in the lungs and the normal liver tissue.Primary determinants of the compartmental dose and activity in the partitionbased activity planning include volume (of compartments involved),shunt fraction, and T/N avidity ratio.This method usually does not consider the heterogeneous dose distribution within the compartments.4However, the voxel-based multi-compartment MIRD method considers the heterogeneity of the microspheres distribution,which may be crucial for intra-tumoral dose distribution.5
Partition-based activity calculation relies on the implementation of99mTc-MAA SPECT/CT,in which the treatment activity in each involved compartment is simulated.The99mTc-MAA is a surrogate of the actual90Y microspheres distribution.Dissimilarities in the90Y microspheres distribution and its surrogate are a limitation of the method6
Despite its promising accuracy and safety,the partition method is not commonly practiced in treatment centers.A limiting factor may be the fact that this approach is inherently demanding due to the labor-intensive volume determination and limited ability to evaluate the T/N ratio.
9.Treatment
After performing preparatory angiography,99mTc-MAA scintigraphy,and activity calculation, the therapeutic microspheres embedded with the beta-emitting isotope90Y are injected via a microcatheter.Like the preparatory angiography, this procedure is performed by an interventional radiologist by using X-ray fluoroscopy.The treatment procedure usually takes place one or two weeks after the preparatory angiography.A trans-femoral or trans-radial approach may be used for entry.The microcatheter (generally 2.7 F) is positioned as selectively as possible(making sure that all the tumors are covered) and in the exact same position as used during preparatory angiography and injection of99mTc-MAA.A vial containing90Y microspheres is then infused through a specific administration system, supplied by the vendor of the microspheres used.
The dosage of glass90Y microspheres typically ranges from 1.2 to 8 million microspheres with a specific activity ranging from>4000 Bq per microsphere to <400 Bq per microsphere.The infusion requires a low volume of saline solution, typically around 100 ml.Continuous fluoroscopic guidance is not necessary as the vascular bed is not completely saturated.The complete infusion usually requires 5 min.7A typical resin90Y microspheres treatment consists of injecting around 20–40 million microspheres(with a specific activity of 50 Bq per microsphere at the day of calibration).Resin microspheres are provided in a vial with water for injection.To ensure safe and effective delivery of resin microspheres,it is essential to administer them slowly, at a rate not exceeding 5 ml/min.Rapid delivery can lead to reflux, which can potentially lead to extrahepatic deposition of activity.During the procedure, the interventional radiologist must continuously monitor the catheter's position to prevent reflux and ensure it remains correctly placed.
For both products, keeping the catheter tip in the same position during the99mTc-MAA procedure and the actual treatment is very important to ensure that the distribution of90Y microspheres after treatment resembles the treatment plan.In the two weeks following radioembolization, more than 95% of the radiation dose is delivered to the surrounding tissues where the microspheres were deposited8
9.1.Post-treatment imaging and dosimetry
After radioembolization, post-treatment imaging with either SPECT or PET is recommended.These scans aim to assess therapy effectiveness,calculate absorbed doses, and correlate results with clinical response(Fig.2).Though SPECT/CT is a widely available modality, its spatial resolution is limited, and energy window-based scatter methods cannot be used for90Y SPECT due to the absence of an identifiable energy peak in the continuous bremsstrahlung energy spectrum measured during90Y SPECT/CT.PET/CT,on the other hand,is generally considered superior in terms of image quality due to its higher spatial resolution.As such,guidelines recommend90Y PET/CT as being the preferred choice for posttreatment imaging since it offers images suitable for visual evaluation and quantitative assessment.9Lhommel et al.first demonstrated the feasibility of using PET/CT for post-treatment imaging after radioembolization,yet many centers often skip this step in the workflow and proceed directly to follow-up imaging despite its importance in contemporary practice.Recent guidelines strongly advocate for post-treatment radioembolization imaging with PET/CT, yet this step remains often neglected.10
In comparison with trans-arterial chemoembolization (TACE), posttreatment imaging after radioembolization relies on different techniques.TACE is usually evaluated using CT or X-ray fluoroscopy to assess the distribution, while radioembolization requires specialized imaging techniques such as90Y SPECT or PET to analyze the microspheres distribution.
9.2.Follow-up imaging
Following radioembolization, a clinical evaluation is usually performed one to three months after treatment to assess side effects.Imaging is usually performed three months post-radioembolization, followed by three-monthly follow-up imaging thereafter.The definition of"treatment response" and the best imaging method to evaluate this response may vary depending on the tumor's characteristics(e.g.,FDG uptake)and the treatment goal.10
For over 20 years,MRI has been utilized for abdominal imaging and has undergone various technical advancements in sequence design and contrast media use, leading to improved diagnostic accuracy.These advancements have notably sharpened image quality and reduced motion and breathing-related artifacts.It should be noted that early performance of MRI after radioembolization may result in enhancement around the treated tumor.It often corresponds to treatment-induced inflammation and is not to be mistaken with a viable tumor or progression.11
At the same time,there has been a dramatic development in CT over the last decade.By utilizing a scanner with 64 or more rows,high spatial and temporal resolution imaging can be performed, enabling the integration of bi- or triphasic liver examinations.As a result of the short acquisition time and high resolution of multidetector CT scanners, they have become the backbone of oncological therapy assessment.Although the imaging technique must be adapted to the underlying tumor entity,a late-arterial and a portal venous phase abdominal CT is generally considered to be standard procedures.
18F FDG-PET(CT)imaging after radioembolization with the calculation of SUVmax(Standardized Uptake Value), FTV (Functional Tumor Volume), and TLG (Total Lesion Glycolysis) and comparing them with the result of pre-treatment functional imaging may be an invaluable method in evaluating the result of treatment in mCRC patients, earlier than other imaging modalities(i.e.,already after 4–6 weeks).12However,FDG-PET has shown limited value for HCC imaging because of its limited sensitivity for HCC lesions.11
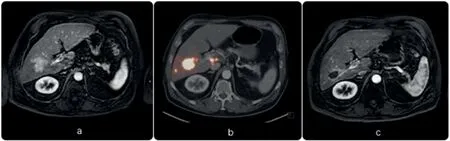
Fig.2.Patient with HCC who received radioembolization with 90Y glass microspheres.(a) The baseline image (MRI) demonstrated enhancing hepatocellular carcinoma in segment V/VI.(b)PET/CT image immediately after the treatment, 90Y glass microspheres were selectively injected into the right hepatic artery.As shown,the microspheres are concentrated in the tumor located in segment V/VI.(c) Follow-up MRI 2 months after radioembolization showing complete response to the treatment as the tumor is no longer enhanced.
10.Tumor response assessment and clinical outcome
The assessment criteria for radioembolization are generally based on tumor size assessment of representative tumors on MRI or CT.Response Evaluation Criteria in Solid Tumors (RECIST) is the assessment tool of choice for solid tumors assessing the tumor response, such as complete response(complete disappearance of lesions)or partial response(at least a 30%decrease in the sum of the longest diameters of target lesions),and stable disease (SD), or progressive disease (PD) to the given treatment.For the particularities of HCC, modified RECIST (mRECIST) criteria are proposed in the literature to assess the tumor response.13This method specifically evaluates the remaining viable tumor, defined as the remaining contrast-enhanced tumor tissue on CT or MRI.
11.Complications and adverse events
Radioembolization is well tolerated by most patients.However,mild side effects generally occur the first 4–6 weeks after radioembolization,for example,nausea,vomiting, fatigue, abdominal pain, and fever.Riaz et al.noted that the incidence of severe complications,grade 3 or higher according to the CTCAE criteria, occur in <10% of patients.14Liver decompensation and extrahepatic deposition of activity (e.g., radiation pneumonitis,pancreatitis,gastric ulceration)are more severe side effects that may develop 2–4 months after treatment,with an incidence of<1%.The most severe complication of radioembolization is radioembolization induced liver disease(REILD),caused by high dose irradiation of healthy liver parenchyma.REILD can be fatal.It is characterized by jaundice and massive ascites 2–4 months after treatment.15Also, hypoalbuminemia and hyperbilirubinemia are signs of liver failure and may direct to REILD,in case biliary obstruction or disease progression is not the explanation for these adverse effects.The incidence of REILD is around 1–3%.16Risk factors for REILD are, e.g., prior chemotherapy, low tumor burden,cirrhotic liver disease,and high baseline bilirubin level.
12.Hepatocellular carcinoma
The treatment philosophy for unresectable advanced primary liver cancer is to prolong survival and improve the quality of life.Accumulating studies have shown the favorable efficacy of90Y microspheres radioembolization in improving the quality of life of patients with unresectable advanced liver cancer.Radioembolization, compared to trans-arterial chemoembolization(TACE),shows similar survival outcomes.However,PVT and bile duct anastomoses are no strict contraindications for radioembolization17Radioembolization for HCC is applied as treatment in a variety of disease states: e.g., ‘ablative’ radiation segmentectomy, conversion therapy, or as palliative therapy.Radiation segmentectomy is applied in patients with a tumor ineligible for ablation or surgical resection.Selective catheterization provides an ablative radiation absorbed dose, aiming to induce necrosis in a (super)selective part of the liver,including the tumor.The LEGACY study (local radioembolization using glass microspheres for the assessment of tumor control with90Y), a multi-center,single-arm,retrospective study,included 162 patients with solitary,unresectable HCC lesions and reported an objective response rate(ORR) of 88.3% with 62.2% exhibiting a duration of response (DoR) of more than 6 months.As much as 86.6%of all patients had a three-year overall survival18The RASER study19(radiation segmentectomy for curative intent of unresectable very early to early-stage hepatocellular carcinoma),a single-center,single-arm study,included 29 patients with early-stage HCC who were not eligible for ablation.The study reported a complete response in 83% of the patients, all patients had an initial objective response and 90%had a sustained complete response.Also,the study reported a low incidence of high-grade adverse events.These results contributed to recommending radiation segmentectomy as a viable treatment option for patients with solitary small HCC.
The aim of conversion therapy is to accomplish a reduction of tumor size,an increase of the residual non-treated liver volume,and to provide patients with the opportunity of radical resection after radioembolization, thereby ensuring that the remaining liver volume after tumor resection can maintain adequate liver function, improving the success rate of surgery,and increasing the survival rate of patients.Gaba et al.included 24 patients who were eligible for surgery after a so-called radiation lobectomy.Their results showed that the contralateral hepatic lobe of all patients increased after 9 months, with a maximum residual liver volume increase of 45% and an average residual liver volume increase of 26%.Therefore, it is recommended that radiation lobectomy can be used as a conversion therapy before surgical resection.20
Bridging therapy can retard tumor progression and enable patients to overcome the waiting period for liver transplantation due to the shortage of donor livers.According to the results of Ettorre et al.,2178.9%of patients who received radioembolization before transplantation successfully experienced tumor reduction.Salem et al.22compared the effects of radioembolization and TACE as bridging therapy before HCC transplantation and found that the time-to-progression of patients in the radioembolization group was significantly longer than that in the TACE group (26 months vs.6.8 months).Based on this study, the European Society for Medical Oncology(ESMO)guidelines for liver cancer in 2018 recommended that radioembolization could replace TACE as bridging therapy to prevent patients from losing the chance of liver transplantation due to tumor progression.23
In addition, radioembolization can potentially also be used in combination with sorafenib or immune checkpoint inhibitors to prolong the survival of patients with advanced HCC (BLCL B or C).Ricke et al.24carried out a prospective,randomized,controlled phase II clinical study to compare the survival of HCC patients receiving sorafenib treatment combined with radioembolization and sorafenib alone.The subgroup analysis found that the OS of HCC patients in the subgroup without cirrhosis and HCC patients ≤65 years old was significantly prolonged after sorafenib treatment combined with radioembolization.Furthermore, Chew et al.25suggested that radioembolization induced immune activation in the tumor microenvironment of HCC and exerted a synergistic effect with immune checkpoint inhibitors, which enhanced the effect of immunotherapy.
13.Intrahepatic cholangiocarcinoma (iCCA)
iCCA is part of the invasive growing biliary tract carcinomas with poor prognosis26Encouragingly,radioembolization has shown safety and efficacy in the treatment of iCCA.Saxena et al.conducted a prospective cohort study of 25 patients with unresectable iCCA to evaluate the efficacy of radioembolization.Most included patients had received chemotherapy and surgery,with multiple lesions involving more than two liver lobes, and 48%of the patients had extrahepatic metastasis.All enrolled patients received radioembolization treatment,with an ORR of 24%and a median OS of 9.3 months.27A phase II clinical trial for which patients were treated with radioembolization,combined with chemotherapy,as a first-line treatment of unresectable iCCA,reported ORR of 39%and OS of 22 months.28According to the ESMO practical guideline for biliary tract cancer,radioembolization in combination with chemotherapy is recommended for selective patients.26
14.Liver metastases from colorectal cancer
At present, numerous studies have proven the efficacy and safety of radioembolization in patients with metastatic colorectal cancer(mCRC).In the ESMO guideline on mCRC, radioembolization is indicated when liver-limited disease and metastases are unresectable,and chemotherapy is not indicated.29In 2001, Gray et al.30carried out a phase III clinical study of radioembolization combined with hepatic arterial infusion of chemotherapy (HAIC) in the treatment of mCRC.It was found that radioembolization in combination with HAIC significantly elevated the ORR of patients compared with HAIC treatment alone(44.0%vs.17.6%),prolonged the TTP(15.9 months vs.9.7 months),and improved the 1-,2-,3-,and 5-year survival rates.There was no significant increase in adverse reactions in the radioembolization plus HAIC group.
In 2021,Mulcahy et al.31reported a randomized,multi-center study on radioembolization in treating mCRC.The study included 428 patients with liver metastasis from colorectal cancer who failed first-line systemic treatment.The results showed that the TTP(8.0 months vs.7.2 months)and ORR(34%vs.21.2%)of the combined treatment group were better than those of the control group (chemotherapy alone).It is confirmed that the combination of radioembolization can locally control the liver metastasis of mCRC patients,effectively prolong the survival period,and improve the quality of life.
15.Liver metastases from neuroendocrine tumor
Preliminary clinical studies32–34and retrospective studies have shown that radioembolization is well-tolerated in patients with liver metastasis from unresectable neuroendocrine tumors (NET), which can achieve persistent liver tumor responses and alleviate symptoms.NET is a disease with a lower incidence than the tumor types mentioned above.Hepatic involvement in NET occurs in many cases and is one of the major factors related to survival.Rhee et al.carried out a non-blinded,non-randomized phase II clinical trial and recruited 42 patients with metastatic NET who experienced standard treatment failure, 74% of whom had carcinoids, and the rest (26%) had pancreatic tumors.Standard treatment failure was defined as tumor progression after the use of octreotide, tumor resection, ablation, or embolization.32This study reported the safety and effectiveness of radioembolization in treating metastatic NET.After radioembolization, only six patients had grade 3 toxic reactions related to serological changes in liver function tests.According to the criteria of response evaluation criteria in solid tumors(RECIST), the ORR of radioembolization was around 50%, and patients with stable disease (SD) accounted for around 40%.These findings demonstrated that radioembolization is safe and has a high tumor response in metastatic NET.
16.Liver metastases from breast cancer
Breast cancer is one of the most common malignant tumors in women.Although the overall 5-year survival rate can be as high as 90%, the survival rate of patients with metastasis is only 3–20%.Breast cancer metastases most frequently occur in the bone,liver,lung,and brain,and about 61%of metastatic patients are complicated with liver metastasis35Radioembolization has been explored as an option for the treatment of liver metastases from breast cancer.Fendler et al.applied radioembolization to treat 81 patients with breast cancer liver metastasis and found that the standardized uptake value of positron emission tomography (PET) imaging in 52% of patients decreased significantly after treatment.36The median OS after radioembolization was 8 months.Pieper et al.reported the outcomes of 44 patients with breast cancer liver metastasis after the treatment with radioembolization.The samples in this study represented a group of extremely advanced patients,of whom 73% had received more than five lines of systemic chemotherapy, and most patients had multi-focal or diffuse tumors in the liver.37According to the evaluation criteria of RECIST,29%of patients achieved objective response after radioembolization,and the median OS after treatment was 6 months.The above-mentioned studies showed that radioembolization could provide clinical survival benefits for breast cancer patients with liver metastasis after primary chemotherapy failure.
17.Conclusion
Radioembolization is a trans-arterial procedure that delivers embolic microspheres loaded with a high radiation absorbed dose to the tumor while normal hepatocytes surrounding the tumor are minimally exposed.Clinical studies over the past two decades have established a solid foundation for the efficacy and safety of radioembolization in treating primary and metastatic liver cancer.As a result,various guidelines have endorsed radioembolization as a valid treatment option.Nevertheless,further clinical evidence is necessary to advance the treatment of primary and secondary liver cancer and ultimately enhance patient survival.
Declaration of competing interest
Feng Duan is the youth editorial board member for Journal of Interventional Medicine and was not involved in the editorial review or the decision to publish this article.All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Journal of Interventional Medicine的其它文章
- Mechanisms and therapeutic strategies to combat the recurrence and progression of hepatocellular carcinoma after thermal ablation
- Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods
- Embolization of brain arteriovenous malformations with squid co-polymer embolic material: Initial experience
- A novel cerebrovascular drug-coated balloon catheter for treating symptomatic intracranial atherosclerotic stenosis lesions:Study protocol for a prospective, multicenter, single-arm, target-value clinical trial
- Combination of transarterial radioembolization with atezolizumab and bevacizumab for intermediate and advanced staged hepatocellular carcinoma: A preliminary report of safety and feasibility
- Argon-helium cryoablation treatment of undifferentiated pleomorphic sarcoma of the thyroid: A case report and literature review
