Prediction of potential targets and molecular mechanisms of Tripterygium hypoglaucum for oral lichen planus based on network pharmacology and molecular docking
2023-03-06MEIJieSUNYuWANGZhenganKUANGHuifangZENGXiaomeiCAIHongxuanFUQiyaLUOWen
MEI Jie, SUN Yu, WANG Zheng-an, KUANG Hui-fang, ZENG Xiao-mei, CAI Hongxuan, FU Qi-ya✉, LUO Wen✉
1. Department of Stomatology, the First Affiliated Hospital of Hainan Medical University, Haikou 570102, China
2. School of Stomatology, Hainan Medical University, Haikou 571199, China
Keywords:
ABSTRACT Objective: To explore the potential targets and molecular mechanisms of tripterygium hypoglaucum for oral lichen planus (OLP) by applying network pharmacology and molecular docking technology.Methods: The active ingredients and targets of tripterygium hypoglaucum were screened.OLP-related targets were predicted.The Protein-Protein Interaction (PPI)network was constructed for the intersection targets of tripterygium hypoglaucum and OLP.The “OLP-target-molecule-Tripterygium hypoglaucum” network was constructed and visualized.The intersection genes were screened for gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses.Molecular docking analysis and visualization were performed.Results: 15 active ingredients and 78 targets of tripterygium hypoglaucum were obtained.9109 OLP-related targets were screened,and 54 intersection genes of tripterygium hypoglaucum with OLP were obtained.The top 10 key targets were screened from the constructed PPI network.The top 10 active ingredients of tripterygium hypoglaucum were screened from the constructed “OLP-targets of actionactive ingredients-tripterygium hypoglaucum” network.The GO and KEGG analyses of the 54 intersection targets indicated that tripterygium hypoglaucum may play a therapeutic role by regulating one carbon pool by folate, pathways in cancer, et al.Molecular docking analysis showed that dihydrofolate reductase (DHFR), phosphoribosylglycinamide formyltransferase(GART), estrogen receptor 1 (ESR1), et al are the key targets for the treatment of OLP in tripterygium hypoglaucum.Conclusion: The potential key targets and molecular mechanisms of tripterygium hypoglaucum in treating OLP provide a theoretical basis for new drug development and clinical applications.
1.Introduction
Oral lichen planus (OLP) is a chronic disease of unknown etiology and pathogenesis that is usually considered to be a T-cellmediated inflammatory disease with an overall global prevalence of approximately 1.01%[1].OLP is a potentially malignant disease of the oral cavity and carries a certain risk of becoming cancerous,with approximately 1.14% of OLP eventually transforming into squamous cell carcinoma of the oral cavity[2, 3].OLP can cause discomfort such as a burning sensation, severe pain, and difficulty eating, thus hindering activities such as chewing, swallowing, and speech and seriously affecting patients’ oral health and quality of life[4].Some patients with OLP develop lesions of the skin or genitalia, resulting in conjunctival scarring, vaginal stenosis, vulvar destruction, and narrowing of the esophagus, urethra, and external auditory canal[5-7].Treatment for OLP includes corticosteroids,immunosuppressive drugs, antifungal drugs, surgery, and lasers[8-10].Current research suggests that corticosteroids are the first line of treatment for OLP[11, 12], however, some serious side effects can occur with long-term use, such as oral candidiasis, adrenal insufficiency, Cushing’s syndrome, hypertension, hyperglycemia,osteoporosis, etc., which adversely affect the lives and work of OLP patients[13-15].In view of the many serious side effects associated with the use of corticosteroids, traditional Chinese medicines,represented by tripterygium hypoglaucum, have received increasing attention in the clinical application of OLP in recent years.
Tripterygium hypoglaucum is the root or whole plant of the deciduous lanceolate or trailing shrub plant tripterygium hypoglaucum of the genus Lei Gong Teng of the family Weseraceae,which has anti-inflammatory, anti-tumor, and immunosuppressive effects[16-18].The ingredients contained in tripterygium hypoglaucum do not go through the pituitary-adrenocortical system to exert their medicinal effects, and there is no drug withdrawal reaction, so it has fewer side effects, lower toxicity, and better efficacy when applied in small dosages and short courses in OLP treatment[19-22].OLP diagnostic and treatment guidelines[23] recommend the discretionary use of tripterygium hypoglaucum for those who are ineffective on glucocorticoid therapy or for those who have contraindications to glucocorticoid therapy.Although tripterygium hypoglaucum has a good basis for clinical use as an effective agent for the treatment of OLP[19 20, 24], however, its molecular mechanism of action in the treatment of OLP remains to be confirmed by further experimental studies.At the same time, in view of the fact that tripterygium hypoglaucum is a traditional Chinese medicine with a variety of ingredients mixed together, the complexity of the ingredients increases the uncontrollability of the medication; for example, there is no consensus on the way to use the medication and the level of evidence is still lacking, and at the same time, the long-term use of the medication can lead to the accumulation of toxicity, including renal cytotoxicity and hepatotoxicity, and the low bioavailability of the medication, which has resulted in its application being greatly restricted[25-27].Therefore, it is necessary to study the potential targets, bioactive components, and molecular mechanisms of tripterygium hypoglaucum for the treatment of OLP so as to lay a relevant foundation for carrying out basic experiments in this area and to improve the therapeutic efficacy, reduce the toxicity, and increase the bioavailability of the drug by modifying it.
The traditional pharmacological concept of “single gene, single target, single disease” and drug research methodology can no longer be adapted to the formation of multi-gene, multi-functional protein interaction disorders of the disease, and the same is not suitable for the study of the complex mechanism of action of traditional Chinese medicine.With the rapid development of bioinformatics,systems biology, and polypharmacology, as well as based on publicly available data, network pharmacology is a novel, promising,and low-cost approach to the discovery of bioactive ingredients,prediction of drug action targets, analysis of drug mechanisms of action, etc.At the same time, network pharmacology emphasizes the multi-channel regulation of signaling pathways and is therefore particularly suitable for explaining the mechanism of traditional Chinese medicines with a wide range of chemical compositions and molecular targets[28, 29].Molecular docking is a method of drug design based on the properties of the receptor and the interaction between the receptor and the drug molecule[30].This study intends to use network pharmacology and molecular docking technology comprehensively, from cytology to molecular, from pathway enrichment to biological processes, to multi-dimensionally explore the pharmacological material basis of tripterygium hypoglaucum for the treatment of OLP and research the biologically active components of tripterygium hypoglaucum for the treatment of OLP,so as to provide a theoretical basis for the development of novel targeted drugs for the treatment of OLP and lay a foundation for its clinical application.
2.Information and methodology
2.1 Screening of active ingredients and targets of tripterygium hypoglaucum
The BATMAN-TCM online analysis tool library (http://bionet.ncpsb.org.cn/batman-tcm/) is the first online bioinformatics analysis tool dedicated to the prediction of potential targets, formulas, and components of traditional Chinese medicines (TCM).It can be used in the Kyoto encyclopedia of genes and genomes (KEGG)and gene ontology (GO) analyses and provides a score for each target gene that correlates with the reliability of the target gene.genes and genomes (KEGG) and gene ontology (GO) analyses, and provides a score for each target gene, which positively correlates with the reliability of the target gene, providing a good prediction of the mechanism of TCM[31].In this study, we proposed to use the BATMAN-TCM online analysis tool library to retrieve the active ingredients and related targets of tripterygium hypoglaucum(the parameters were set as: score cutoff > 20, adjusted P-value <0.05), “Organism” was selected as “Homo sapiens”, and input the related targets into the Uniprot database[32] (https://www.uniprot.org/), correct their names, and finally combine and de-weight all the potential target genes related to tripterygium hypoglaucum to derive the active ingredients and predicted targets.
2.2 Prediction of OLP-related targets
Using “oral lichen planus, Lichen Planus, Oral, lichen planus of the mouth, lichen planus of mouth” as the keywords, “Organism”was selected as “Hapo sapiens”.“Homo sapiens” was selected from the databases GeneCards[33] (https://www.genecards.org/),OMIM[34] (Online Mendelian Inheritance in Man (OMIM))(https://www.omim.org/), DisGeNET[35] (https://www.disgenet.org/), DrugBank[36] (https://go.drugbank.com/), TTD[37](Therapeutic Target Database, TTD) (https://db.idrblab.net/ttd/), NCBI[38](National Center for Biotechnology Information, NCBI) (https://www.ncbi.nlm.nih.gov/), and CTD[39] (Comparative Toxicogenomics Database, CTD) (http://ctdbase.org/) to retrieve OLP-related targets.After merging the above seven database targets and de-emphasizing them using the online mapping tool of Microbiotics[40] (http://www.bioinformatics.com.cn/), the relevant target genes of OLP were obtained.The intersecting targets of the potential targets of action of tripterygium hypoglaucum were obtained by the online mapping tool Venny2.1[41] (https://bioinfogp.cnb.csic.es/tools/venny/) with OLPrelated targets.
2.3 Protein Interaction Network Construction
Import the intersection genes of tripterygium hypoglaucum and OLP into the String database[42] (https://cn.string-db.org/).To create a visual protein-protein interaction (PPI) network, select “Homo sapiens” for “Organism”, set “minimum required interaction score”to “medium confidence (0.4)”, choose “hide disconnected nodes in the network” for “network display options”, and other parameters are kept at their default settings to derive the PPI relationship, and the data is imported into Cytoscape 3.9.2 software[43].The CytoNCA plugin[44] is used to filter based on degree, betweenness, and closeness values to select the top 10 key targets.
2.4 Construction and Visualization of the Disease-Target-Gene-Drug Network
Import the relevant data into Cytoscape 3.9.2 software[43] to create a “disease-drug-molecule-target” regulatory network diagram for treating OLP with tripterygium hypoglaucum.Use the Network Analyser[45] to analyze the results and obtain the top 10 effective components of tripterygium hypoglaucum.
2.5 Function analysis of GO and pathway enrichment analysis of KEGG
Import the intersection targets of potential targets of tripterygium hypoglaucum and OLP-related targets into Metascape[46] (https://metascape.org/gp/index.html) for GO functional analysis and KEGG pathway enrichment analysis and obtain relevant data, selecting the top 10 results for analysis.Next, the corresponding data will be imported into the online plotting tool, bioinformatics[40] (http://www.bioinformatics.com.cn/), to obtain GO functional analysis, including biological processes, cellular components, and molecular functions,as well as a KEGG enrichment analysis network diagram.All results will be presented in the form of bubble charts.
2.6 Molecular docking analysis and visualization
Perform molecular docking analysis between the top 10 core targets selected and the effective ingredients of the top 10 tripterygium hypoglaucum, download the 3D structures of the top 10 target proteins from the UniProt database[32] (https://www.uniprot.org/)as protein receptors, and download the 3D structures corresponding to the active ingredients of the top 10 tripterygium hypoglaucum compounds from the PubChem database[47] (https://pubchem.ncbi.nlm.nih.gov/) as small molecular ligands.Use PyMOL software[48] to perform dehydrating, hydrogenating, and other operations on protein receptors and small molecule ligands and convert them into pdb format, then use AutoDockTools[49] for molecular docking analysis,and finally import the molecular docking results into PyMOL software[48] for visualization analysis to obtain the corresponding docking mode diagram.According to previous literature reports,combining energies smaller than -5.0 kcal/mol indicates good binding affinity.
3.Results
3.1 Analysis of the Effective Components and Targets of tripterygium hypoglaucum
In the BATMAN-TCM database, using “kun ming shan hai tang”as the search term and a score cutoff > 20 and an adjusted p-value <0.05 as the screening criteria, we obtained 15 active ingredients and 123 target points.After removing duplicates, we obtained 15 active ingredients and 78 remaining target points, as shown in Table 1.
3.2 Predictive results for OLP-related targets
Using the keyword “oral lichen planus、Lichen Planus, Oral、lichen planus of the mouth、lichen planus of mouth”; 577, 12, 257,8, 1, 137, and 8865 target genes related to OLP were retrieved from the GeneCards, OMIM, DisGeNET, DrugBank, TTD, NCBI, and CTD databases, respectively.After merging and removing duplicate targets using a bioinformatics online tool, a total of 9109 OLPrelated target genes were obtained, as shown in Figure 1.After inputting 78 potential target points of tripterygium hypoglaucum into Venny2.1 together with 9109 OLP-related target points, we obtained 54 intersecting target points, as shown in Figure 2 and Table 2.
3.3 PPI network construction for screening key targets
Using the STRING database, PPI analysis was conducted on the 54 intersecting targets of tripterygium hypoglaucum and OLP according to the above settings, resulting in 45 interacting targets and 118 PPI relationships.Further analysis was conducted on the relationship between tripterygium hypoglaucum and OLP’s PPI (protein-protein interaction).The obtained data was imported into Cytoscape 3.9.2 software to generate the PPI network diagram of the target protein,as shown in Figure 3.In the diagram, the size and color of the circles representing the proteins are determined by their degree values, with larger degree values corresponding to bigger and darker circles.By using the CytoNCA plugin, we conducted a joint screening based on the degree, betweenness, and closeness values and identified the top 10 potential targets of tripterygium hypoglaucum on OLP.These targets include dihydrofolate reductase (DHFR), fibroblast growth factor 2 (FGF2), estrogen receptor 1 (ESR1), thymidylate synthetase (TYMS), methylenetetrahydrofolate dehydrogenase 1 (MTHFD1), mammalian target of rapamycin (MTOR),phosphoribosylglycinamide formyltransferase (GART), methionine synthase (MTR), methylenetetrahydrofolate reductase (MTHFR),androgen receptor (AR).
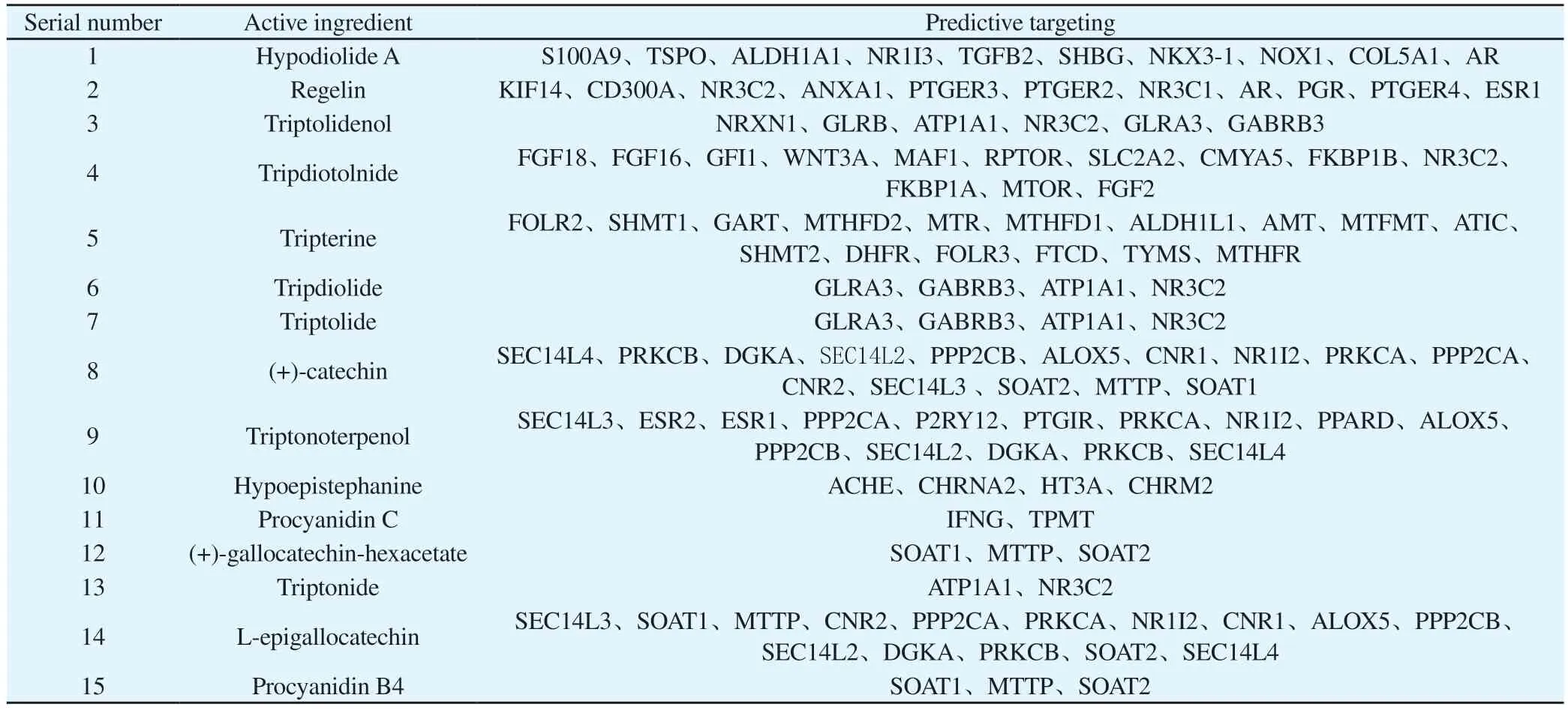
Tab 1 Active components and predicted target of tripterygium hypoglaucum

Fig1 OLP related targets
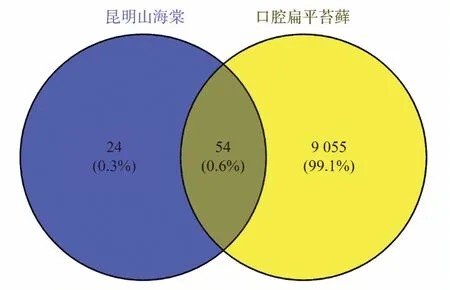
Fig 2 Venn diagram of the intersection target of tripterygium hypoglaucum and OLP
3.4 Construction and Visualization Analysis of the “OLPTarget-Active Ingredient-Tripterygium Hypoglaucum”Network
Use cytoscape3.9.2 software for network construction and visualization analysis of “OLP-target-active ingredient-tripterygium hypoglaucum”.Inclusion of 54 interaction targets between tripterygium hypoglaucum and OLP and 15 effective components of tripterygium hypoglaucum.In order to make the network diagram more intuitive, OLP and tripterygium hypoglaucum are added as well, as shown in Figure 5.Hexagons represent tripterygium hypoglaucum, triangles represent OLP, ellipses represent the interaction targets between tripterygium hypoglaucum and OLP,and rectangles represent the effective components of tripterygium hypoglaucum.Using the Network Analyzer, we analyzed the results and found that the top 10 active components in tripterygium hypoglaucum are Tripterine, Regelin, Hypodiolide A, Tripdiotolnide,L-epigallocatechin, Triptonoterpenol, Triptolide, Hypoepistephanine,Triptolidenol, and Procyanidin B4.
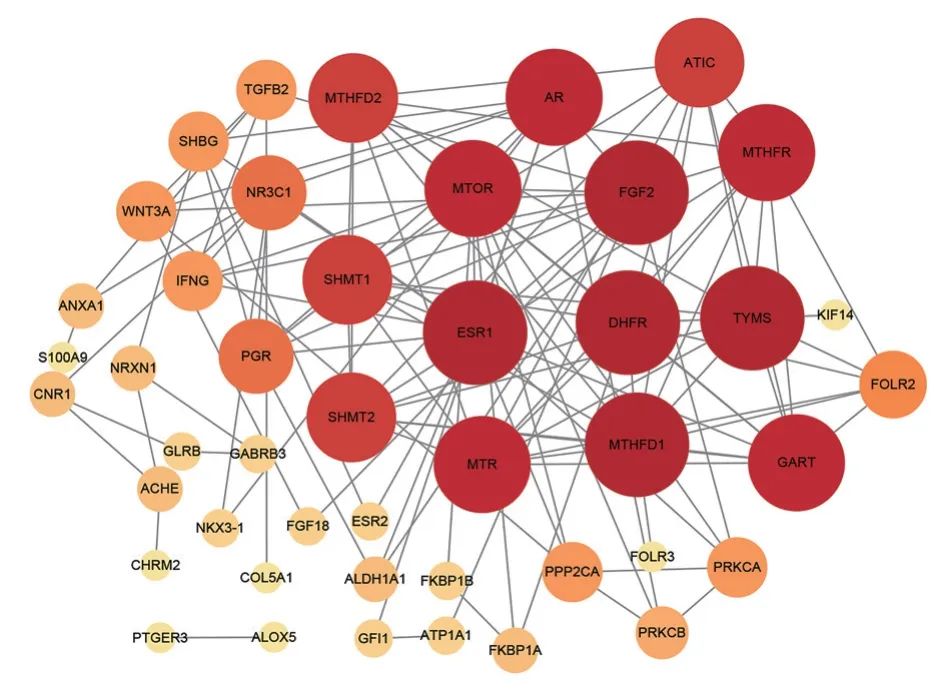
Fig 3 PPI network diagram of tripterygium hypoglaucum and OLP
3.5 Functional analysis of GO and enrichment analysis of KEGG pathways
In order to elucidate the biological functions of 54 tripterygium hypoglaucum genes that intersect with OLP target genes, GO functional and KEGG pathway enrichment analyses were performed, as shown in Figure 6.The enrichment analysis results of the GO function suggest that the biological processes mainly involve tetrahydrofolate metabolism, folate-containing compound metabolism, pyridine-containing compound metabolism, hormone level modulation, response to steroid hormones,etc (as shown in Figure 6a).Cell composition mainly involves synaptic membranes,extracellular matrix containing collagen protein, extracellular matrix, external encapsulation structures, presynaptic structures,etc (as shown in Figure 6b).The molecular functions mainly focus on nuclear receptor activity, ligand-activated transcription factor activity, steroid binding, carboxylic acid binding, organic acid binding, vitamin binding, etc (as shown in Figure 6c).The results of the KEGG pathway enrichment analysis showed that the main pathways enriched by 54 key targets are the one-carbon folate cycle,the tumour signalling pathway, resistance to antiretroviral drugs,chemical carcinogenesis-receptor activation, etc(as shown in Figure 6d).
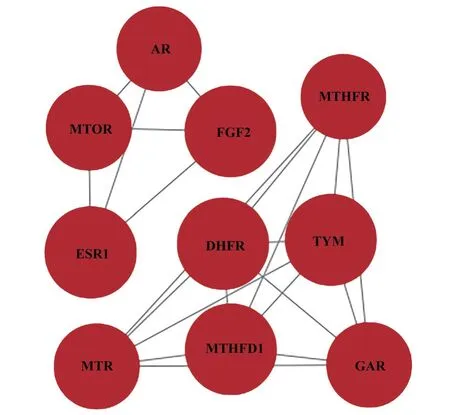
Fig 4 Screened PPI network diagram

Tab 3 The top 10 potential targets of tripterygium hypoglaucum for OLP
3.6 Molecular docking analysis and visualization results
Perform molecular docking analysis between the top 10 identified core targets and the top 10 active ingredients of tripterygium hypoglaucum.Select the results with the lowest binding energy among the dockings of the top 10 core targets, as shown in Table 4.The results indicate that the binding energies between tripterygium hypoglaucum and the 10 core target points are all less than -5.0 kcal/mol, suggesting that tripterygium hypoglaucum has a good binding affinity with the first 10 core target points.Visual analysis of the docking results was performed using PyMOL software.The top 6 binding energies were visualized in 3D in Figure 7, as shown in Table 4.
4.Discussion
Research has confirmed that tripterygium hypoglaucum is highly effective in treating OLP clinically[19 20], but its composition is complex and the medication is uncontrollable.This study aims to identify the interaction between tripterygium hypoglaucum and OLP by constructing a PPI network of tripterygium hypoglaucum and OLP and an “OLP-target-active ingredient-Tripterygium Hypoglaucum” network.The research investigates the bioactive components of tripterygium hypoglaucum for treating OLP.The ultimate goal is to enhance the clinical efficacy, reduce the toxicity,and improve the bioavailability of tripterygium hypoglaucum by modifying it, so that its medication can be more controllable.

Fig 6 GO function and KEGG path enrichment analysis
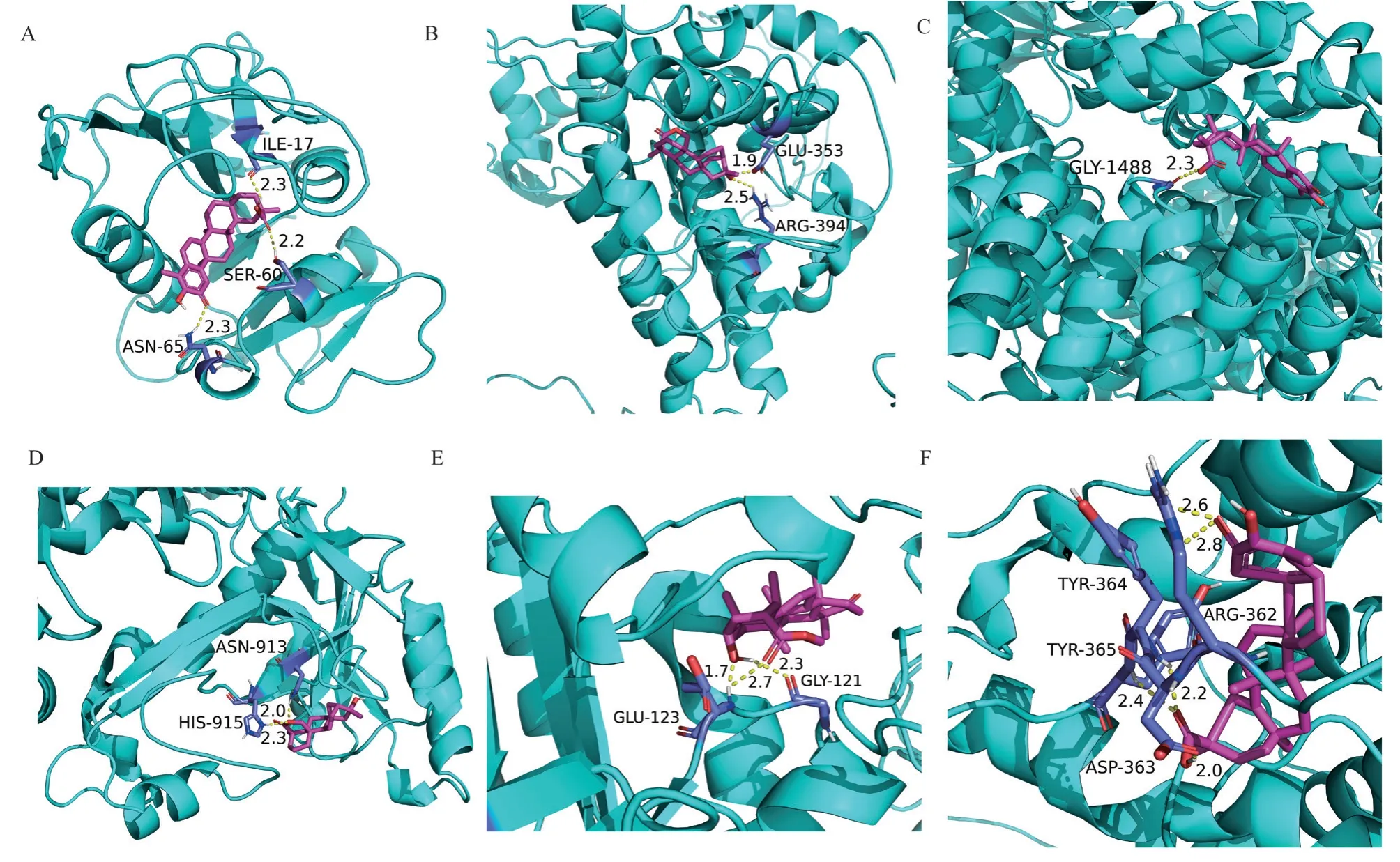
Fig 7 3D visualization of the molecule docking

Tab 4 Top 10 core target genes and their corresponding minimum binding energies of OLP and tripterygium hypoglaucum
To screen the 54 intersecting genes, GO functional analysis and KEGG pathway enrichment analysis revealed that the mechanism of tripterygium hypoglaucum in treating OLP mainly focuses on pathways such as the one-carbon folate cycle, tumor signaling pathways, and other pathways.A literature search found that patients with OLP have disturbances in the one-carbon folate cycle.The folate cycle can affect the proliferation and metabolism of T cells,and OLP is a T-cell-mediated inflammatory disease.This suggests that tripterygium hypoglaucum may exert its therapeutic effects on OLP by regulating the one-carbon folate cycle[50].Common tumor signaling pathways include PI3K/Akt, p53, etc.Li Ting et al.’s[51]research indicates that triptolide can inhibit the PI3K/AKT signaling pathway, inhibit the proliferation of human oral squamous cell carcinoma cells, and promote apoptosis of human oral squamous cell carcinoma cells.OLP is considered a potential malignant oral condition with a certain risk of carcinogenesis.Research has shown that the occurrence and development of OLP are related to the activation of cancer-promoting signal pathways.It is suggested that tripterygium hypoglaucum may inhibit the carcinogenesis of OLP by regulating tumor signaling pathways such as MAPK, PI3K/Akt,and p53[52, 53].Therefore, tripterygium hypoglaucum may treat OLP by regulating the one-carbon folate cycle, tumor signaling pathways,and other pathways.
The docking results of the PPI network and molecules revealed that DHFR, GART, ESR1, and other proteins are key target points for the treatment of oral lichen planus using tripterygium hypoglaucum.Both DHFR and GART regulate folate metabolism, affecting DNA synthesis and thus impacting the growth and development of organisms[54, 55].At present, research indicates that folic acid can inhibit T-cell apoptosis, increase the circulating number and proliferation of T-cells, and enhance innate immune responses[56].OLP is an oral mucosal inflammatory disease mediated by T cells[57].Therefore, it is inferred that DHFR and GART may mediate the growth and development of T cells and participate in the pathogenesis of OLP by regulating folate metabolism.ESR1 is a ligand-activated transcription factor belonging to the superfamily of nuclear hormone receptors and responsible for encoding estrogen receptor alpha.Estrogen promotes synaptic formation, induces the production of growth factors, and reduces oxidative stress.It can regulate the neurotransmission in brain systems associated with cognition and emotions (such as serotonin, norepinephrine, and acetylcholine).Research indicates a significant association between ESR1 polymorphism and anxiety or depression symptoms[58-60].Currently, research has found that the occurrence of OLP is related to anxiety or depressive symptoms caused by stress[61-63],suggesting that ESR1 may be involved in the development process of OLP.Therefore, tripterygium hypoglaucum may participate in the treatment of OLP by regulating key targets such as DHFR, GART,and ESR1.
In conclusion, this study utilized network pharmacology and molecular docking techniques to discover that tripterygium hypoglaucum may be involved in the treatment of OLP through pathways such as the one-carbon folate cycle and tumor signaling pathways.DHFR, GART, ESR1, etc.are potential key target proteins.The top 10 active ingredients of tripterygium hypoglaucum are: Tripterine, Regelin, Hypodiolide A, Tripdiotolnide,L-epigallocatechin, Triptonoterpenol, Triptolide, Hypoepistephanine,Triptolidenol, and Procyanidin B4.This study provides a theoretical basis for the development of new drugs targeting the abovementioned targets and their clinical applications.
Authors’ contribution
This article was written by Mei Jie, Wang Zheng’an, Kuang Huifang, Zeng Xiaomei, and Cai Hongxuan.Sun Yu, Fu Qiya, and Luo Wen reviewed the article.
Conflict of Interest
The article content does not involve any relevant conflicts of interest.
Ethical Requirement
Content that does not involve ethical conflicts.
杂志排行
Journal of Hainan Medical College的其它文章
- Meta-analysis of the impact of hyperuricemia on contrast agent-related acute kidney injury after percutaneous coronary intervention
- Research progress on the drug action and resistance mechanism in Mycobacterium tuberculosis
- Macrophage-derived exosomes mediate mir-222 targeting Caspase-10 to promote glioma proliferation
- Mechanism of action of verbascoside in mice with Parkinson's disease based on molecular docking, molecular dynamics and in vivo experiments
- Mechanism of Sanshi decoction inhibits macrophage pyroptosis by inhibiting BRD4/NF-κB/ NLRP3 pathway in the treatment of gouty arthritis
- Effects of female body mass index on embryo development and ART outcomes
