Oxidation of ciprofloxacin by the synergistic effect of DBD plasma and persulfate:reactive species and influencing factors analysis
2023-03-06ShilinSONG宋世林YuyueHUANG黄玉月YanshengDU杜彦生SisiXIAO肖思思SongHAN韩松KunHU胡坤HuihuiZHANG张会会HuijuanWANG王慧娟ChunduWU吴春笃andQiong阿琼
Shilin SONG (宋世林),Yuyue HUANG (黄玉月),Yansheng DU (杜彦生),2,Sisi XIAO(肖思思),2,Song HAN(韩松),2,Kun HU(胡坤),Huihui ZHANG(张会会),Huijuan WANG (王慧娟),2,*,Chundu WU (吴春笃),2 and Qiong A (阿琼)
1 School of the Environment and Safety Engineering,Jiangsu University,Zhenjiang 212013,People’s Republic of China
2 Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment,Suzhou 215009,People’s Republic of China
3 Environmental Science Institute of Tibet Autonomous Region,Lhasa 850000,People’s Republic of China
Abstract A synergistic system of water falling film dielectric barrier discharge (DBD) plasma and persulfate(PS)was set up and used for oxidizing ciprofloxacin(CIP)in water.Results of reactive species formation in the DBD-only system as well as the DBD-PS system verified the PS activation in the DBD system.Influencing factors on CIP degradation and the degradation process were also been studied.The obtained results showed that the presence of PS could greatly improve the degradation and mineralization of CIP and that the degradation efficiency could reach 97.73%after only 40 min treatment with 4 mM PS addition.The increase of PS concentration,the lower CIP concentration,the acidic solution pH and the addition of metal ions(Fe2+and Cu2+)enhanced the CIP degradation,while the existence of Cl-and had a negative effect.The experiments related to scavenger addition confirmed the contribution of the main reactive species to the CIP oxidation.Three probable degradation pathways were proposed by analyzing the inorganic ions and organic byproducts formed during the CIP degradation.The toxicity evaluation results of the CIP and its intermediates confirmed the effectiveness of the DBD-PS synergistic system.
Keywords: dielectric barrier discharge plasma,persulfate,reactive species,ciprofloxacin oxidation,influencing factors
1.Introduction
As a third-generation quinolone antibiotic,ciprofloxacin (CIP)has been frequently detected in surface water,wastewater and even drinking water in recent years [1].CIP that enters the aquatic environment eventually takes the form of a prototype or a metabolite,posing a significant threat to human health and aquatic ecosystems even at trace levels due to its high toxicity and persistence [2-4].
As antibiotics cannot be oxidized effectively through various conventional methods,advanced oxidation technologies(AOTs)have been developed to remove these pollutants.Among the various types of AOT,non-thermal plasma technologies have attracted extensive attention as there are multiple physical (UV irradiation and shock wave) and chemical processes [various reactive oxygen species (ROS)]involved in pollutant degradation [5-8].However,using discharge plasma for wastewater treatment has the problem of a relatively low mineralization efficiency for organic pollutants.Although efficient removal of organic pollutants could be achieved by enhancing the discharge power and prolonging the treatment time,the energy consumption would also increase significantly.Therefore,setting up synergistic processes based on the non-thermal plasma technologies is an effective solution to overcome the problem.
Persulfate(PS)activation technology has attracted a great deal of attention for pollutant removal in the past few years due to the formation of sulfate radicals (·),which have a strong oxidation capacity (2.5-3.1 V).Studies have proved that·and a series of ROS produced by PS activation can dissociate various organic pollutants [9].Many methods,including those using heat,light and chemicals (transition metal ions,zerovalent iron or activated carbon),have been proven to activate PS to successfully form· [10-12].Recently,non-thermal plasma has shown excellent PS activation performance and consequently improved the mineralization efficiency of pollutant degradation [13-15].On one hand,the heat,UV light and high-energy electrons (e*) produced in the plasma system activate the PS to form various radicals which then strengthen the degradation; on the other hand,the main species of O3formed during the discharge process provide another route of PS activation.The extra·OH and·results in further enhancement of organic compound degradation [16].
Based on the above analysis,a synergistic system of dielectric barrier discharge (DBD) plasma and PS was set up in this work to treat CIP wastewater.A water falling film DBD plasma system,which was considered having more O3formation [17,18],was used to activate the PS.The combining activation of the PS induced by the radiation and O3formed concurrently in the DBD process was utilized to promote the more radicals’ formation and then the better degradation of the CIP.The reactive species formation and influencing factors analysis were carried out to verify the synergistic effect of the DBD plasma and the PS activation.Other detection techniques,such as chemical oxygen demand(COD) and total organic carbon (TOC) removal,UV-vis spectroscopy,three-dimensional fluorescence spectrum analysis,degradation pathways,and the corresponding toxicity analysis,were also implemented to illustrate the CIP degradation in the synergistic system.
2.Experiment
2.1.Chemical materials
Sodium persulfate (Na2S2O8,≥98%),sodium chloride (NaCl,≥99.5%),sodium hydrogen carbonate (NaHCO3,≥99.5%),ethanol (EtOH,≥99.8%),tert-butyl alcohol (TBA,≥99%),triethylenediamine (TEDA,≥96%),sodium dihydrogen phosphate (DHP,≥98%),p-benzoquinone (BQ,≥98%),sodium hydroxide (NaOH,≥96%),titanyl sulfate and 30% hydrogen peroxide were purchased from the China National Pharmaceutical Group Corporation.CIP(≥98%),5,5-dimethyl-1-pyrroline N-oxide(DMPO,≥97%),2,2,6,6-tetramethylpiperidine(TEMP,98%),ferrous sulfate (FeSO4,≥98%) and copper sulfate(CuSO4,≥99%) were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.Acetonitrile (C2H3N,≥99.9%)was purchased from Shanghai Macklin Biochemical Technology Co.,Ltd.All reagents were used as purchased without further purification.
2.2.Experimental system and methods
The water falling film DBD plasma system used in the study as well as the detailed parameters of the system were described in our earlier research[19].A schematic diagram is also shown in figure S1 (available online at stacks.iop.org/PST/25/025505/mmedia).The ice bath cooling system and the fan shown in the figure were used to control the solution temperature.The solution temperature change during the reaction period is from 19 °C to 34 °C.An oscilloscope(Tektronix DPO 2014b) and probes (Tektronix,P6015A and TPP0200) were combined together to record the voltage and current waveforms as well as the Lissajous figure for the discharge power calculation.In each experiment,the discharge power was set at 0.4 kW,and 3 l CIP solution or deionized water was circulated in the experimental system using a peristaltic pump(China,Baoding Lead Fluid,BT600/YZ15)at a flow rate of 1.5 l min-1.A magnetic stirrer(China,Xinrui,DJ-1) was used to ensure uniform mixing of the solution.The total reaction time was 40 min and samples were taken every 10 min for the following analysis.
2.3.Analytic methods
High-performance liquid chromatography apparatus (China,Lunan Ruihong,P1010)assembled with a UV detector and a Symmetry C18 column (Φ4.6 × 250 mm) was used to measure the CIP concentration at a wavelength of 277 nm.An acetonitrile and water(0.1%formic acid) 13:87 v/v solution at a flow rate of 1 ml min-1was used as the mobile phase.The CIP degradation efficiency (DE) was calculated according to formula (1),and the energy yield (EY) of CIP degradation [G50,mg (kWh)-1]was calculated using formula (2).The synergetic factor(SF)was also calculated(formula(3))to illustrate the synergetic effect of the DBD plasma and the PS[20].
Here,C0(mg l-1) is the initial concentration of CIP;Ct(mg l-1) is the CIP concentration obtained aftertmin reaction;V(L) is the solution volume (3 l in this study);f(Hz) is the applied discharge frequency;C(F) is the internal sampling capacitance of the power supply;Sis the calculated area according to the obtained Lissajous figure;t50(h) is the time taken for 50% CIP degradation (obtained from the kinetic analysis results of CIP degradation).kDBD+PSis the kinetic constant of CIP degradation in the DBD-PS system;kDBDis the kinetic constant of CIP degradation in the DBD-only system;kPSis the kinetic constant of CIP removal by only the addition of PS.
The initial pH value of solution was adjusted using 0.1 M NaOH and 0.1 M HCl solutions.During the experiments,no more than two drops of HCl solution were used to adjust the solution pH value,and the corresponding Cl-concentration was about 0.03 mM,so the effect of the Cl-on the collection of radicals was overlooked.A pH meter (China,Shanghai INESA,PHS-3E) was used to measure the pH values of the different solutions,and the solution conductivity was monitored by a conductivity analyzer (China,Shanghai INESA,DDS-307A).Full-band scanning of the CIP solutions during degradation at different reaction times was recorded with a UV-visible spectrophotometer (Japan,Shimadzu,UV-2600).Solution COD and TOC values were detected by a COD analysis meter(China,Lianhua,5B-3A)and a TOC-measuring instrument (German,German element,Vario TOC select)respectively.Molecular fluorescence properties of the degradation intermediates were characterized by a three-dimensional fluorescence spectrophotometer(Hitachi,F-4600).An electron spin resonance instrument (ESR; Germany,Bruker,Micro ESR) was used to determine the relative amount of HO· and· radicals.H2O2concentration was determined by the titanyl sulfate method[7]with a UV spectrophotometer(China,Shanghai Yidian,N4S).O3concentration in the liquid phase was measured using an ozone detector (China,Shanghai Haiheng,CY-1A).The byproducts formed during the CIP degradation process were identified via liquid chromatography-mass spectrometry (LC-MS; Thermo LXQ).All experiments were repeated three times and the results are shown with an error bar.
3.Results and discussion
3.1.Reactive species formation
The concentrations or relative concentrations of critical reactive species formation,including O3,H2O2,·OH,·and1O2,in the DBD-only system and the DBD-PS system with deionized water were first analyzed to verify the synergetic effect of the DBD plasma and the PS.Figure 1(a)reveals that the O3concentration in the DBD-PS system was higher than that in DBD-only system.This might be due to the higher solubility of O3in the solution with a lower pH value,which was caused by the addition of PS [18].In addition,during the first 30 min reaction time,O3accumulated and the concentration increased,and the concentrations decreased at 40 min.This might be due to the slight increase of solution temperature during the reaction,which led to O3decomposition.Figure 1(b) lists the change of H2O2concentrations in the DBD-only system as well as the DBD-PS system during the reaction process.Evidently,H2O2concentration increased gradually by prolonging the reaction time in the DBD-only system,which was due to the accumulation of the active substances accompanying the reaction.However,the H2O2concentration in the DBD-PS system decreased with the extension of reaction time and the concentrations were lower than those in the DBD-only system.The results might be attributed to the consumption of H2O2induced by the PS activation (reactions (4) and (5)) [21].

Figure 1. Reactive species formation: (a) O3,(b) H2O2,(c) ESR (DBD-only),(d) ESR (DBD-PS),(e) ESR (DBD-PS).Experimental conditions: P = 0.4 kW,deionized water,PS concentration is 4 mM.

Figure 2. Effect of the PS concentration.(a) Degradation efficiency,(b) kinetic analysis,(c) energy yield,(d) SF value.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1.

Figure 3.Effect of initial concentration of the CIP.(a)Degradation efficiency,(b)kinetic analysis,(c)energy yield.Experimental conditions:P = 0.4 kW,PS concentration is 4 mM.

Figure 4. Effect of the initial pH value.(a) Degradation efficiency,(b) kinetic analysis,(c) energy yield.Experimental conditions:P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.
3.2.Influencing factor analysis
3.2.1.PS concentration.The effect of PS concentration on CIP degradation in the DBD-PS system was investigated in the study and the results are summarized in figure 2,including degradation efficiency,kinetic analysis,energy yield andSFvalues.The reviewed PS concentrations were 1,2,3,4 and 5 mM,respectively.
As an increase of PS concentration could lead to more radical formation and accordingly more efficient degradation of pollutants [24],figure 2(a) shows that the degradation efficiency of CIP was enhanced by the gradual increase of PS concentration and the enhancement was dramatically evident compared with those in the DBD-only and PS-only systems.The degradation efficiency reached 97.73% after 40 min treatment in the DBD-PS system with 4 mM PS addition.The results confirmed the synergistic effect of the DBD plasma and the PS on CIP degradation,which was mainly due to the PS activation induced by the physical and chemical effects formed in the plasma system.In the activation process,·and the extra·OH(as shown in figure 1)were formed through a series of reactions and then contributed to the further degradation of the organic compounds.Comparing the CIP degradation in the DBD-PS system with different additive concentrations of PS,it can be seen that the increase of CIP degradation was more obvious in the 1-4 mM PS addition range than with 5 mM PS addition.The persulfate anion reacted with the formed· when excessive PS was added into the reaction system (reaction (13)) [25],which accordingly resulted in a moderate increase of CIP degradation when the additive concentration of the PS exceeded 4 mM.
Combining the results of the degradation efficiency and energy yield of the CIP degradation under different amounts of PS addition,4 mM was chosen as the optimal PS additive concentration for investigating synergistic effects in the subsequent experiments.
3.2.2.CIP concentration.Figure 3 shows the CIP degradation in both the DBD-PS system and the DBD-only system when changing the initial concentration of the CIP.Apparently,significant removal of CIP was observed in the DBD-PS system and higher degradation could be obtained at a lower initial CIP concentration in the DBD-PS system as well as the DBD-only system(figure 3(a)).Furthermore,it can be seen from the results of the corresponding kinetic analysis(figure 3(b))that the kinetic constants increased by 12.53 times,10.68 times and 3.30 times,when the initial concentrations were 5 mg l-1,10 mg l-1and 20 mg l-1,respectively.The results were due to the relatively stable reactive species formation in both the DBD-PS system and the DBD-only system as the input power was constant.The limited reactive species could only oxidize a certain amount of target molecules,which led to a decrease of degradation percentage in accordance with the increase of the initial concentration of CIP.The slightly higher energy yield in the case of 10 mg l-1CIP solution (figure 3(c)) was caused by the relatively higher collision probability between the reactive species and the CIP molecules under the condition of a higher solution concentration.

Figure 5.Effect of inorganic ion addition.Experimental conditions:P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.
3.2.3.Initial pH value.The effect of the initial solution pH on the CIP degradation in the DBD-PS system was reviewed by adjusting the solution pH value from 2.45 to 11.03.The results are shown in figure 4.It can be obtained from the results that the degradation efficiency,thekvalue as well as the energy yield were relatively higher under the condition of initial pH of 2.45.It can also be seen that there was a synergistic effect of the DBD plasma and the PS under different solution pH values and theG50was highest under the condition of an initial pH of 2.45.Under the condition of acidic solution,PS could be decomposed into·(reactions(14)and(15))and then attack organic pollutants(such as CIP)rapidly [26].Under neutral or alkaline pH conditions,·could undergo a quenching reaction,which reduced the formation of active substances and slowed down the CIP degradation (reactions (16)-(19)).Although· can be converted to·OH,the presence of various ions(such asand)in the solution might play an inhibitory role in the reactivity of ·OH or· [26-28].
3.2.4.Inorganic ion addition.To broaden the possible application of the synergistic system in practical wastewater treatment,the effects of adding different metal ions (Fe2+,Cu2+)and inorganic anions(Cl-,HC-O3)on CIP degradation were also studied and the results are described in figure 5.The corresponding chemicals representing the ions were FeSO4,CuSO4,NaCl and NaHCO3(2 mM).
Figure 5 shows that the CIP degradation performance was significantly improved within the first 10 min reaction when Fe2+was added into the DBD-PS system.The concurrent addition of Fe2+and PS in the DBD plasma system could contribute to increased production of· and·OH through PS activation by the Fe2+and the Fenton reaction (reactions (20) and (21)) [14],and then lead to an rapid increase of CIP degradation in the primary stage.CIP removal was also enhanced by addition of Cu2+,the CIP degradation percentage reached 100% after 40 min reaction.The results might be due to the PS activation induced by the Cu2+and the catalysis of Cu2+for the H2O2decomposition(reactions (22)-(24)) [29].Figure 5(c) illustrates the CIP degradation in the DBD-PS system with Cl-addition.It can be seen from the figure that the degradation efficiency was reduced by 13.13%after 40 min reaction when 2 mM Cl-was added into the synergistic system,which was mainly due to the formation of Cl· by the reactions between the Cl-and· or ·OH (reactions (25) and (26)) [30].Furthermore,the CIP degradation efficiency decreased with a further extension of reaction time whenwas added,which was due to the inhibition reactions of· and ·OH(reactions (27) and (28)) [31,32].
It can be observed from figure 6(a) that the CIP degradation exhibited an obvious decline from 97.73% (no scavenger) to 41.31% (TBA addition) and 61.48% (EtOH addition),respectively,indicating that the CIP degradation obeyed the following inhibition order: TBA > EtOH.Combining the different reaction rate constants of TBA and EtOH with ·OH and·,it could be concluded that there was more ·OH in the DBD-PS system,which led to the stronger CIP degradation.The addition of BQ,DHP and TEDA also had negative effects on CIP degradation (shown in figure 6(b)),which indicated the positive effects of·,e*and1O2on CIP oxidation.
3.3.Degradation of the CIP
3.3.1.UV-vis analysis.Figure 7 illustrates the UV-vis spectra of the CIP solutions at different reaction times in the DBD-only and DBD-PS systems.It can be seen from the figure that the characteristic peak of CIP appears at 277 nm,and the intensity of the characteristic peak decreases with the prolonged reaction time in the DBD system with or without PS addition.The decrease is more significant in the DBD-PS system,which confirms the synergetic effect of DBD plasma and PS,and beyond that,the byproducts formed by CIP degradation were responsible for the gradual increase of the absorption intensity around 200-250 nm.The byproducts with a conjugated double bond,benzene ring and an unsaturated carbonyl group had absorption peaks at 190-230 nm [33],and further oxidation of these byproducts would cause the continuous accumulation of substances with absorption peaks of 210-230 nm.

Figure 6. Effect of radical scavenger addition.(a) Quenching tests for ·OH and· (200 mM),(b) quenching tests for·,e* and 1O2(5 mM).Experimental conditions: P = 0.4 kW, C = 10 mg l-1,PS concentration is 4 mM.

Figure 7. UV-vis analysis.(a) DBD-only system,(b) DBD-PS system.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.
3.3.2.Three-dimensional fluorescence spectrum analysis.The conjugated heterocyclic structures in organic compounds show different fluorescence intensities in three-dimensional fluorescence spectra [34].The fluorescence in different regions also represents different components,and the corresponding change of intensities can reflect the relative concentration change of the organic compounds [35].Therefore,the evolution process of the CIP solution during the degradation process was analyzed by three-dimensional fluorescence spectroscopy,as shown in figure 8 (obtained at 0 min,DBD-only 40 min and DBD-PS 40 min,respectively).Figure 8(a)presents that the fluorescent peaks were located in the range ofEx/Em= (376-550 nm)/(200-375 nm),which belong to humic acid-like and fulvic acid-like substances.With the extension of reaction time,the fluorescence intensities within the above range decreased (figure 8(b)),which indicated that the conjugated heterocyclic structures of the CIP were destroyed and the heterocyclic structure intermediates were oxidized.After 40 min DBD-PS treatment,the fluorescence in the position significantly reduced (figure 8(c)),which illustrated that the conjugate heterocyclic structures of the CIP were largely destroyed.The results verified the more effective CIP degradation in the DBD-PS system.
3.3.3.Variation of solution pH and conductivity.Figure 9 displays the variation of the CIP solution pH and conductivity during reactions in the DBD-only system and the DBD-PS system.Figure 9(a) presents that the solution pH decreased from the initial value of pH 3.83 to 2.25 during the 40 min reaction time in the DBD-only system and the corresponding solution conductivity increased from 0.0099 to 1.596 mS cm-1,while the solution pH value decreased from pH 3.47 to 2.00 and the solution conductivity increased from 0.607 to 3.407 mS cm-1in the DBD-PS system(figure 9(b)).With the mineralization of CIP,some byproducts,such as organic acids and inorganic ions (i.e.,etc),were produced in the system [4],which resulted in the decrease of solution pH and the increase of solution conductivity.

Figure 9. Variation of the solution pH and conductivity during the reaction.(a) DBD-only system; (b) DBD-PS system.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.

Figure 10.TOC and COD removal.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.

Figure 11.Anion formation.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.
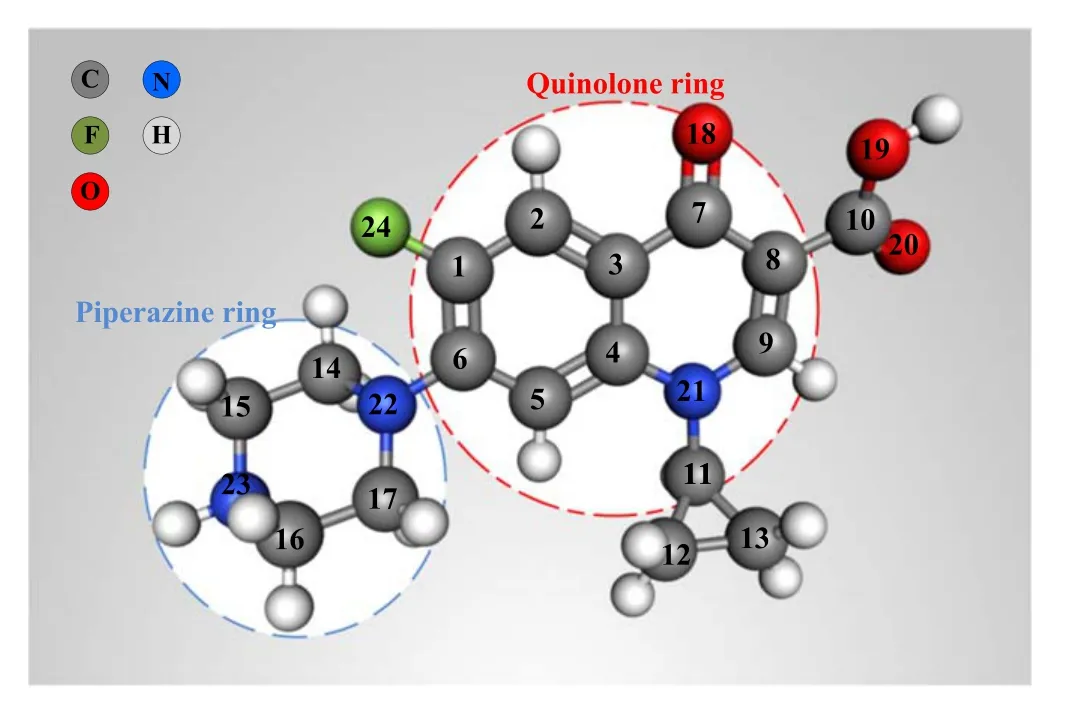
Figure 12.Ball-and-stick model of CIP.

Figure 13.Three probable degradation pathways of CIP in the DBD-PS system.Experimental conditions: P = 0.4 kW, C0 = 10 mg l-1,PS concentration is 4 mM.

Figure 14.Toxicity evaluation of CIP and its byproducts.(a) Pathway I,(b) Pathway II,(c) Pathway III.
Furthermore,nitric acid and carbonic acid could also be produced by the gas ionization and then cause a change of solution properties [9].The lower solution pH and higher conductivity in the DBD-PS system after the reaction illustrated the deeper mineralization of the CIP by the synergistic effect of the DBD plasma and the PS activation.
3.3.4.TOC and COD removal.In order to evaluate the mineralization and degradation degree of the CIP solution in the DBD plasma system with or without PS addition,TOC and COD removal was examined.It can be observed from figure 10(a)that the TOC removal in the DBD plasma system was only 13.81%and the value was enhanced to 25.75%after adding PS.In addition,the calculated energy yield of the TOC reduction in the DBD-PS system was 136.71 mg(kWh)-1,which was 58.81 mg (kWh)-1higher than that in the DBD-only system (77.9 mg (kWh)-1).The results indicate an accelerated mineralization process with the addition of PS.Meanwhile,the COD removal results shown in figure 10(b) display that COD removal was 29.59% in the DBD-only plasma system,while it rose to 49.40% after adding the PS.The results indicated that DBD plasma with added PS could relieve water pollution more effectively.
3.4.Degradation byproducts and pathway analysis
3.4.2.Degradation pathway analysis.The organic intermediates of CIP degradation were identified by LC-MS and the possible degradation pathways in the DBD-PS system were then proposed.By analyzing the LC-MS spectra obtained at different time points (10,30,40 min),the substances with high content were selected to speculate the degradation path.The corresponding spectra are shown in figure S2.The obtained mass charge ratio (m/z),molecular formula and different chemical structures of the byproducts formed during the CIP degradation are summarized in table S1.According to the theoretical calculation of the Fukui functions and frontier electron densities (FEDs) of CIP reported by Liuet aland Xuet al[38,39],the CIP reaction sites were preferentially attacked by ROS.The O18,C9,C1,N22,O14 and C6 atoms shown in figure 12 were more vulnerable to attack by·OH and·.The N22,C10 and F24 positions were vulnerable to attack by1O2and·radicals,which can preferentially react with organic compounds through the electron transfer mechanism.The N1 and N4 atoms on the piperazine ring contained lone pair electrons[40],which might be attacked by· and then produce ammonium cation radicals through the electron transfer mechanism,and then the cracking of organic compounds could be finally realized.
According to the above analysis of the possible CIP oxidation reactions,three probable degradation pathways were proposed,as shown in figure 13.Pathway I: the piperazine ring of CIP (m/z= 332) suffered from cleavage and oxidation to produce E1 (m/z= 305) due to the attack by strong oxidizing substances (· or ·OH) [40,43],and the E1 was further transformed to E2(m/z= 262)by losing the piperazine ring and a decarbonization reaction.E2 then underwent a hydroxyl substitution reaction and a desorption reaction by the·OH attack to form E3(m/z= 261).Finally,E4(m/z= 201)was formed by losing HO-and a quinolone decarboxylation [41].Pathway II: E5 (m/z= 274) could be formed by the attack of·and·OH on the piperazine ring and a simultaneous defluorination reaction on the quinolone ring[40].Then,E6 (m/z= 245) was produced through decarboxylation and hydroxyl substitution reactions [41].Under the continuous attack of ROS,the ring-side group of the quinolone ring was opened and E6 transformed into E7(m/z= 177)[42];then,-COOH groups were cracked to form E8 (m/z= 149).Pathway III: the piperazine ring of CIP was oxidized and cracked to produce E9 (m/z= 361).Then,E10 (m/z= 333)was generated via the missing of one carbonyl group from E9.Subsequently,E11 (m/z= 290) was formed by deamination and a carbonylation reaction,and the intermediate product of E12 (m/z= 260) was derived from decarboxylation and a hydroxyl substitution reaction of E11[4].The further oxidative cleavage of the cyclopropane could result in the loss of -CH2and the formation of E13 (m/z= 220) [40].Finally,E14(m/z= 181) was generated through the cleavage of the quinolone rings and the loss of N22 [42].These intermediate products then might undergo a series of reactions and be eventually mineralized to CO2,H2O,F-,[1,13].
3.5.Toxicity evaluation
The toxicity of the byproducts produced by organic pollutant degradation has important theoretical and practical significance for improving water environment quality.Acute LC50(LD50) is a statistic of a substance that can be expected to cause death in 50% of the animals when given by a specified route as a single dose and the animals observed for a specified period of time [44].Developmental toxicity represents the harmful effects of exogenous physical and chemical factors on offspring before they reach adulthood,including structural deformity,growth retardation,dysfunction and death[20].Mutagenicity refers to the ability of a substance to change the chromosome base sequence of biological (especially human)cells.Therefore,the developmental toxicity and mutagenicity of CIP and its degradation byproducts (formed through the three pathways) on minnow (LC50),daphnia(LC50) and rat (LD50) were analyzed using the Toxicity Estimation Software Tool (TEST) software to evaluate the effectiveness of the DBD-PS oxidation process.The results are presented in figure 14.
As displayed in figure 14(a)(Pathway I),the toxicity of E1,E2,E3 and E4 was lower than that of CIP to the three animals.The developmental toxicity and mutagenicity of the four byproducts were also lower than those of CIP.Similar results could also be obtained from figures 14(b) and (c) (Pathway II and Pathway III).Based on the toxicity analysis results,it can be concluded that CIP could not only be mineralized by the DBDPS treatment,but also the toxicity of the initial CIP solution decreased,indicating the effectiveness of the synergistic effect of the DBD plasma and the PS activation.
3.6.Economic analysis
To illustrate the economy of the DBD-PS technology,its energy yield for CIP degradation was compared with that of other technologies [8,20,45,46]and the results are summarized in table S2.It can be seen from the table that the energy yield of the DBD-PS process was significantly higher than that of other technologies.The comparison proved the economy of the technology used in the research.
4.Conclusions
CIP degradation using water falling film DBD plasma in the presence of PS in this work verified that PS could be activated by the DBD plasma,and then improved the degradation and mineralization of the CIP.The chemical and ESR analysis proved the production of reactive species,including O3,H2O2,·OH,·and1O2,in the DBD-PS synergistic system.A higher PS additive amount,a lower initial concentration of CIP and an acidic pH solution were favorable for CIP degradation in the DBD-PS system.The presence of Fe2+and Cu2+ions could accelerate the degradation,while the presence of Cl-andhad an inhibitory effect.The radical scavenger addition verified the roles of ·OH,·,·,e*and1O2in the CIP degradation.The UV-vis and three-dimensional fluorescence spectrum analysis proved the better degradation by the DBD-PS system.Furthermore,three probable pathways of CIP degradation were proposed according to the results of the detected inorganic ions as well as the organic intermediates.The toxicity evaluation results showed that the organic byproducts usually have lower toxicities than its parent CIP.A laboratory study could provide proof of the synergistic effect of the DBD plasma and the PS for organic compound degradation,and a scaling up of treatment capacity for real wastewater could be conducted in the future based on this study.
Acknowledgments
The authors thank National Natural Science Foundation of China (No.21876070) for their support of this study.
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Efficient combination and enhancement of high-power mid-infrared pulses in plasmas
- Realization of Te0>10 keV long pulse operation over 100 s on EAST
- Solving Poisson equation with slowingdown equilibrium distribution for global gyrokinetic simulation
- Experimental studies of cusp stabilization in Keda Mirror with AXisymmetricity (KMAX)
- BITS: an efficient transport solver based on a collocation method with B-spline basis
- Comparing simulated and experimental spectral line splitting in visible spectroscopy diagnostics in the HL-2A tokamak
