纤维素纳米晶的空间受限自组装:从胶体液晶到功能材料
2023-02-25段一雄李云峰
段一雄, 杨 柏, 李云峰
(吉林大学化学学院, 超分子结构与材料国家重点实验室, 长春 130012)
1 Introduction
Cellulose nanocrystals(CNCs) have become promising building blocks for the preparation of many nanomaterials due to their inherent renewability, sustainability, and abundance[1—3]. The functional materials based on CNCs have been widely used in the field of plasmonic chiral materials[4], sensors[5,6],biomedicine[7—10], energy[11,12], catalysis[13], and environmental science[14—18]. CNCs are mainly preparedviathe acid hydrolysis of the amorphous domains of cellulose fibers to obtain crystalline domains[1]. CNCs have rod-like structures with a high aspect ratio, which makes them ideal building blocks for the formation of cholesteric(chiral nematic) liquid crystals(LCs). In general, the CNCs suspension in a glass vial undergoes phase separation into a cholesteric LC bottom phase and an isotropic top phase[19]. Initially, CNCs assemble into a small anisotropic droplet(tactoid) with cholesteric packing of CNCs[19]. These tactoids then coalesce and merge into the cholesteric LC phase[20,21].
Chiral nematic LCs self-assembled from CNCs have been extensively studied as photonic materials[22],templates[23], responsive materials[24]and reconfigurable materials[25]. Recently, the self-assembly of CNCs in confined spaces has attracted many interests in the fundamental research of soft matter and the exploration of innovative materials. In particular, the spatially confined assembly of CNCs provides a valuable platform for the fundamental studies of particle packing[26], self-assembly[27,28], relaxation of colloidal liquids[29,30]and topological defects[31,32]. Furthermore, confined assembly of the CNC cholesteric LCs provides a unique host template for the nanoparticle organization to prepare complex nanostructures[31—33]. The self-assembly of CNCs in confined spaces also enables the fabrication of cholesteric microgels[13], microfibers[34]and microparticles[31]with photonic properties.
Although recently some reviews have summarized the macroscopic LCs of CNCs and photonic crystals of CNCs[16,19], there are few research summaries of the self-assembly of CNCs in spatial confinement. In this review, we provide a comprehensive review of the self-assembly of CNCs in spatial confinement. We first review the preparation of CNCs and the research progress of the macroscopic LCs and photonic films assembled from CNCs. Subsequently, we summarize the self-assembly of CNCs in the spatial confinement and their co-assembly with other nanoparticles. We also review the fixation methods and their emerging applications of CNCs in spatial confinement, and discuss the current challenges and prospects of the self-assembly of CNCs in spatial confinement.
2 Cellulose Nanocrystals
The cellulose in the cell wall of plants shows the fibril form. The cellulose fibers consist of a bundle of nanofibers which are composed of elongated parts of a single crystal of cellulose separated by amorphous cellulose regions[14]. Amorphous domains in cellulose nanofibers are more vulnerable and preferentially attacked by an acid to release crystal domains to obtain CNCs[1]. Normally, the CNCs were extracted by using acid hydrolysis with 64% sulfuric acid. The CNCs show nanorod-like structure with a high aspect ratio(>10)(Fig.1)[35—37]. CNCs have excellent mechanical properties with high axial tensile strength(ca.7.5 GPa) and stiffness(ca.150 GPa)[19].
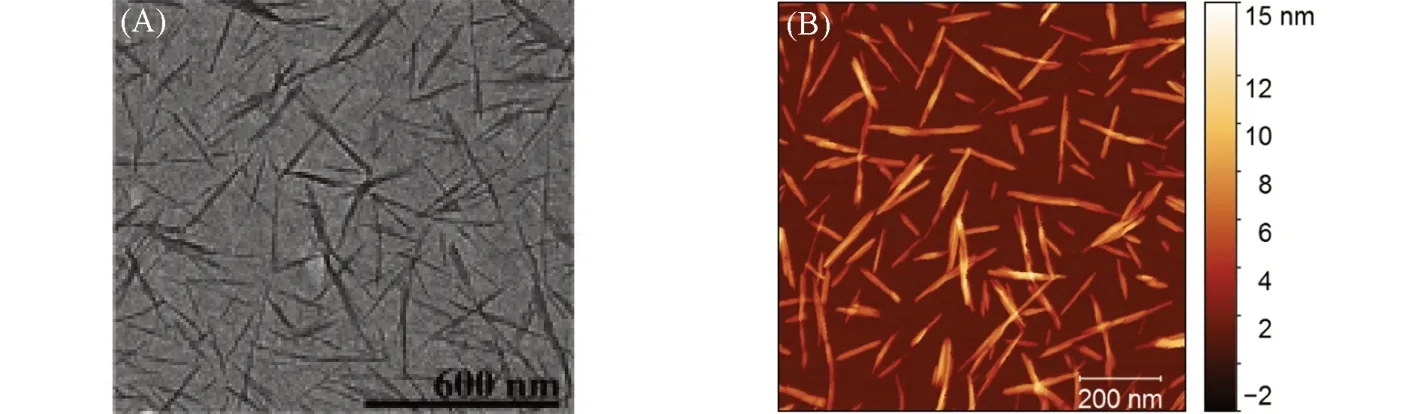
Fig.1 Representative transmission electron micrographs of CNCs(A)[36] and representative atomic force microscope images of CNCs(B)[37]
The size, morphology, and yield of CNCs depend not only on the cellulose sources and the acid hydrolysis procedure but also on the pretreatment after cellulose purification. Pretreatment of cellulose fibers with ionic liquids[38]or combined with ultrasound[39]can improve the thermal stability of CNCs. In addition,these procedures can also greatly reduce the amount of sulfuric acid used for hydrolysis, thereby reducing the number of anionic sulfate ester groups(—OSO3-). Enzymatic treatment of cellulose fibers can also reduce the sulfur content of CNCs and increase the yield and particle sizes of the CNCs[40].
The colloidal stability of CNCs depends on the electrostatic repulsion of the nanoparticle surfaces. The hydroxyl groups on the surface of CNCs are esterified to anionic sulfate ester groups which further enable the stable suspensions[41]. Conductometric titration can be used to quantify the sulfate content on the surface of CNCs, which can largely reflect the surface charge[42]. The thermal stability of CNCs is reduced due to the sulfate groups on the surface[43]. Pretreatment procedures[38,39]or hydrolysis by another acid such as hydrochloric acid[44]can improve the thermal stability of CNCs. In addition, other processes used to generate cellulose such as treatment by ionic liquids, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl(TEMPO) oxidation, and treatment by acidic deep eutectic solvents result in CNCs with different surface chemistries, charge densities, and morphologies[45].
A large number of hydroxyl groups and high surface area on the nanoparticle surface enable the facile functionalization of CNCs[46—48]. The surface of nanoparticles can be modified by small molecules or hydrophobic oligomers to enable the effective dispersion of CNCs in non-aqueous solvents or polymers[45]. The polyol chains were modified on the surface of CNCs by periodate or TEMPO oxidation to improve the steric stability and the ability to resist aggregation in a high-salt environment[49]. The surface charge not only affects the colloidal stability and thermal stability of CNCs but also is a key factor to be considered for tuning the pitch of chiral nematic LCs of CNCs[19].
3 Cholesteric Liquid Crystals of Cellulose Nanocrystals
The one-dimensional(1D) rod-like structure makes CNCs an ideal candidate for the formation of LCs[50].Similarly, the 1D anisotropic structures can also form LCs such as semiconductors nanorods, single-walled carbon nanotubes(SWNTs), and tobacco mosaic virus[51]. The CNC suspension results in lyotropic LCs, with phase separation into isotropic and anisotropic phases above a critical concentration[Fig.2(A) and (B)][52].The formation of the cholesteric LCs of CNCs starts with the tactoid. Tactoids are considered to be intermediate transition states of phase separation from isotropic dispersions to macroscopic anisotropic phases[53,54]. The CNC tactoids coalesce and merge into a multidomain macroscopic cholesteric phase with alternating dark and bright stripes[Fig.2(C)][52]. The CNC helix along an axis is left-handed possibly because of the intrinsic chirality of crystalline cellulose. The pitch value of the CNC cholesteric LCs varies from less than 1 to 50 μm and beyond.
Many parameters control the pitch value, including the CNC quality, its concentration, ionic strength of the suspension, and the temperature[14]. The CNC morphology, size, charge, and chemical modification on the surface of CNCs greatly affect their dispersion in solvents and their self-assembly of cholesteric LCs[1,55].The length and the aspect ratio of CNCs affect the formation of the cholesteric LCs, with longer CNCs preferentially forming cholesteric LCs[19]. The surface charge and the type and concentrations of electrolytes in solvents affect the helical pitch of cholesteric LCs. For example, in aqueous solution, the polymer-grafted CNCs formed similar cholesteric LCs in comparison with the unmodified CNCs, however, the pitch of the cholesteric LCs decreased[56]. In addition, the addition of surfactants is favorable for the dispersion of CNCs in non-polar solvents, and the pitch of the assembled chiral nematic structures isca.4 μm, which is much smaller than that in aqueous solutions[57].
Interestingly, the phase separation of CNCs was used to separate the nanoparticles of different sizes. The anisotropic tactoids of CNCs have a size-selective repulsion effect on foreign nanoparticles due to the different sizes of the LC lattice gap[54]. The gold nanoparticle partitioning occurred because of the phase separation of CNCs, leading to a nanoparticle enriched isotropic top phase and a nanoparticle poor cholesteric bottom phase[Fig.2(D)]. The amount of the gold nanoparticles enriched in the isotropic top phase increased as the sizes of the nanoparticles increased[Fig.2(D)]. In addition, superparamagnetic magnetite-doped nanoparticles were also separated by using the same procedure[21].

Fig.2 Cholesteric liquid crystals of CNCs
The chiral nematic structures of CNC suspension can be preserved in solid films to achieve photonic crystals[58]. The CNCs photonic crystals show unique optical properties such as selective reflection of lefthanded circularly polarized light[58]. When the pitch of the cholesteric CNCs approaches the wavelength length of visible light, the iridescent colors will appear due to Bragg scattering[16,59]. The CNCs chiral iridescent films can be ground into particles of various sizes to produce pigments for food coloring because of their inherent biocompatibility[60]. The optical properties of cholesteric CNC films are closely related to the helical pitch of the cholesteric LCs phase. For example, the dispersion of CNCs upon ultrasonic treatments showed a larger pitch in comparison with the dispersion without ultrasonic treatments, leading to CNCs cholesteric films showing red-shifted colors[61]. The adding salt in the dispersion of CNCs causes a decrease of the pitch,resulting in CNCs cholesteric films with blue-shifted colors[55]. These photonic materials can be used in colorimetric sensors due to their red-shifted or blue-shifted colors to different external stimuli[19].
The mechanical and optical properties of chiral nematic CNC films can be regulated by the introduction of neutral polymer, such as PEG, into CNC LCs. PEG can improve the mechanical stability of iridescent CNC coatings and the adhesion to hydrophobic surfaces without disrupting the liquid crystal structure[62]. The photonic flexible films of CNCs with uniformly structural colors were also fabricated and exhibited reversible swelling and dehydration by adjusting the content of PEG[63—65].
The combination of responsive polymers and cholesteric LC CNCs enabled the fabrication of responsive photonic materials. Several parameters can change the arrangement and the pitch of the cholesteric CNCs,including the type and concentration of the electrolyte chemicals[66], the electric[67], and magnetic fields[68],and thus affecting their optical properties.
Various CNCs-based photonic sensors have been developed and were used to detect humidity[69,70],chemicals[71], solvents[6], and mechanical pressure[70]. For example, the high-performance humidity sensor has been fabricated by using the composite of cholesteric CNCs and polyacrylamide. When the photonic film was exposed to water, the pitch of the chiral nematic structure increased due to the expansion of polyacrylamide in water, leading to the color changes of the composite photonic films[69].
A photonic elastomer based on the composite of CNCs and polymers has been prepared and showed reversible color changes upon the application of mechanical stress. With the elongation of film changing from 0 to 300%, the colors of the photonic elastic film changed from red to blue due to the decreasing pitch upon the stretch[Fig.3(A) and (B)][72].

Fig.3 Photonic materials derived from cholesteric LCs of CNCs
Recently, mesoporous photonic films were prepared by removing the CNCs in the composites of CNCs and urea-formaldehyde resins. These mesoporous photonic films showed dual sensitivity to both solvent and pressure[70]. The swelling degree of these films was very sensitive to the solvent. These mesoporous photonic films showed different colors when immersed in solutions of different ratios of ethanol/water due to the change of pitch during swelling. The colors of these photonic films changed from blue to red when the concentration of the ethanol decreased from 100% to 60%[Fig.3(C)]. In addition, these photonic films showed reversible piezochromic properties. The pressure reduces the pitch of the chiral nematic structure, leading to a blue shift.
Except for the photonic sensors, mesoporous photonic films were also used as a photonic actuator. This photonic actuator was composed of bilayer mesoporous composite films derived from cholesteric CNCs. Bilayer composite film was prepared by embedding phenolic resin with CNCs as a template and subsequent removal of CNCs[70]. Upon drying, the bilayer mesoporous photonic films bended. When the concave side of the bilayer films was treated with water vapor, the curled films straightened because of the film expansion caused by the selective swelling[Fig.3(D)][73]. The mesoporous photonic film was cut into the shape of a hand. The“fingers” of the hand-shaped films were straight when they were swollen in water. The selective dropping of the acetone on the fingers led to the bending of the films quickly with concomitant color changes[Fig.3(D)].
4 Self-assembly of CNCs in the Cylindrical Capillary
Self-assembly of CNCs in confined spaces such as spherical droplets or narrow channels provides a valuable way to generate novel structures and materials with potentially useful properties and functions.Confined self-assembly of cholesteric LCs of CNCs in the narrow capillaries has been used for the fundamental studies of relaxation dynamics[30], the formation of defects[74,75], and the exploration of optical materials[34,76,77].For example, the relaxation dynamics of CNCs confined into a cylindrical capillary were studied by a combination of experimental measurements and theoretical calculations[30]. Cholesteric LCs of CNCs in the cylindrical confinement showed a fast equilibration through smooth relaxation dynamics. To investigate the defects in the cholesteric LCs of CNCs in the cylindrical capillary, the cholesteric phase of CNCs was introduced into a glass capillary with an inner diameter of 100 μm[75]. The two-dimensional confinement led to phase separation of cholesteric CNC LCs into an isotropic core thread running parallel to the long axis of the capillary and a cholesteric shell of concentric CNC pseudo-layers with the helicoidal axis perpendicular to the inner surface of the capillary[Fig.4(A) and (B)].

Fig.4 Confined self-assembly of CNCs in the capillary
Recently, the self-assembly of cholesteric LCs of CNCs in a microscale alginate hydrogel sheath(liquid metacrystal) was studied by using a simple microfluidic spinning[34]. A mixed solution of CNCs, alginate, and glucose was injected into a CaCl2solution by using a microfluidic device[Fig.4(C)]. The shear forces during the extrusion of the mixed solution resulted in the alignment of the CNCs in the injection direction[Fig.4(D)]. Once the thread of the mixed solution exited from the nozzle, the alginate moved outward and formed a hydrogel layer triggered by Ca2+ions at the liquid-liquid interfaces, meanwhile, the unwinding phase of CNCs relaxed back to cholesteric CNC LCs. The cross-section of the liquid metacrystal fiber showed a distinct Maltese cross pattern corresponding to the radial helicoidal packing of CNCs[Fig.4(E)]. Moreover,an awl-shaped topological defect was observed in the hydrogel fibers of the liquid metacrystal[Fig.4(F)]. The fibers of the liquid metacrystal were woven into flexible fabrics which had applications in the polarizationbased encryption and recognition.
5 Self-assembly of CNCs in the Spherical Droplets
The spherical confinement of cholesteric CNCs in droplets offers an ideal geometry for self-assembly, in which the high curvature(elastic energy) and interface energy lead to very interesting phenomena and architectures. To date, solvent stabilization[78], emulsification[79], and droplet-based microfluidics[31,52]were usually used to confine the cholesteric CNCs in droplets. Despite the difficulty in controlling droplet homogeneity,solvent stabilization and emulsification are relatively simple and scalable[80]. In contrast, droplet-based microfluidic techniques were applied to produce homogenous droplets of CNCs with monodisperse size[31—33]. For example, the cholesteric self-assembly of CNCs in spherical droplets was reported by Liet al. by using a microfluidic flow-focusing droplet generator[31]. Prior to the confinement of CNCs in the droplets, the cholesteric phase of CNCs was separated from the two-phase system of an isotropic top phase and a cholesteric bottom phase. The cholesteric CNCs were emulsified in a microfluidic flow-focusing droplet generator to obtain uniformly sized droplets with a polydispersity ofca.2.5%[Fig.5(A)].

Fig.5 Confined self-assembly of CNCs in the droplets[31]
The size of the droplets was tuned from tens of micrometers to hundreds of micrometers by changing the flow rates of fluorinated oil(F-oil) and CNCs, as well as using microfluidic devices with different orifices[31,33].In this work, the confined self-assembly of CNCs in droplets showed characteristic structures of the cholesteric liquid crystal with a Maltese cross with alternating dark and bright concentric rings[Fig.5(B—D)][31]. This liquid crystalline structure of CNCs in the droplet is related to the tangential alignment of CNCs at the interface of droplet and F-oil with a radial orientation of the helical axis of the CNCs twists in space[Fig.5(G)][31]. The average pitch of the cholesteric CNCs with a volume fraction of 0.048 in droplets, measured as a double distance between two adjacent stripes wasca. 6 μm, which is close to the pitch of the macroscopic cholesteric CNC phase. Interestingly, the confined self-assembly of CNCs led to the phase separation in the droplets with an isotropic core(topological defect) and a cholesteric CNC shell[Fig.5(C)][31]. Moreover, a radial disclination that connected the core to the droplet surface was generated under the planar anchoring of CNCs over the whole droplet surface[Fig.5(C) and (D)][31].
The size of the droplets plays an important role in the self-assembled structures of cholesteric CNCs in the droplets. For example, the droplets with a radius over 115 μm showed multidomain cholesteric structures,being similar to the macroscopic cholesteric CNCs[31]. For the droplets with their radius in the range from 40 to 115 μm, most of the droplets exhibited an isotropic core and a cholesteric CNC shell with a concentric packing of CNC pseudo-layers[Fig.5(B—D)]. The droplets with the radius changing from 10 to 40 μm displayed a transitional pattern of ellipsoidal concentric layers with planar cholesteric CNC pseudo-layers in the center and tangential CNC packing at the droplet periphery[Fig.5(E) and(H)]. When the radius of the droplets was smaller than 10 μm, the droplets showed a bipolar stripe pattern with the flattened cholesteric layers trapped between the two diametrically opposite poles[Fig.5(F) and (I)].
The confined self-assembly of CNCs in droplets usually occurs initially at the spherical interface[31]. Due to the interfacial energy, the cholesteric CNCs are tangentially anchored at the two-phase interface[31,32]. With the release of the internal elastic energy, the equilibrium structure of CNCs gradually develops from the shell to the core center along the radial direction[31,33]. Furthermore, the equilibration time and the final structure after equilibration are heavily dependent on the droplet size[31]. In contrast, in capillary confinement, CNCs preferentially forms tactoids in the capillary and multiple tactoids merged to form equilibrium structure[30].Moreover, the confined self-assembly of CNCs in droplets leads to more controllable topological defects, such as disclination, dislocation, and point defect[75].
6 Co-assembly of Nanoparticles with Cholesteric CNCs in the Droplets
The topological defects in the cholesteric CNC droplets can be used as templates to organize the nanoparticles in the droplets. The co-assembly of functional nanoparticles with cholesteric CNCs in the droplets paved a valuable way for the preparation of the functional soft materials with liquid crystalline structures. For instance, the co-assembly of cholesteric CNCs with polymers, metals, carbon, or metal oxide nanoparticles,led to size-dependent nanoparticle partitioning in the isotropic cores of the droplets[31,32]. Because the average distance between the CNCs layers isca.30 nm[50], smaller nanoparticles were less disruptive to the cholesteric packing of CNCs and showed relatively homogenous distribution in the whole droplets. The increase in the nanoparticle size resulted in the nanoparticle enriching in the droplet cores[Fig.6(A—D)][31,32]. Moreover,the extent of nanoparticle segregation in the core increased with the increase of nanoparticle concentrations.The co-assembly of CNCs with other nanoparticles provided a route for the CNC droplets with plasmonic, fluorescent, and magnetic properties, accelerating the design of stimuli-responsive liquid crystalline materials.
The confined self-assembly of CNCs in the spherical droplets also led to a radial disclination line(topological defect) in the droplets. This disclination line in the droplets of cholesteric CNC templated the selfassembly of the nanoparticles[32], because of the reduction of the associated elastic energy in the system. Upon the co-assembly of the polymer nanoparticles with cholesteric CNC in the droplets, the nanoparticles formed a periodic array of discrete beads in the disclination line[Fig.6(B—D)][32]. Interestingly, the periodic interbead spacing was equal to the half-pitch of the cholesteric CNCs. Through the ultrasonic treatments to the CNC suspension, the pitch of the cholesteric CNCs increased from 3.1 to 5.1 μm. Accordingly, the average interbead spacing increased from 3.0 to 5.0 μm[Fig.6(E—G)][32]. These results in this work shed the light on our understanding of the hierarchical assembly of nanoparticles in complex liquid crystal environments,thus providing a useful strategy for the discovery of soft composite materials.
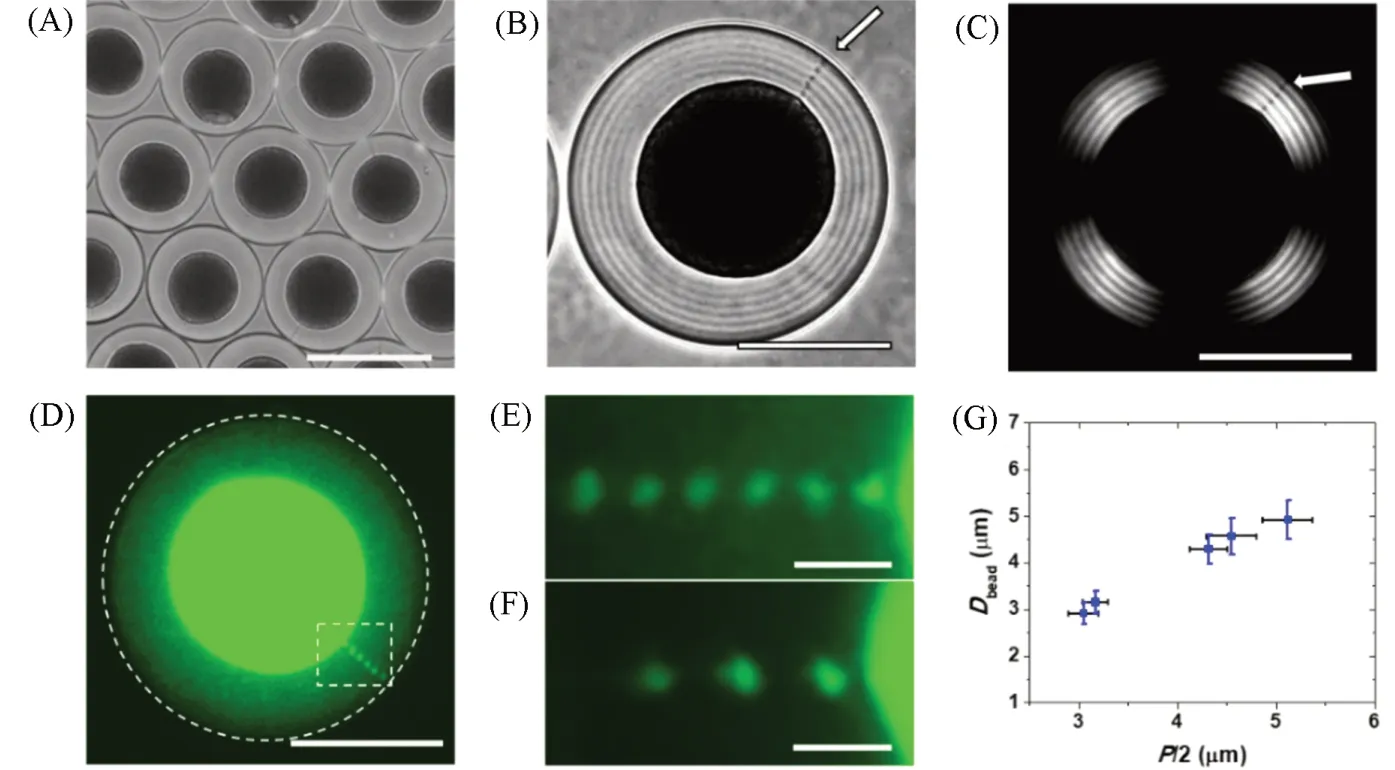
Fig.6 Confined coassembly of CNCs and nanoparticles in the droplet
In addition to the templating assembly of the nanoparticles in the droplets of the cholesteric LCs, the interplay between the cholesteric CNC packing and the nanoparticle organization endowed programmable nanoparticle assemblies with different compositions, shapes, and dimensions. The co-assembly of guest nanoparticles and host cholesteric CNCs in the droplets with flat-ellipsoidal packing of CNC pseudo-layers led to interactive morphogenesis of nanoparticle assemblies and a CNC host of a cholesteric LCs[33]. In this work,with the increase in the nanoparticle concentration, both the host CNCs and the guest nanoparticles experienced significant changes in assembled structures. The nanoparticles with low concentration formed two coneshaped structures at droplet poles[Fig.7(A—C)] or toroidal ring assemblies[Fig.7(D—F)], while the CNCs exhibited a flat-ellipsoidal packing of cholesteric pseudo-layers in the droplets. A significantly structural transformation in the droplets was triggered by the increase of the nanoparticle concentration to obtain a coreshell droplet with an isotropic core and a cholesteric CNC shell, with the nanoparticle partitioning in the core and disclinations[Fig.7(G—I)]. Using the droplets carrying magnetic nanoparticles as building blocks, the active assembly of the structural droplets was observed, yielding linear[Fig.7(J) and (K)][33]and staggered chains[Fig.7(L) and(M)] of droplet assembly. This work paves a way for nanoparticle organization in the complex anisotropic medium to achieve new types of soft nanostructured materials.
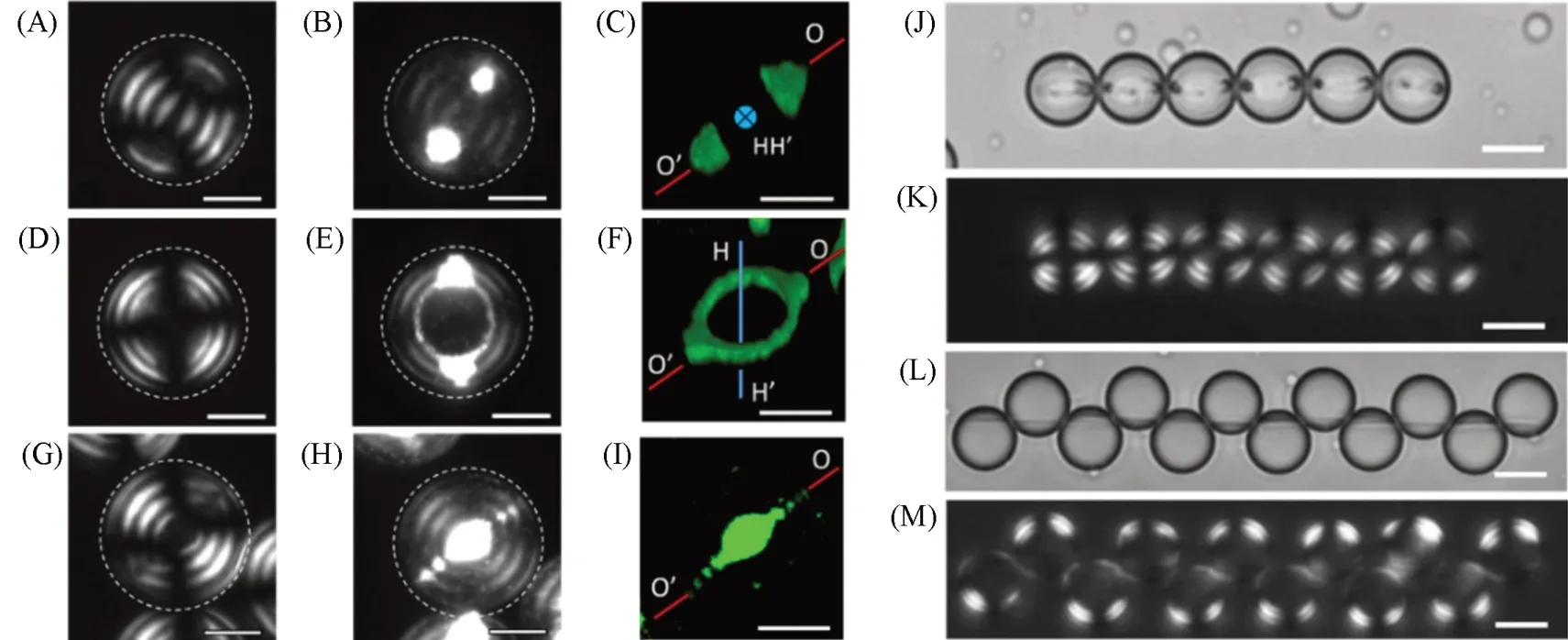
Fig.7 Confined coassembly of CNCs and nanoparticles in the droplet with flat-ellipsoidal packing of CNCs[33]
7 Solidification of Cholesteric CNCs in the Confinement
The self-assembly of cholesteric CNCs in the confined space resulted in hierarchical structures with useful properties, however, they usually showed poor stability. To extend their practical applications, solidification techniques were used to fix the structures of the cholesteric CNCs in the confined space. The structures of the cholesteric CNCs in the spherical droplets were fixed by either photopolymerization[13]or sol-gel chemistry[79]. For example, stimuli-responsive microgels with cholesteric structures were prepared by the photopolymerization of the droplets with cholesteric CNCs, a monomer, and a cross-linker[13]. The structures of the microgels were controlled by their size and were altered from the concentric packing to the bipolar planar packing of the cholesteric CNCs with reducing the droplet sizes. The microgels were used as microreactors for catalytic hydrolysis reactions and for the synthesis of metal nanoparticles. To prepare the microgels with cholesteric structures on large scale, the microdroplets containing cholesteric CNCs, a monomer, and a cross-linker were generated by the inverse emulsion method and then were polymerized by the photopolymerization[79]. Combining the sol-gel chemistry and photopolymerization, the cholesteric microparticles with the double matrix of silica gel and polymer were prepared. Upon complete drying and calcination, mesoporous silica microspheres with cholesteric order were achieved.
One of the promising applications of the self-assembly of cholesteric CNCs in the droplets was to obtain solid microparticles with angular independent colors[81]. These photonic microparticles had important applications in renewable and sustainable pigments[82]. Recently, cellulose photonic pigments were successfully explored by drying the cholesteric CNC droplets emulsified in a microfluidic droplet generator[81]. In this work, the slow drying made the randomly oriented tactoids of CNCs merge and form monodomain structures of cholesteric CNCs in the droplets. An arrested shell of cholesteric CNCs was formed because of the further water loss at the interface of the droplets. Subsequently, the cholesteric shell of CNCs buckled because of the interplay between compressive capillary forces and the mechanical resistance of the solidifying cholesteric shell. The pitch reduction in the arrested droplet was achieved because of the significant buckling, resulting in microparticles with red colors[Fig.8(A—C)]. Interestingly, through the thermal or solvent treatments, the red microparticles could be further buckled to reduce the pitch, leading to CNC microparticles that displayed the green[Fig.8(D—F)] or blue colors[Fig.8(G—I)].
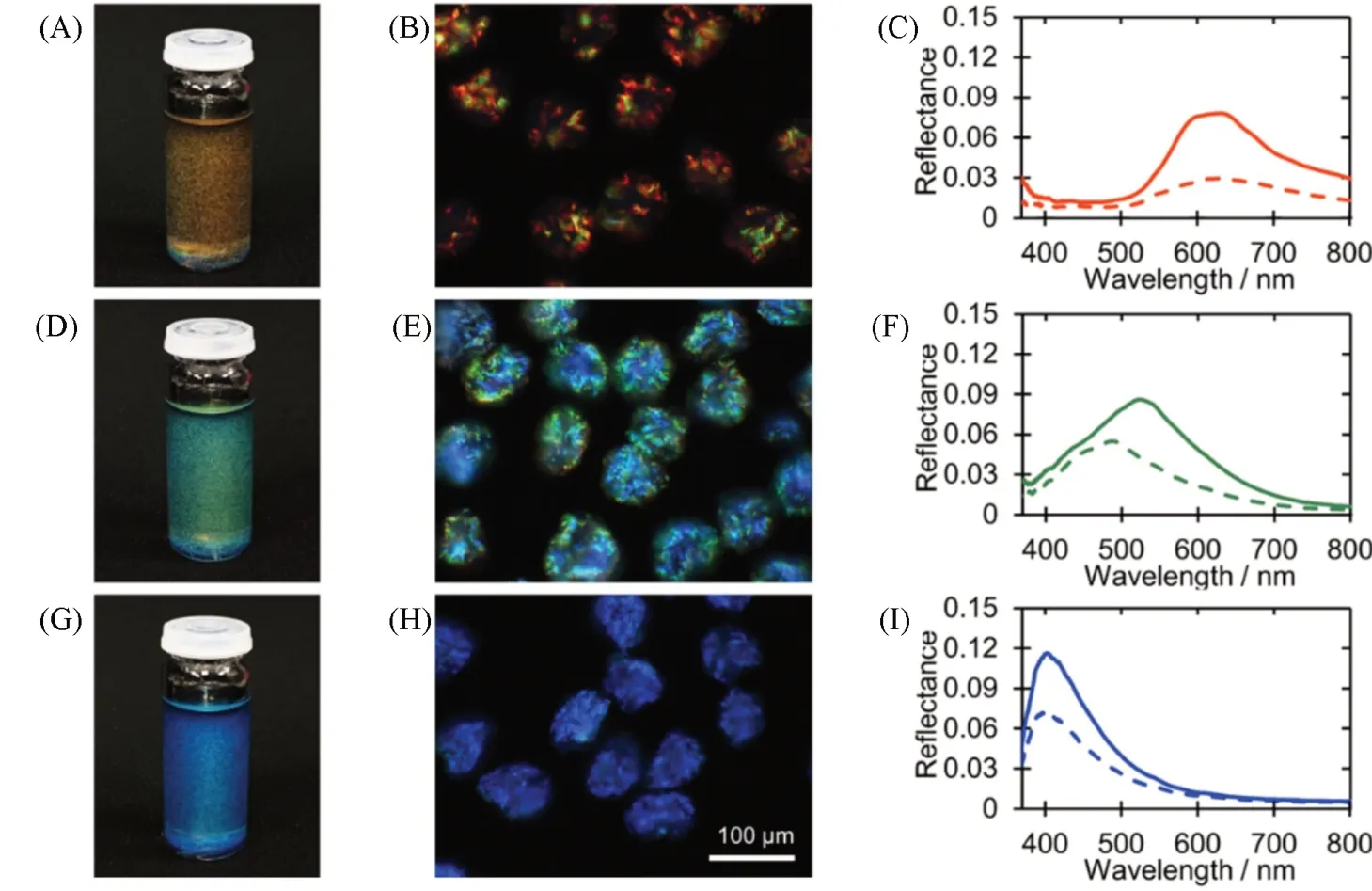
Fig.8 Photonic particles derived from the CNC droplets[81]
Recently, large area monodomain photonic crystals were reported by confined self-assembly of a CNC suspension in a rectangular capillary[76,77]. In this strategy, after the CNC suspension was filled in the capillary by wetting-induced upward flow, asymmetric water evaporation at the top and the bottom of the capillary resulted in the fast formation of a liquid crystal film at the bottom. The formation of the CNC photonic films took hours, in comparison with the weeks needed for film formation by a traditional dish-cast approach.
The large and uniformly oriented chiral CNC solid films fabricated under such asymmetric drying conditions have superior optical properties compared to conventional methods[76]. In addition, appropriately increasing the intermolecular interactions between the components, such as hydrogen bonds, is beneficial to improve the mechanical properties of the film while maintaining the optical properties[77].
8 Conclusions and Perspectives
The versatile usages of CNCs paved a way for the development of innovative materials that provided a broad range of important applications, such as photonic pigments, photonic sensors and actuators, energy devices, and biomedical materials. In particular, the self-assembly of CNCs into cholesteric LCs templated many porous inorganic photonic materials with tunable bandgaps and polymer photonic materials with stimuliresponsive properties. Notably, the self-assembly of CNCs in geometrically confined spaces attracted significant interest. The confined self-assembly of CNCs offered a favorable tool for fundamental studies on the relaxation kinetics and defect formation in LC systems. More importantly, the spatially confined assembly of CNCs provided a unique template for nanoparticle organization into functional structures and dynamic soft materials.
CNCs showed a very broad size distribution, which limits their self-assembly into other LC phases and ordered soft materials. Further work should focus on the useful preparative techniques of CNCs with a narrow size distribution. The CNCs with uniform size would result in new LC phases, such as the smectic or columnar phase of CNCs, and newly ordered nanocomposite materials with useful functions. The cholesteric LCs selfassembled from CNCs are thermodynamically stable and lack responsive properties. The hydroxyl groups on the surface of CNCs enable the versatile chemical-modifications of CNCs by using stimuli-responsive polymers. This modification would lead to a responsive LC phase of CNCs triggered by chemical stimuli or external fields. To understand the role of the geometrical shapes on the self-assembly of CNCs, further efforts should focus on CNC assemblies in other confined geometry, such as a toroid, an ellipsoid, a cube, etc. 3D printing is a very useful technology to fabricate the complicated geometry in which the CNCs would selfassemble into new structures with interesting properties and functions. Through rationally programming the process of 3D printing, it may be possible to simultaneously achieve confined self-assembly of CNCs in customized geometry with different self-assembly structure in different geometric locations of the samples.These geometrically constrained assemblies of CNCs will lead to responsive soft materials for chiroptical sensing, polarization-based encryption, and photonics. Recently, the photonic microparticle pigments based on cholesteric CNC droplets have been reported[60,81]. The microparticles showed buckles on the particle surfaces, which led to the reflection of both left-circularly polarized and right-circularly polarized light. To achieve the photonic microparticles from cholesteric CNC droplets, an innovative strategy of controlled drying and alternative solvent removal is highly desired to suppress the surface buckling, thus resulting in photonic microparticles with the reflection of left-circularly polarized light only.
