云南萝芙木根中吲哚生物碱及其抗菌活性
2023-02-21宋京风秦马龙胡炜彦郭瑞荣张荣平李玉鹏于浩飞丁彩凤
吴 昊,宋京风,范 堃,秦马龙,胡炜彦,郭瑞荣,高 雯,张荣平,李玉鹏,于浩飞*,丁彩凤*
云南萝芙木根中吲哚生物碱及其抗菌活性
吴 昊1,宋京风1,范 堃1,秦马龙1,胡炜彦1,郭瑞荣1,高 雯1,张荣平2,李玉鹏1,于浩飞1*,丁彩凤1*
1. 昆明医科大学药学院暨云南省天然药物药理重点实验室,云南 昆明 650500 2. 云南中医药大学,云南 昆明 650500
研究云南萝芙木根中的吲哚类生物碱化学成分及抗菌活性,明确该植物抗菌活性成分。采用硅胶柱色谱、RP-C18、Sephadex LH-20、半制备HPLC等方法进行分离纯化,结合波谱数据及文献参数对化合物进行结构鉴定,并采用微量肉汤稀释法对单体化合物进行抗菌活性评价。从云南萝芙木根醋酸乙酯部位共分离得到20个吲哚类生物碱化合物,分别鉴定为维诺任碱(1)、霹雳萝芙木碱(2)、四叶萝芙木新碱(3)、萝加灵(4)、西特斯日钦碱(5)、缝籽木醇(6)、柯楠醇(7)、二氢柯楠醇(8)、毛茶碱(9)、异毛茶碱(10)、佩立任碱(11)、10-羟基-16-表-花菊醇(12)、育亨宾(13)、降马枯星碱B(14)、洛柯碱(15)、阿枯米定碱(16)、去乙酰阿枯米灵(17)、利血平(18)、哈尔满碱(19)、梅林诺宁F(20)。其中化合物20对白色念球菌表现出较好的抗菌活性,最低抑菌浓度(minimum inhibitory concentration,MIC)值为3.12 μg/mL,与临床抗真菌药物氟康唑的MIC值相同。化合物1、11对大肠杆菌,化合物11、13、20对枯草芽孢杆菌均表现出一定的抗菌活性,MIC值为6.25~12.50 μg/mL,活性与植物源抗菌药物小檗碱相当。化合物20为首次从萝芙木属中分离得到,化合物6、8~13、16~18均为首次从该植物中分离得到,部分单体化合物表现出潜在抗菌活性。
云南萝芙木;吲哚类生物碱;抗菌活性;梅林诺宁F;维诺任碱;佩立任碱;二氢柯楠醇;育亨宾
云南萝芙木Tsiang为夹竹桃科(Apocynaceae)萝芙木属L.植物,别名麻三端、羊屎果,主要分布于我国云南、贵州和广西等地[1]。其味苦、微辛,性凉,具有清风热、降肝火、消肿解毒的作用[2]。云南萝芙木作为我国重要的南药,民间以根入药,用于治疗风痒疮疥、咽喉肿痛、腹痛吐泻等疾病[3-4]。现代研究表明,吲哚类生物碱是其主要药效物质成分,具有降压、免疫抑制、抗肿瘤等作用[5-6]。目前,云南萝芙木中的主要吲哚生物碱成分利血平,已经被开发成复方利血平以及复方利血平氨苯蝶啶片在国内广泛应用,主要用于治疗顽固性高血压[7]。而萝芙木碱临床上也应用于治疗高血压、精神病及心悸等疾病[8]。云南萝芙木植物的药理学研究发现,其醇提浸膏具有良好的抗菌活性[9],但是关于其单体化合物的抗菌活性报道较少。为了丰富云南萝芙木中的化学成分,探寻该植物的抗菌药用价值。该研究以云南萝芙木干燥根为研究对象,运用经典的色谱分离手段和现代结构鉴定方法,共从其醋酸乙酯部位分离得到20个吲哚类生物碱(图1),分别鉴定为维诺任碱(vinorine,1)、霹雳萝芙木碱(perakine,2)、四叶萝芙木新碱(tetraphyllicine,3)、萝加灵(raugalline,4)、西特斯日钦碱(sitsirikine,5)、缝籽木醇(geissoschizol,6)、柯楠醇(corynantheol,7)、二氢柯楠醇(dihydrocorynantheol,8)、毛茶碱(antirhine,9)、异毛茶碱(isoantirhine,10)、佩立任碱(pelirine,11)、10-羟基-16-表-花菊醇(10-hydroxy-16--affinine,12)、育亨宾(yohimbine,13)、降马枯星碱B(normacusine B,14)、洛柯碱(lochnerine,15)、阿枯米定碱(akuammidine,16)、去乙酰阿枯米灵(deacetylakuammiline,17)、利血平(reserpine,18)、哈尔满碱(harman,19)、梅林诺宁F(melinonine F,20)。其中,化合物20为首次从萝芙木属中分离得到,化合物6、8~13、16~18均为首次从该植物中分离得到;并对分离得到的单体化合物进行了抗菌活性测定。该研究结果不仅丰富了云南萝芙木的化学物质基础,也为日后开发利用该植物资源提供了理论依据。
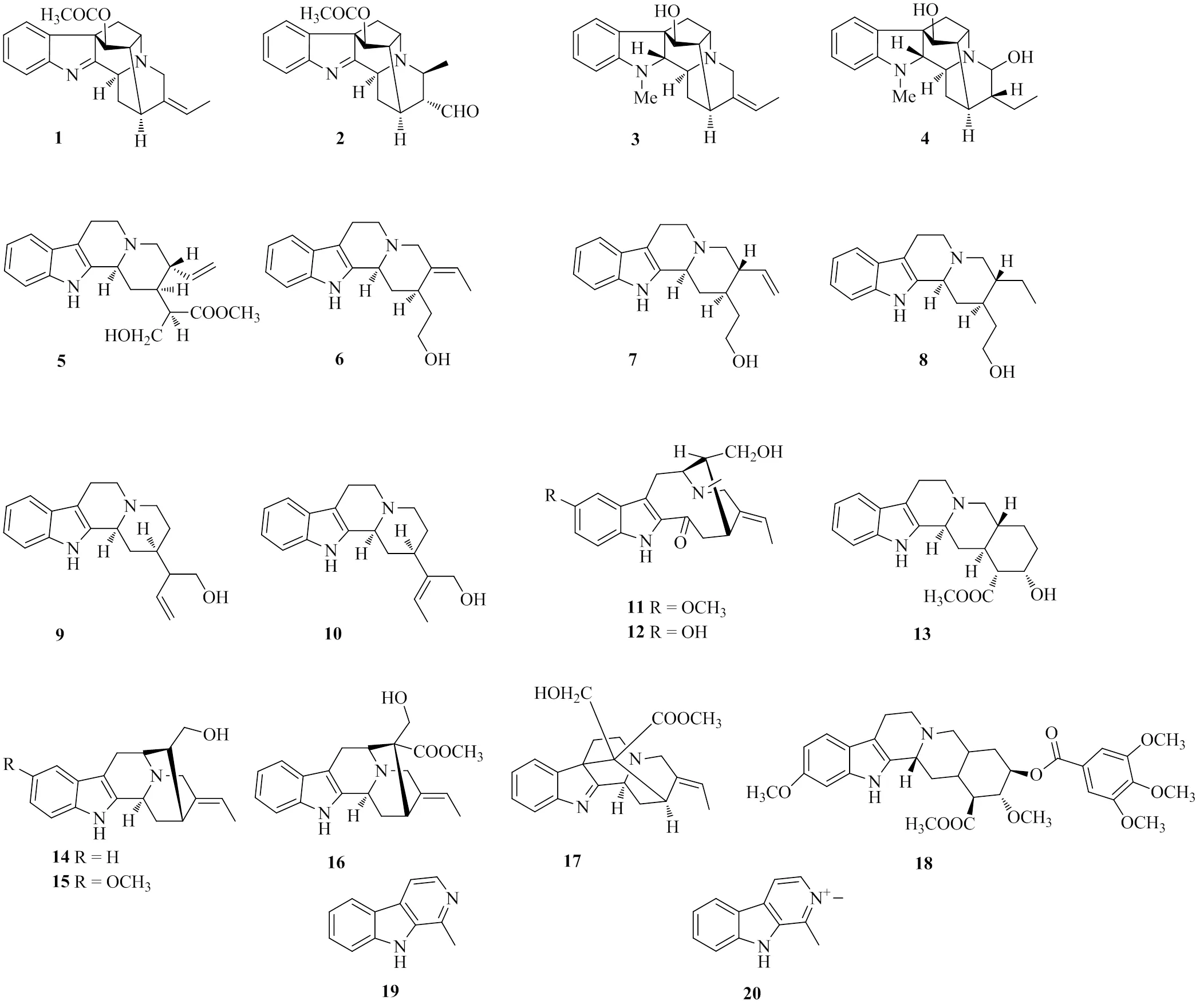
图1 化合物1~20的结构
1 材料与仪器
1.1 材料
云南萝芙木药材于2019年9月采自云南省德宏州,经昆明医科大学胡炜彦副教授鉴定为云南萝芙木Tsiang的干燥根,标本(20190910)现存于昆明医科大学药学院暨云南省天然药物药理重点实验室。白色念球菌(ATCC 2091),大肠杆菌(ATCC 8739),金黄色葡萄球菌(ATCC 2592),枯草芽孢杆菌(ATCC 6633)均来自美国典型培养物保藏中心(Rockville USA)。小檗碱(上海源叶生物科技有限公司,批号B21379),氟康唑(上海麦克林公司,批号C11990398)。
1.2 仪器与试剂
AVANCE-400/AVANCE-600型核磁共振波谱仪(德国布鲁克公司),Waters Xevo TQ-S三重四级杆质谱仪(美国沃特斯公司),LC-52制备型高效液相色谱仪(北京赛谱锐斯科技有限公司),C18-MS-Ⅱ色谱柱(250 mm×10 mm,5 μm,日本半井公司),Sephadex LH-20葡聚糖凝胶(瑞典安发玛西亚生物技术公司,粒径40~70 μm,批号10276077),硅胶GF254预制薄层色谱板(青岛海洋化工厂,100~200目,批号20200601),柱色谱硅胶(200~300目,青岛海洋化工厂,批号0190170),RP-18柱色谱材料(德国默克公司,粒径40~63 μm,批号KU00503),HBS-1096B型酶标仪(南京德铁公司),高效液相所用乙腈为分析纯(西格玛奥德里奇贸易有限公司,批号WXBD4328V),其他试剂均为工业级(重蒸后使用)。
2 方法
2.1 提取与分离
云南萝芙木干燥根(13.0 kg)粉碎,过100目筛。用80%甲醇回流提取5次(每次溶剂80 L,回流3 h),提取液减压回收溶剂,得到总浸膏1.0 kg,总浸膏用pH=2~3的盐酸-水分散后滤过,滤液用氨水调pH=9~10,醋酸乙酯萃取,共得醋酸乙酯层浸膏(60.0 g)。醋酸乙酯层浸膏(60.0 g)经硅胶(200~300目)柱色谱分离,氯仿-甲醇(1∶0、10∶1、9∶1、8∶1、7∶1、5∶1、0∶1)梯度洗脱,合并组分得5个流分(Fr.A~Fr.E)。Fr.A(5.0 g)经硅胶柱色谱,氯仿-丙酮(10∶1、5∶1、3∶1、1∶1)梯度洗脱,得5个流分(Fr. A1~A5)。Fr. A3(3.2 g)经RP-18柱色谱,甲醇-水(10∶90~100∶0)梯度洗脱,得化合物1(100.0 mg)、5(20.0 mg)。Fr. B(11.0 g)经硅胶柱色谱,石油醚-丙酮(3∶1、2∶1、1∶1)梯度洗脱,得6个流分(Fr. B1~B6)。Fr. B1(2.5 g)经Sephadex LH-20柱色谱(甲醇)洗脱及半制备高效液相色谱纯化得化合物2(4.0 mg,乙腈-水70∶30,2 mL/min,R=18 min)、16(4.0 mg,乙腈-水60∶40,2 mL/min,R=14 min)、19(1.2 mg,乙腈-水65∶35,2 mL/min,R=17 min)、20(1.5 mg,乙腈-水65∶35,2 mL/min,R=20 min)。Fr. C(5.0 g)经硅胶柱色谱,氯仿-丙酮(5∶1、3∶1、1∶1)梯度洗脱,得6个流分(Fr. C1~C6)。Fr. C4(53.1 mg)经Sephadex LH-20柱色谱纯化(甲醇)洗脱,得化合物3(5.0 mg)、4(10.0 mg)。Fr. C5(326.3 mg)经RP-18柱色谱甲醇-水(10∶90~100∶0)梯度洗脱,Sephadex LH-20柱色谱(甲醇)洗脱及半制备高效液相色谱纯化得化合物13(2.0 mg,乙腈-水55∶45,2 mL/min,R=18 min)。Fr. C6(17.0 mg)经Sephadex LH-20柱色谱(甲醇)洗脱及半制备高效液相色谱纯化得化合物6(2.0 mg,乙腈-水55∶45,2 mL/min,R=21 min)、8(5.0 mg,乙腈-水55∶45,2 mL/min,R=25 min)。Fr. D(3.2 g)经硅胶柱色谱,氯仿-甲醇(20∶1、10∶1)梯度洗脱,得5个流分(Fr. D1~D5)。Fr. D4(38.4 mg)以Sephadex LH-20柱色谱(甲醇)洗脱及半制备液相纯化,得化合物14(6.0 mg,乙腈-水50∶50,2 mL/min,R=21 min)、15(4.0 mg,乙腈-水55∶45,2 mL/min,R=27 min)。Fr. D5(300.7 mg)经RP-18柱色谱甲醇-水(10∶90~100∶0)梯度洗脱,再经硅胶柱色谱,氯仿-丙酮-二乙胺(1∶1∶0.1)等度洗脱得化合物11(2.0 mg)、18(3.0 mg)。Fr. E(1.0 g)经硅胶柱色谱,氯仿-甲醇(10∶1、5∶1)梯度洗脱,得2个流分(Fr. E1~E2)。Fr. E1(600.0 mg)经RP-18柱色谱甲醇-水(10∶90~100∶0)梯度洗脱,Sephadex LH-20柱色谱(甲醇)洗脱,得化合物7(30.0 mg)、12(10.0 mg)。Fr. E2(317.3 mg)经Sephadex LH-20柱色谱(甲醇)洗脱及半制备高效液相色谱纯化得化合物17(11.0 mg,乙腈-水40∶60,2 mL/min,R=21 min)、9(4.0 mg,乙腈-水40∶60,2 mL/min,R=27 min)、10(2.0 mg,乙腈-水40∶60,2 mL/min,R=31 min)。
2.2 抗菌活性测定
2.2.1 菌悬液的制备 取待测菌株大肠杆菌、枯草芽孢杆菌、金黄色葡萄球菌、白色念球菌接种到琼脂固体平板上,待菌落长出后挑取单个菌落转接到琼脂液体培养基中,置于37 ℃、180 r/min条件下震荡培养至对数期。在紫外分光光度计下测定菌液在600 nm的吸光度()值,并调整无菌生理溶液的光密度在0.10(0.5麦氏法兰标准),最后用MHB肉汤培养基将菌液稀释到1×106CFU/mL[10]。
2.2.2 待测样品的制备 取待测样品(化合物1~20及阳性药物氟康唑和小檗碱),加入10%二甲基亚砜(DMSO)溶液,配制样品母液质量浓度为200 μg/mL备用。
2.2.3 最低抑菌浓度(minimum inhibitory concentration,MIC)的测定 选用无菌的96微孔板,在每孔中各加入100 μL肉汤培养基,然后往第1排小孔中加入100 μL已调配好的测试样品,准备好双重稀释的样品,随后加入100 μL稀释后的菌液,用移液枪吹吸打匀,使测试样品及阳性药物的最终质量浓度在0.78~100 μg/mL。混匀后将96微孔板加盖后细菌置于37 ℃下培养24 h,白色念球菌置于28 ℃下培养48 h。经过恒温培养后,用酶标仪测定菌液在600 nm处的值,并观察96孔板的浑浊度,待测样品组的数值小于等于空白对照组的数值或者未出现明显浑浊的样品孔即为待测样品的MIC值,如果待测样品的MIC值大于100 μg/mL,视为该样品没有抗菌活性,每个实验平行3次。
3 结果与分析
3.1 结构鉴定
化合物1:白色粉末,ESI-MS/335 [M+H]+,分子式为C21H22N2O2,1H-NMR (400 MHz, CD3OD): 7.62 (1H, d,= 7.6 Hz, H-9), 7.46 (1H, d,= 7.6 Hz, H-12), 7.39 (1H, t,= 7.6 Hz, H-10), 7.22 (1H, t,= 7.6 Hz, H-11), 5.31 (1H, q,= 6.8 Hz, H-19), 5.04 (1H, d,= 0.8 Hz, H-17), 4.22 (1H, t,= 4.8 Hz, H-3), 3.54 (2H, m, H-21), 3.39 (1H, t,= 6.8 Hz, H-5), 3.27 (1H, m, H-15), 2.74 (1H, dd,= 11.6, 4.8 Hz, H-6a), 2.43 (1H, t,= 6.8 Hz, H-16), 2.17 (3H, s, H-23), 1.93 (2H, t,= 4.8 Hz, H-14), 1.69 (1H, m, H-6b), 1.67 (3H, d,= 6.8 Hz, H-18);13C-NMR (100 MHz, CD3OD): 183.8 (C-2), 169.9 (C-22), 156.5 (C-13), 137.9 (C-20), 136.4 (C-8), 128.6 (C-11), 125.4 (C-10), 123.8 (C-9), 120.9 (C-12), 115.6 (C-19), 77.7 (C-17), 64.3 (C-7), 58.0 (C-3), 56.1 (C-5), 54.1 (C-21), 48.9 (C-16), 37.3 (C-6), 27.4 (C-15), 26.4 (C-14), 21.1 (C-23), 12.9 (C-18)。以上数据与文献对比[11],鉴定化合物1为维诺任碱。
化合物2:白色粉末,ESI-MS/351 [M+H]+,分子式为C21H22N2O3,1H-NMR (600 MHz, CDCl3): 9.85 (1H, s, H-21), 7.62 (1H, d,= 7.8 Hz, H-9), 7.49 (1H, d,= 7.8 Hz, H-12), 7.40 (1H, t,= 7.8 Hz, H-10), 7.23 (1H, t,= 7.8 Hz, H-11), 4.95 (1H, s, H-17), 4.18 (1H, d,= 9.6 Hz, H-3), 3.63 (1H, t,= 6.0 Hz, H-5), 3.33 (1H, m, H-19), 2.88 (1H, t,= 6.0 Hz, H-15), 2.80 (1H, dd,= 12.0, 4.8 Hz, H-6a), 2.47 (1H, t,= 6.0 Hz, H-16), 2.18 (1H, m, H-20), 2.17 (3H, s, H-23), 1.77 (1H, m, H-14a), 1.65 (1H, d,= 12.0 Hz, H-14b), 1.64 (1H, d,= 12.0 Hz, H-6b), 1.27 (3H, d,= 6.0 Hz, H-18);13C-NMR (150 MHz, CDCl3): 201.6 (C-21), 182.7 (C-2), 170.1 (C-22), 156.5 (C-13), 136.2 (C-8), 128.8 (C-11), 125.6 (C-10), 123.9 (C-9), 121.0 (C-12), 78.0 (C-17), 64.9 (C-7), 56.9 (C-3), 56.4 (C-19), 51.6 (C-5), 49.9 (C-16), 48.7 (C-20), 37.4 (C-6), 26.2 (C-15), 22.6 (C-14), 21.1 (C-23), 18.9 (C-18)。以上数据与文献对比[12],鉴定化合物2为霹雳萝芙木碱。
化合物3:白色粉末,ESI-MS/309 [M+H]+,分子式为C20H24N2O,1H-NMR (600 MHz, CDCl3): 7.47 (1H, d,= 7.8 Hz, H-9), 7.14 (1H, t,= 7.8 Hz, H-10), 6.77 (1H, t,= 7.8 Hz, H-11), 6.63 (1H, d,= 7.8 Hz, H-12), 5.19 (1H, q,= 6.6 Hz, H-19), 4.42 (1H, brs, H-17), 3.47 (1H, d,= 9.6 Hz, H-3), 3.41 (1H, d,= 15.6 Hz, H-21a), 3.34 (1H, d,= 15.6 Hz, H-21b), 3.13 (1H, t,= 6.0 Hz, H-15), 3.05 (1H, t,= 6.0 Hz, H-5), 2.79 (3H, s, N-CH3), 2.65 (1H, s, H-2), 2.34 (1H, m, H-16), 2.05 (1H, t,= 12.0 Hz, H-14a), 1.92 (1H, d,= 7.8 Hz, H-6a), 1.84 (1H, t,= 12.0 Hz, H-6b), 1.68 (3H, d,= 6.6 Hz, H-18), 1.61 (1H, m, H-14b);13C-NMR (150 MHz, CDCl3): 154.4 (C-13), 140.0 (C-20), 132.8 (C-8), 127.3 (C-11), 122.8 (C-10), 119.3 (C-9), 114.9 (C-19), 109.4 (C-12), 79.4 (C-17), 76.7 (C-2), 56.1 (C-3), 55.3 (C-21), 54.9 (C-7), 52.2 (C-5), 49.2 (C-16), 35.0 (C-6), 34.6 (N-CH3), 29.5 (C-14), 28.1 (C-15), 12.8 (C-18)。以上数据与文献对比[13],鉴定化合物3为四叶萝芙木碱。
化合物4:白色粉末,ESI-MS/327 [M+H]+,分子式为C20H26N2O2,1H-NMR (600 MHz, CDCl3): 7.52 (1H, d,= 7.2 Hz, H-9), 6.67 (1H, t,= 7.2 Hz, H-10), 7.05 (1H, t,= 7.2 Hz, H-11), 6.62 (1H, d,= 7.2 Hz, H-12), 4.63 (1H, d,= 7.2 Hz, H-21), 4.40 (1H, s, H-17), 4.20 (1H, m, H-15), 3.60 (1H, d,= 7.2 Hz, H-3), 2.96 (1H, m, H-5), 2.80 (3H, s, N-CH3), 2.62 (1H, s, H-2), 2.20 (1H, m, H-16), 2.01 (1H, m, H-14b), 1.95 (2H, m, H-6), 1.55 (1H, dd,= 13.2, 5.4, Hz, H-20), 1.41 (1H, m, H-14a), 1.39 (1H, m, H-19a), 1.34 (1H, m, H-19b), 0.98 (3H, t,= 6.1 Hz, H-18);13C-NMR (150 MHz, CDCl3): 154.0 (C-13), 134.5 (C-8), 126.7 (C-11), 123.1 (C-10), 118.5 (C-9), 109.1 (C-12), 87.6 (C-21), 81.4 (C-17), 79.4 (C-2), 57.7 (C-7), 54.7 (C-3), 50.5 (C-5), 46.9 (C-16), 44.4 (C-20), 35.3 (C-6), 34.3 (N-CH3), 31.6 (C-19), 28.4 (C-15), 25.5 (C-14), 12.3 (C-18)。以上数据与文献对比[14],鉴定化合物4为萝加灵。
化合物5:白色针晶(甲醇),mp 239~241 ℃,ESI-MS/355 [M+H]+,分子式为C21H26N2O3,1H-NMR (400 MHz, CDCl3): 8.64 (1H, brs, N-H), 7.45 (1H, d,= 7.6 Hz, H-9), 7.32 (1H, d,= 7.6 Hz, H-12), 7.13 (1H, t,= 7.6 Hz, H-10), 7.07 (1H, t,= 7.6 Hz, H-11), 5.58 (1H, m, H-19), 5.23 (1H, dd,= 17.2, 1.6 Hz, H-18a), 5.18 (1H, dd,= 10.0, 1.6 Hz, H-18b), 4.00 (1H, dd,= 11.2, 7.2 Hz, H-3), 3.77 (1H, dd,= 11.2, 6.0 Hz, H-21a), 3.72 (2H, m, H-17), 3.66 (3H, s, 22-OCH3), 3.34 (1H, m, H-5a), 3.19 (1H, d,= 11.2 Hz, H-15), 3.13 (1H, m, H-6a), 3.05 (1H, m, H-21b), 2.79 (1H, m, H-6b), 2.52 (1H, m, H-5b), 2.40 (2H, m, H-14), 2.20 (1H, m, H-16), 1.41 (1H, d,= 7.6 Hz, H-20);13C-NMR (100 MHz, CDCl3): 174.4 (C-22), 138.1 (C-19), 136.2 (C-13), 136.1 (C-2), 127.1 (C-8), 121.5 (C-11), 119.4 (C-10), 118.5 (C-9), 118.1 (C-18), 111.0 (C-12), 107.6 (C-7), 61.9 (C-21), 60.8 (C-17), 59.7 (C-3), 52.6 (C-5), 51.7 (22-OCH3), 47.9 (C-16), 44.3 (C-20), 40.3 (C-15), 31.5 (C-14), 21.8 (C-6)。以上数据与文献对比[15],鉴定化合物5为西特斯日钦碱。
化合物6:白色针晶(甲醇),mp 216~218 ℃,ESI-MS/297 [M+H]+,分子式为C19H24N2O,1H-NMR (400 MHz, CD3OD): 7.37 (1H, d,= 7.6 Hz, H-9), 7.28 (1H, d,= 7.6 Hz, H-12), 7.03 (1H, t,= 7.6 Hz, H-10), 6.96 (1H, t,= 7.6 Hz, H-11), 5.39 (1H, q,= 6.6 Hz, H-19), 4.47 (1H, m, H-17a), 3.91 (1H, m, H-3), 3.71 (1H, d,= 7.6 Hz, H-17b), 3.40 (1H, m, H-21a), 3.18 (1H, m, H-5a), 3.05 (1H, m, H-6a), 2.83 (1H, m, H-21b), 2.78 (1H, m, H-6b), 2.51 (1H, m, H-5b), 2.01 (1H, m, H-14a), 1.90 (1H, m, H-14b), 1.67 (3H, dd,= 6.6, 1.2 Hz, H-18), 1.55 (1H, m, H-16a), 1.40 (1H, m, H-16b), 1.33 (1H, m, H-15);13C-NMR (100 MHz, CD3OD): 136.8 (C-13), 136.6 (C-20), 134.2 (C-2), 126.9 (C-8), 120.0 (C-11), 118.4 (C-10), 117.2 (C-9), 116.1 (C-19), 110.5 (C-12), 106.2 (C-7), 60.3 (C-3), 59.3 (C-17), 55.0 (C-21), 52.4 (C-5), 37.5 (C-16), 35.8 (C-14), 33.8 (C-15), 20.8 (C-6), 11.7 (C-18)。以上数据与文献对比[16],鉴定化合物6为缝籽木醇。
化合物7:白色粉末,ESI-MS/297 [M+H]+,分子式为C19H24N2O,1H-NMR (600 MHz, CDCl3): 10.66 (1H, s, N-H), 7.27 (1H, d,= 7.8 Hz, H-9), 7.22 (1H, d,= 7.8 Hz, H-12), 6.93 (1H, t,= 7.8 Hz, H-10), 6.86 (1H, t,= 7.8 Hz, H-11), 5.59 (1H, m, H-19), 4.99 (2H, m, H-18), 4.34 (1H, brs, H-17a), 3.75 (1H, brs, H-3), 3.70 (1H, m, H-17b), 3.51 (1H, dd,= 10.2, 4.2 Hz, H-21a), 3.42 (1H, dd,= 10.2, 4.2 Hz, H-5a), 2.95 (1H, dd,= 11.4, 4.8 Hz, H-5b), 2.76 (1H, m, H-21b), 2.68 (1H, m, H-6a), 2.54 (1H, m, H-6b), 2.19 (1H, m, H-14a), 1.96 (1H, m, H-14b), 1.80 (1H, m, H-16a), 1.51 (1H, m, H-16b), 1.34 (1H, m, H-20), 1.28 (1H, m, H-15);13C-NMR (150 MHz, CDCl3): 140.5 (C-19), 135.7 (C-13), 135.6 (C-2), 127.5 (C-8), 120.6 (C-10), 118.6 (C-11), 117.7 (C-9), 116.6 (C-18), 111.4 (C-12), 106.6 (C-7), 63.3 (C-3), 62.9 (C-17), 54.6 (C-21), 52.3 (C-5), 48.5 (C-20), 35.0 (C-14), 37.2 (C-15), 35.8 (C-16), 25.9 (C-6)。以上数据与文献对比[17],鉴定化合物7为柯楠醇。
化合物8:白色针晶(甲醇),mp 175~177 ℃,ESI-MS/299 [M+H]+,分子式为C19H26N2O,1H-NMR (400 MHz, CDCl3): 8.24 (1H, brs, N-H), 7.45 (1H, d,= 7.6 Hz, H-9), 7.30 (1H, d,= 7.6 Hz, H-12), 7.12 (1H, t,= 7.6 Hz, H-10), 7.07 (1H, t,= 7.6 Hz, H-11), 3.73 (1H, t,= 7.6 Hz, H-17a), 3.08 (1H, m, H-3), 3.07 (1H, m, H-17b), 3.04 (1H, m, H-5a), 3.00 (1H, m, H-6a), 2.71 (1H, dd,= 11.2, 4.4 Hz, H-6b), 2.51 (1H, m, H-5b), 2.24 (1H, d,= 12.4 Hz, H-16a), 2.05 (1H, m, H-21a), 1.96 (1H, m, H-14a), 1.90 (1H, m, H-16b), 1.62 (1H, m, H-19a), 1.44 (1H, m, H-21b), 1.42 (1H, m, H-20), 1.31 (1H, m, H-14b), 1.28 (1H, m, H-15), 1.10 (1H, m, H-19b), 0.89 (3H, t,= 7.6 Hz, H-18);13C-NMR (100 MHz, CDCl3): 136.1 (C-13), 127.3 (C-2), 124.1 (C-8), 121.3 (C-10), 119.3 (C-11), 118.1 (C-9), 110.9 (C-12), 107.8 (C-7), 60.5 (C-21), 60.2 (C-17), 59.8 (C-3), 53.0 (C-5), 41.6 (C-20), 36.9 (C-15), 35.4 (C-16), 35.2 (C-14), 23.4 (C-18), 21.5 (C-6), 11.0 (C-19)。以上数据与文献对比[18],鉴定化合物8为二氢柯楠醇。
化合物9:白色粉末,ESI-MS/297 [M+H]+,分子式为C19H24N2O,1H-NMR (600 MHz, CDCl3): 8.01 (1H, brs, N-H), 7.47 (1H, d,= 7.8 Hz, H-9), 7.34 (1H, d,= 7.8 Hz, H-12), 7.15 (1H, t,= 7.8 Hz, H-10), 7.10 (1H, t,= 7.8 Hz, H-11), 5.60 (1H, m, H-19), 5.22 (1H, d,= 10.2 Hz, H-18a), 5.16 (1H, d,= 10.2 Hz, H-18b), 4.17 (1H, brs, H-3), 3.74 (1H, m, H-21a), 3.60 (1H, dd,= 10.2, 7.8 Hz, H-21b), 3.21 (1H, d,= 7.8 Hz, H-5a), 3.03 (1H, m, H-5b), 3.00 (1H, m, H-6a), 2.77 (1H, m, H-6b), 2.75 (1H, m, H-17a), 2.65 (1H, m, H-14a), 2.62 (1H, m, H-17b), 2.25 (1H, m, H-20), 2.01 (1H, m, H-14b), 1.74 (1H, m, H-16a), 1.54 (1H, m, H-15), 1.52 (1H, m, H-16b);13C-NMR (150 MHz, CDCl3): 138.1 (C-19), 135.8 (C-13), 133.5 (C-2), 127.6 (C-8), 121.4 (C-10), 119.5 (C-11), 118.6 (C-18), 118.0 (C-9), 111.0 (C-12), 108.0 (C-7), 63.7 (C-21), 54.1 (C-3), 51.8 (C-5), 49.7 (C-20), 47.1 (C-17), 31.8 (C-14), 31.7 (C-15), 28.8 (C-16), 18.5 (C-6)。以上数据与文献对比[19],鉴定化合物9为毛茶碱。
化合物10:白色粉末,ESI-MS/297 [M+H]+,分子式为C19H24N2O,1H-NMR (600 MHz, CDCl3): 8.30 (1H, brs, N-H), 7.47 (1H, d,= 7.8 Hz, H-9), 7.33 (1H, d,= 7.8 Hz, H-12), 7.14 (1H, t,= 7.8 Hz, H-10), 7.09 (1H, t,= 7.8 Hz, H-11), 5.51 (1H, q,= 7.2 Hz, H-19), 4.21 (1H, brs, H-3), 3.63 (2H, m, H-21), 3.55 (1H, m, H-5a), 3.25 (1H, m, H-5b), 3.07 (1H, m, H-6a), 3.04 (1H, m, H-17a), 3.02 (1H, m, H-6b), 2.95 (1H, m, H-17b), 2.66 (1H, dd,= 14.4, 4.8 Hz, H-14a), 2.17 (1H, dt,= 14.4, 4.8 Hz, H-14b), 2.03 (1H, m, H-16a), 1.64 (3H, d,= 7.8 Hz, H-18), 1.54 (1H, m, H-15), 1.52 (1H, m, H-16b);13C-NMR (150 MHz, CDCl3): 137.2 (C-20), 135.0 (C-13), 133.0 (C-2), 127.5 (C-8), 121.5 (C-10), 119.5 (C-11), 118.1 (C-9), 115.4 (C-19), 111.0 (C-12), 107.5 (C-7), 61.8 (C-21), 53.9 (C-3), 53.5 (C-5), 51.2 (C-17), 35.9 (C-14), 32.8 (C-15), 31.6 (C-16), 18.2 (C-6), 12.9 (C-18)。以上数据与文献对比[20],鉴定化合物10为异毛茶碱。
化合物11:黄色粉末,ESI-MS/355 [M+H]+,分子式为C21H26N2O3,1H-NMR (400 MHz, CDCl3): 9.07 (1H, brs, N-H), 7.29 (1H, d,= 8.8 Hz, H-12), 7.08 (1H, d,= 2.4 Hz, H-9), 7.02 (1H, dd,= 8.8, 2.4 Hz, H-11), 5.51 (1H, q,= 6.4 Hz, H-19), 3.89 (3H, s, 10-OCH3), 3.73 (1H, d,= 13.6 Hz, H-21a), 3.63 (2H, m, H-17), 3.51 (1H, m, H-6a), 3.40 (1H, m, H-5), 3.38 (1H, m, H-15), 3.31 (1H, m, H-21b), 3.10 (1H, m, H-6b), 3.07 (1H, m, H-14a), 2.66 (1H, dd,= 8.8, 2.4 Hz, H-14b), 2.62 (3H, s, N-CH3), 1.70 (3H, d,= 8.8 Hz, H-18), 1.25 (1H, m, H-16);13C-NMR (100 MHz, CDCl3): 191.1 (C-3), 154.5 (C-10), 136.0 (C-2), 135.0 (C-20), 132.2 (C-13), 128.5 (C-8), 121.1 (C-19), 120.0 (C-7), 118.6 (C-12), 113.5 (C-11), 100.4 (C-9), 67.0 (C-17), 56.5 (C-5), 55.8 (10-OCH3), 52.3 (C-21), 43.5 (C-14), 41.9 (-CH3), 38.4 (C-16), 31.4 (C-15), 19.4 (C-6), 12.1 (C-18)。以上数据与文献对比[21],鉴定化合物11为佩立任碱。
化合物12:黄色粉末,ESI-MS/341 [M+H]+,分子式为C20H24N2O3,1H-NMR (600 MHz, DMSO-6): 7.29 (1H, d,= 8.8 Hz, H-12), 6.99 (1H, d,= 1.8 Hz, H-9), 6.90 (1H, dd,= 8.8, 1.8 Hz, H-11), 5.51 (1H, m, H-19), 3.60 (1H, m, H-21a), 3.58 (2H, m, H-17), 3.50 (1H, m, H-6a), 3.46 (1H, m, H-5), 3.40 (1H, m, H-15), 3.37 (1H, m, H-21b), 3.20 (1H, m, H-6b), 3.14 (1H, m, H-14a), 2.68 (1H, dd,= 8.8, 2.4 Hz, H-14b), 2.59 (3H, s,-CH3), 1.70 (3H, d,= 8.8 Hz, H-18), 1.26 (1H, m, H-16);13C-NMR (150 MHz, DMSO-6): 191.5 (C-3), 151.0 (C-10), 135.9 (C-2), 135.0 (C-20), 132.5 (C-13), 128.8 (C-8), 126.0 (C-19), 121.2 (C-7), 117.7 (C-11), 112.8 (C-12), 102.6 (C-9), 64.2 (C-17), 55.6 (C-5), 51.9 (C-21), 42.9 (C-14), 40.9 (N-CH3), 39.9 (C-16), 29.9 (C-15), 19.5 (C-6), 10.8 (C-18)。以上数据与文献对比[22],鉴定化合物12为10-羟基-16-表-花菊醇。
化合物13:白色粉末,ESI-MS/355 [M+H]+,分子式为C21H26N2O3,1H-NMR (600 MHz, CDCl3): 7.46 (1H, d,= 7.8 Hz, H-9), 7.32 (1H, d,= 7.8 Hz, H-12), 7.14 (1H, t,= 7.8 Hz, H-11), 7.08 (1H, t,= 7.8 Hz, H-10), 4.23 (1H, d,= 2.4 Hz, H-17), 3.60 (3H, s, 22-OCH3), 3.21 (1H, d,= 12.0 Hz, H-3), 3.08 (1H, dd,= 12.0, 5.2 Hz, H-5a), 2.99 (1H, m, H-6a), 2.94 (1H, m, H-21a), 2.78 (1H, m, H-6b), 2.71 (1H, m, H-5b), 2.61 (1H, t,= 7.8 Hz, H-14a), 2.20 (1H, m, H-16), 2.17 (1H, m, H-21b), 2.13 (1H, m, H-15), 2.07 (1H, m, H-18a), 1.74 (1H, d,= 13.2 Hz, H-18b), 1.52 (1H, m, H-20), 1.41 (2H, m, H-19), 1.37 (1H, m, H-14b);13C-NMR (150 MHz, CDCl3): 172.4 (C-22), 136.3 (C-2), 136.0 (C-13), 127.7 (C-8), 121.4 (C-11), 119.5 (C-10), 118.2 (C-9), 110.8 (C-12), 108.2 (C-7), 67.3 (C-17), 62.2 (C-3), 60.4 (C-21), 53.3 (C-5), 51.5 (C-16), 50.9 (22-OCH3), 39.4 (C-20), 36.9 (C-15), 33.9 (C-14), 28.7 (C-18), 23.7 (C-19), 21.6 (C-6)。以上数据与文献对比[23],鉴定化合物13为育亨宾碱。
化合物14:白色粉末,ESI-MS/295 [M+H]+,分子式为C19H22N2O,1H-NMR (600 MHz, CDCl3): 7.39 (1H, d,= 7.8 Hz, H-9), 7.28 (1H, d,= 7.8 Hz, H-12), 7.05 (1H, t,= 7.8 Hz, H-10), 6.91 (1H, t,= 7.8 Hz, H-11), 5.45 (1H, q,= 6.0 Hz, H-19), 4.17 (1H, d,= 7.8 Hz, H-3), 3.59 (1H, m, H-21a), 3.47 (2H, m, H-17), 3.43 (1H, m, H-21b), 3.02 (1H, dd,= 15.6, 5.4 Hz, H-6a), 2.86 (1H, brs, H-15), 2.73 (1H, t,= 6.0 Hz, H-5), 2.70 (1H, d,= 15.6 Hz, H-6b), 2.10 (1H, t,= 7.8 Hz, H-14a), 1.85 (1H, m, H-16), 1.75 (1H, m, H-14b), 1.64 (3H, d,= 6.6 Hz, H-18);13C-NMR (100 MHz, CDCl3): 137.8 (C-2), 136.8 (C-13), 134.8 (C-20), 127.4 (C-8), 120.6 (C-11), 118.4 (C-10), 117.2 (C-9), 116.8 (C-19), 110.6 (C-12), 102.9 (C-7), 63.7 (C-17), 55.2 (C-21), 54.7 (C-5), 50.4 (C-3), 44.1 (C-16), 33.1 (C-14), 27.3 (C-15), 26.4 (C-6), 11.6 (C-18)。以上数据与文献对比[24],鉴定化合物14为降马枯星碱B。
化合物15:白色粉末,ESI-MS/325 [M+H]+,分子式为C20H24N2O2,1H-NMR (400 MHz, CD3OD): 7.17 (1H, d,= 9.0 Hz, H-12), 6.91 (1H, d,= 1.8 Hz, H-9), 6.72 (1H, dd,= 9.0, 1.8 Hz, H-11), 5.44 (1H, m, H-19), 4.14 (1H, d,= 9.0 Hz, H-3), 3.80 (3H, s, 10-OCH3), 3.59 (1H, m, H-21a), 3.49 (1H, m, H-21b), 3.46 (2H, m, H-17), 2.99 (1H, dd,= 15.6, 5.4 Hz, H-6a), 2.85 (1H, brs, H-15), 2.72 (1H, m, H-5), 2.65 (1H, m, H-6b), 2.08 (1H, m, H-14a), 1.85 (1H, m, H-16), 1.74 (1H, m, H-14b), 1.63 (3H, d,= 9.0 Hz, H-18);13C-NMR (100 MHz, CD3OD): 153.7 (C-10), 138.7 (C-20), 134.7 (C-2), 132.0 (C-13), 127.8 (C-8), 116.8 (C-19), 111.2 (C-12), 110.4 (C-11), 102.9 (C-7), 99.3 (C-9), 63.7 (C-17), 55.2 (C-21), 54.9 (C-5), 54.3 (10-OCH3), 50.4 (C-3), 44.1 (C-16), 33.1 (C-14), 27.2 (C-6), 26.4 (C-15), 11.6 (C-18)。以上数据与文献对比[24],鉴定化合物15为洛柯碱。
化合物16:白色粉末,ESI-MS/337 [M+H]+,分子式为C21H24N2O2,1H-NMR (400 MHz, CD3OD): 7.36 (1H, d,= 7.6 Hz, H-9), 7.26 (1H, d,= 7.6 Hz, H-12), 7.03 (1H, t,= 7.6 Hz, H-11), 6.95 (1H, t,= 7.6 Hz, H-10), 5.44 (1H, q,= 7.6 Hz, H-19), 4.21 (1H, d,= 9.6 Hz, H-3), 3.76 (1H, d,= 9.6 Hz, H-17a), 3.65 (1H, m, H-17b), 3.63 (1H, m, H-21a), 3.60 (1H, m, H-21b), 3.52 (1H, m, H-5), 3.40 (1H, d,= 13.2 Hz, H-6a), 3.24 (1H, m, H-15), 2.93 (3H, s, 22-OCH3), 2.81 (1H, m, H-6b), 2.68 (1H, m, H-14a), 1.89 (1H, m, H-14b), 1.62 (3H, d,= 7.6 Hz, H-18);13C-NMR (100 MHz, CD3OD): 174.1 (C-22), 138.6 (C-2), 138.5 (C-13), 137.9 (C-20), 127.9 (C-8), 122.0 (C-11), 119.7 (C-10), 118.6 (C-9), 118.2 (C-19), 112.0 (C-12), 106.1 (C-7), 68.9 (C-17), 59.0 (C-5), 54.7 (C-21), 51.6 (C-3), 50.5 (C-16), 50.1 (22-OCH3), 30.3 (C-14), 30.1 (C-15), 25.0 (C-6), 13.3 (C-18)。以上数据与文献对比[25],鉴定化合物16为阿枯米定碱。
化合物17:白色针晶(甲醇),mp 190~192 ℃, ESI-MS/353 [M+H]+,分子式为C21H24N2O3,1H-NMR (400 MHz, CDCl3): 7.47 (1H, d,= 7.8 Hz, H-9), 7.45 (1H, d,= 7.8 Hz, H-12), 7.25 (1H, t,= 7.8 Hz, H-10), 7.11 (1H, t,= 7.8 Hz, H-11), 5.44 (1H, q,= 7.8 Hz, H-19), 4.36 (1H, d,= 4.8 Hz, H-3), 3.99 (1H, d,= 16.8 Hz, H-21a), 3.70 (3H, s, 17-OCH3), 3.66 (1H, m, H-5a), 3.63 (1H, m, H-15), 3.13 (1H, m, H-21b), 2.81 (1H, d,= 12.0 Hz, H-17a), 2.61 (1H, m, H-6a), 2.59 (1H, m, H-17b), 2.41 (1H, m, H-14a), 2.38 (1H, m, H-6b), 1.88 (1H, dd,= 15.0, 4.2 Hz, H-5b), 1.85 (1H, m, H-14b), 1.65 (3H, dd,= 7.2, 2.4 Hz, H-18);13C-NMR (100 MHz, CDCl3): 190.7 (C-2), 173.2 (C-22), 154.5 (C-13), 144.4 (C-8), 138.5 (C-20), 128.0 (C-9), 126.0 (C-12), 125.2 (C-11), 120.2 (C-19), 120.0 (C-10), 63.0 (C-17), 60.5 (C-7), 58.8 (C-16), 54.3 (C-3), 52.8 (C-21), 51.3 (C-5), 51.0 (17-OCH3), 37.4 (C-6), 33.6 (C-15), 30.0 (C-14), 12.5 (C-18)。以上数据与文献对比[26],鉴定化合物17为去乙酰阿枯米灵。
化合物18:白色针晶(甲醇),mp 264~266 ℃,ESI-MS/609 [M+H]+,分子式为C33H40N2O9,1H-NMR (600 MHz, CDCl3): 7.63 (1H, s, N-H), 7.32 (2H, m, H-2, 6), 7.31 (1H, m, H-9), 6.82 (1H, d,= 2.4 Hz, H-12), 6.78 (1H, dd,= 8.4, 2.4 Hz, H-10), 5.05 (1H, m, H-3), 4.51 (1H, s, H-18), 3.90 (9H, s, 3, 4, 5-OCH3), 3.84 (3H, s, 11-OCH3), 3.83 (3H, s, 17-OCH3), 3.49 (3H, s, 22-OCH3), 3.21 (1H, m, H-17), 3.19 (1H, m, H-21a), 3.01 (1H, m, H-5a), 2.96 (1H, m, H-21b), 2.70 (1H, dd,= 8.4, 2.4 Hz, H-6a), 2.51 (1H, m, H-6b), 3.51 (2H, m, H-19), 2.35 (1H, m, H-5b), 2.31 (1H, m, H-16), 2.08 (1H, m, H-15b), 2.02 (1H, m, H-15a), 1.94 (1H, m, H-14a), 1.84 (1H, m, H-14b);13C-NMR (150 MHz, CDCl3): 172.8 (C-22), 165.5 (C-24), 156.3 (C-11), 153.0 (C-3, C-5), 142.3 (C-4), 136.4 (C-13), 130.2 (C-2), 125.4 (C-1), 122.1 (C-8), 118.6 (C-9), 108.0 (C-10), 107.3 (C-7), 106.8 (C-2, 6), 95.2 (C-12), 77.9 (C-17), 77.8 (C-18), 60.8 (3, 4, 5-OCH3), 56.3 (17-OCH3), 55.8 (22-OCH3), 53.8 (11-OCH3), 53.7 (C-3), 51.5 (C-16), 51.2 (C-5), 49.0 (C-21), 33.9 (C-20), 32.2 (C-15), 29.7 (C-19), 24.2 (C-14), 16.7 (C-6)。以上数据与文献对比[27],鉴定化合物18为利血平。
化合物19:白色粉末,ESI-MS/183 [M+H]+,分子式为C12H10N2,1H-NMR (400 MHz, CDCl3): 8.36 (1H, d,= 3.2 Hz, H-3), 8.12 (1H, d,= 5.2 Hz, H-5), 7.83 (1H, d,= 3.2 Hz, H-4), 7.55 (1H, m, H-7), 7.53 (1H, m, H-8), 7.30 (1H, t,= 3.2 Hz, H-6), 2.84 (3H, s, 1-CH3);13C-NMR (100 MHz, CDCl3):141.7 (C-1), 140.1 (C-8a), 138.7 (C-3), 134.5 (C-9a), 128.4 (C-6), 128.3 (C-4b), 122.1 (C-4a), 121.9 (C-5), 120.2 (C-7), 113.0 (C-4), 111.6 (C-8), 20.2 (1-CH3)。以上数据与文献对比[28],鉴定化合物19为哈尔满碱。
化合物20:白色粉末,ESI-MS/198 [M+H]+,分子式为C13H13N2,1H-NMR (400 MHz, CDCl3): 8.36 (1H, d,= 3.2 Hz, H-3), 8.11 (1H, d,= 5.2 Hz, H-5), 7.84 (1H, d,= 3.2 Hz, H-4), 7.55 (1H, m, H-7), 7.53 (1H, m, H-8), 7.30 (1H, t,= 3.2 Hz, H-6), 3.77 (3H, s,-CH3), 2.84 (3H, s, 1-CH3);13C-NMR (100 MHz, CDCl3):141.6 (C-1), 140.1 (C-8a), 138.5 (C-3), 134.5 (C-9a), 128.5 (C-4b), 128.4 (C-6), 122.0 (C-4a), 121.9 (C-5), 120.2 (C-7), 113.0 (C-4), 111.6 (C-8), 63.7 (-CH3), 20.2 (1-CH3)。以上数据与文献对比[29],鉴定化合物20为梅林诺宁F。
3.2 化合物抗菌活性结果
按“2.2”项方法测试化合物1~20的抗菌活性,结果如表1所示。化合物20对白色念球菌表现出较好的抗菌活性,MIC值为3.12 μg/mL,与临床抗真菌药物氟康唑的MIC值相同。化合物1、11对大肠杆菌表现出一定的抗菌活性,MIC值为6.25 μg/mL,化合物11、13、20对枯草芽孢杆菌表现出一定的抗菌活性,MIC值为12.50 μg/mL,活性与植物源抗菌药物小檗碱相当。
4 讨论
云南萝芙木为提取“降压灵”和“利血平”的原料,具有清热解毒,降压,镇痛的作用。植株含有丰富的吲哚类生物碱成分,因而越来越受到人们的关注。本研究通过对云南萝芙木根的化学成分进行了系统研究,共从其醋酸乙酯部位分离得到20个吲哚类生物碱,包括4个萝芙木碱(ajmaline)型,6个柯楠因碱(corynantheine)型,2个酰基吲哚(vobasine)型,2个育亨宾烷(yohimbine)型,3个沃洛亭碱(sarpagine)型,1个阿古米碱(akuammiline)型,2个β-卡波林(β-carboline)型。其中化合物20首次从萝芙木属中分离得到,化合物6、8~13、16~18首次从该植物中分离得到,这些化合物的分离丰富了云南萝芙木的物质种类。

表1 化合物1~20的MIC
a-氟康唑 b-小檗碱 NA-没有活性,MIC>100 μg·mL−1
a is fluconazole for fungi and b is berberine for bacteria NA-no activity MIC >100 μg·mL−1
当前,随着人类对抗生素及合成抗微生物药物的滥用,出现了前所未有的微生物耐药性及不良反应,使得现有可选择的抗微生物药物束手无策。基于天然产物骨架多样,且具有多靶点、不良反应小的特点,正在成为各国新药研发团队积极开发抗微生物药物的重要来源[30-31]。云南萝芙木民间用于治疗咽喉肿痛、腹痛吐泻等疾病,这些用途可能与提取物的抗菌活性有直接关系。本研究采用了国际公认的测定MIC的方法,筛选了从云南萝芙木中得到的主要单体化合物1~20的抗菌活性,结果表明部分化合物具有抑制细菌增殖的活性。从一定程度上表明吲哚类生物碱可能是云南萝芙木植物抗菌作用的主要活性成分之一。其中化合物20对白色念球菌及枯草芽孢杆菌表现出较好的广谱抗菌活性,从构效关系分析来看,结构中含有的季铵离子可能是其发挥抗菌作用的关键基团。这与植物源广谱抗菌药物小檗碱结构具有一定相似。该研究工作为开发抗菌药物提供了新的研究思路和分子模板。本研究结果初步验证了云南萝芙木植物的药用功效,为云南萝芙木植物的综合开发利用提供了理论参数。
利益冲突 所有作者均声明不存在利益冲突
[1] 中国科学院中国植物志编辑委员会. 中国植物志(第63卷) [M]. 北京: 科学出版社, 1977: 58.
[2] Geng C G, Liu X K. Five new indole alkaloids from the leaves of[J]., 2013, 89: 42-47.
[3] Gao Y, Wang F, Zhou D,. Three new indole alkaloids from[J]., 2011, 1(3): 104-107.
[4] Li L M, Shi S D, Liu Y,. Bioactivity-guided isolation and identification of new and immunosuppressive monoterpenoid indole alkaloids fromtsiang [J]., 2019, 24(24): 4574.
[5] Hu X J, He H P, Zhou H,. New indole alkaloids from[J]., 2006, 89(7): 1344-1350.
[6] Primus P S, Ismail M H, Adnan N E,. Stenophyllols A-C, new compounds from[J]., 2022, 24(2): 146-152.
[7] 施泰来, 唐敏, 苏海. 利血平在高血压治疗中还有一席之地吗? [J]. 中华高血压杂志, 2021, 29(11): 1029-1031, 1028.
[8] 刘洋洋, 许琼情, 汪春牛, 等. 南药萝芙木药理活性研究现状 [J]. 中国药学杂志, 2010, 45(20): 1521-1523.
[9] 陈晓英, 郭晓云, 李翠, 等. 萝芙木属植物药理活性和单萜吲哚生物碱合成途径研究进展 [J]. 中草药, 2019, 50(8): 2004-2012.
[10] Ding C F, Ma H X, Yang J,. Antibacterial indole alkaloids with complex heterocycles from[J]., 2018, 20(9): 2702-2706.
[11] 位翠杰, 谢静, 李紫薇, 等. 鸡骨常山中的一个新生物碱 [J]. 药学学报, 2020, 55(2): 294-297.
[12] Batista C V F, Schripsema J, Verpoorte R,. Indole alkaloids from[J]., 1996, 41(3): 969-973.
[13] 李琳, 何红平, 周华, 等. 催吐萝芙木中生物碱的研究 [J]. 天然产物研究与开发, 2007, 19(2): 235-239.
[14] Chatterjee A, Chakrabarty M, Ghosh K M,. Indole alkaloids of rauwolfia reflexa. the structures of rauflexine and reflexine [J]., 1978, 19(40): 3879-3882.
[15] 龚爽, 熊凤, 张兴杰, 等. 钩藤的生物碱构成及其抗炎活性研究 [J]. 云南大学学报: 自然科学版, 2021, 43(6): 1220-1227.
[16] 李松涛, 白文鑫, 袁孟菲, 等. 药用狗牙花枝叶的生物碱类成分研究 [J]. 中草药, 2019, 50(4): 802-807.
[17] Massiot G, Thépenier P, Jacquier M,. Alkaloids of[J]., 1987, 26(10): 2839-2846.
[18] Zaman K. The isolation and13C-NMR of dihydrocorynantheol and rhazimol (deacetylakuammiline), alkaloids from the roots of[J]., 1986, 52(1): 73-74.
[19] Weniger B, Anton R, Varea T,. Indole alkaloids from[J]., 1994, 57(2): 287-290.
[20] Nunes D S, Koike L, Taveira J J,. Indole alkaloids from[J]., 1992, 31(7): 2507-2511.
[21] Sakai S I, Wan A S C, Yokota M,. Structure of pelirine and chemical conversion of gardnerine to 11-methoxy-16-epiaffinine [J]., 1987, 26(5): 1211.
[22] Zhan G Q, Miao R K, Zhang F X,. Monoterpene indole alkaloids with acetylcholinesterase inhibitory activity from the leaves of[J]., 2020, 102: 104136.
[23] Wenkert E, Chang C J, Chawla H P S,. General methods of synthesis of indole alkaloids. 14. Short routes of construction of yohimboid and ajmalicinoid alkaloid systems and their carbon-13 nuclear magnetic resonance spectral analysis [J]., 1976, 98(12): 3645-3655.
[24] 程春雷, 袁孟菲, 谢静, 等. 药用狗牙花的单萜吲哚生物碱类成分 [J]. 暨南大学学报: 自然科学与医学版, 2019, 40(4): 288-294.
[25] 位翠杰, 桑晨晨, 昂松, 等. 鸡骨常山枝叶的生物碱类成分研究 [J]. 时珍国医国药, 2020, 31(9): 2049-2052.
[26] 陈秋铃, 范春林, 敖运林, 等. 普约狗牙花枝叶的生物碱类成分研究 [J]. 中草药, 2022, 53(6): 1680-1687.
[27] Stork G, Tang P C, Casey M,. Regiospecific and stereoselective syntheses of (+/-)-reserpine and (-)-reserpine [J]., 2005, 127(46): 16255-16262.
[28] 黄艳丽, 项伟, 宋启示. 美丽蛇根草的化学成分研究 [J]. 中草药, 2009, 40(4): 519-521.
[29] Brandt V, Tits M, Geerlin V,. Β-carboline glucoalkaloids from[J]., 1999, 51(8): 1171-1176.
[30] 贾丽阳, 邓冬, 孙丽华, 等. 中药治疗耐药菌感染作用机制研究进展 [J]. 中国实验方剂学杂志, 2020, 26(16): 228-234.
[31] Newman D J, Cragg G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019 [J]., 2020, 83: 770-803.
Indole alkaloids from roots ofand its antibacterial activities
WU Hao1, SONG Jing-feng1, FAN Kun1, QIN Ma-long1, HU Wei-yan1, GUO Rui-rong1, GAO Wen1, ZAHNG Rong-ping2, LI Yu-peng1, YU Hao-fei1, DING Cai-feng1
1. Yunnan Key Laboratory of Pharmacology for Natural Products, School of Pharmaceutical Science, Kunming Medical University, Kunming 650500, China 2. Yunnan University of Traditional Chinese Medicine, Kunming 650500, China
To study the indole alkaloids from the roots of, and its antibacterial activities, so as to clarify the antibacterial active ingredients of the plant.The constituents were isolated and purified by silica gel column chromatography (CC), RP-C18, Sephadex LH-20 and semi-preparative chromatography, their structures were identified by the combination of physicochemical properties, spectral data and literature comparison. Then, the antibacterial activities of these compounds were detected by the method of micro broth dilution.Totally twenty indole alkaloids were obtained and identified as vinorine (1), perakine (2), tetraphyllicine (3), raugalline (4), sitsirikine (5), geissoschizol (6), corynantheol (7), dihydrocorynantheol (8), antirhine (9), isoantirhine (10), pelirine (11), 10-hydroxy-16--affinine (12), yohimbine (13), normacusine B (14), lochnerine (15), akuammidine (16), deacetylakuammiline (17), reserpine (18), harman (19) and melinonine F (20). Moreover, compound 20 showed significant antibacterial activity againstwith MIC value of 3.12 μg/mL. Compounds 1 and 11 showed certain antimicrobial activity against, and compounds 11, 13, and 20 showed certain antimicrobial activity against, the MIC values were 6.25—12.50 μg/mL respectively. And their activities were comparable to antibiotics berberine.Compound 20 was firstly reported from the genera, and compounds 6, 8—13, 16—18 were firstly reported from this plant. Some compounds showed potential antimicrobial activities.
Tsiang; indole alkaloid; antimicrobial activity; melinonine F; vinorine; pelirine; dihydrocorynantheol; yohimbine
R284.1
A
0253 - 2670(2023)04 - 1033 - 10
10.7501/j.issn.0253-2670.2023.04.003
2022-05-08
国家自然科学基金项目(32000274);国家自然科学基金项目(31900290);云南省教育厅科研基金项目(2020J0139);云南省基础研究项目(202101AU070069);云南省有毒药用植物创新平台研究项目(202005AE160004)
吴 昊(1997—),男,硕士研究生,从事植物化学研究。E-mail: 1326069381@qq.com
丁彩凤,博士,讲师,硕士生导师,从事天然药物化学物质基础与生物活性研究。E-mail: dingcaifeng@kmmu.edu.cn
于浩飞,博士,副教授,硕士生导师,从事天然药物化学物质基础与生物活性研究。E-mail: yaohaofei@kmmu.edu.cn
[责任编辑 王文倩]
