广藿香内生真菌撕裂耙齿菌A878次级代谢产物研究
2023-02-21王诺依刘洪新陈玉婵章卫民高晓霞1
王诺依,刘洪新,陈玉婵,章卫民,高晓霞1
•化学成分 •
广藿香内生真菌撕裂耙齿菌A878次级代谢产物研究
王诺依1, 2,刘洪新2,陈玉婵2,章卫民2*,高晓霞1*
1. 广东药科大学药学院,广东 广州 510006 2. 广东省科学院微生物研究所华南应用微生物国家重点实验室,广东省菌种保藏与应用重点实验室,广东 广州 510070

广藿香;内生真菌;撕裂耙齿菌;次级代谢产物;生物活性;撕裂耙齿菌内酯A;撕裂耙齿菌内酯B;细胞松弛素J;狄瑟酚A
真菌次级代谢产物具有结构新颖、活性显著等优点,是开发新药的重要来源之一[1]。植物内生真菌是指生存在健康的植物组织内,在长期共生和遗传进化过程中与宿主植物形成互利共生的一类特殊菌群[2-3],因其特殊的生长环境进化出独特的代谢途径。众多的科学研究表明,植物内生真菌能够产生丰富的活性次级代谢产物,如生物碱、萜、聚酮、肽等多种类型的天然产物[4],在医药、工业、农业和环境等行业有着广阔的应用前景。
广藿香(Blanco) Benth.是连花清瘟胶囊等30多种中成药的主要原料,在临床应用、化妆品、香料的原材料等领域有着巨大的市场潜能。前人化学成分研究表明,广藿香植物含有甾体、醇、萜等多种类型次级代谢产物[5],这些次级代谢产物具有抗氧化、抗炎、抗肿瘤、抗菌等多种药理活性[6]。植物内生真菌可以产生与宿主植物相同或相似的化合物,以往对广藿香内生真菌次级代谢产物的研究中已经发现了许多具有生物活性的次级代谢产物,如抑菌活性化合物链格孢毒素VII(altertoxin VII)[7]、抗肿瘤化合物小穴壳菌酮O(dothiorelone O)和异旋孢腔醌(isocochlioquinones)D~E等[8-9]。
1 仪器与材料
1.1 仪器
AVANCE III型500 MHz核磁共振波谱仪(瑞士Bruker公司);LC-20A半制备型高效液相色谱仪(日本岛津公司);EasySep-1050全制备型高效液相色谱仪(上海通微分析技术有限公司);PZ1000B型旋转式大容量普通摇床(武汉瑞华仪器设备有限公司);AX224ZH/E电子天平(OHAUS公司);MD-S2显微熔点仪(Yanagimoto Seisakusho 有限公司)。
1.2 试剂
正相柱色谱硅胶(100~200、200~300目,青岛海湾精细化工有限公司);C18反相硅胶(40~75 μm,Fuji Silysia Chemical Ltd.);Sephadex LH-20(18~110 μm,Amersham Biosciences Ltd.);YMC-pack ODS-A色谱柱(250 mm×10 mm,5 μm,12 nm,YMC公司);所有化学试剂均为分析纯(广州化学试剂厂)或色谱纯(美国BCR公司)。
1.3 菌株与细胞
实验所用菌株A878是从广东省阳春市广藿香叶中分离得到,广藿香由广东药科大学中药学院严寒静教授鉴定为广藿香(Blanco) Benth.。分离培养基采用麦芽糖培养基,经ITS序列鉴定为撕裂耙齿菌(N. Maek., Suhara & R. Kondo) C.C. Chen & Sheng H. Wu,基因登录号为OP748374,菌株保存于广东省科学院微生物研究所。
SF-268细胞、MCF-7细胞、HepG2细胞和A549细胞购自中国科学院细胞研究所。
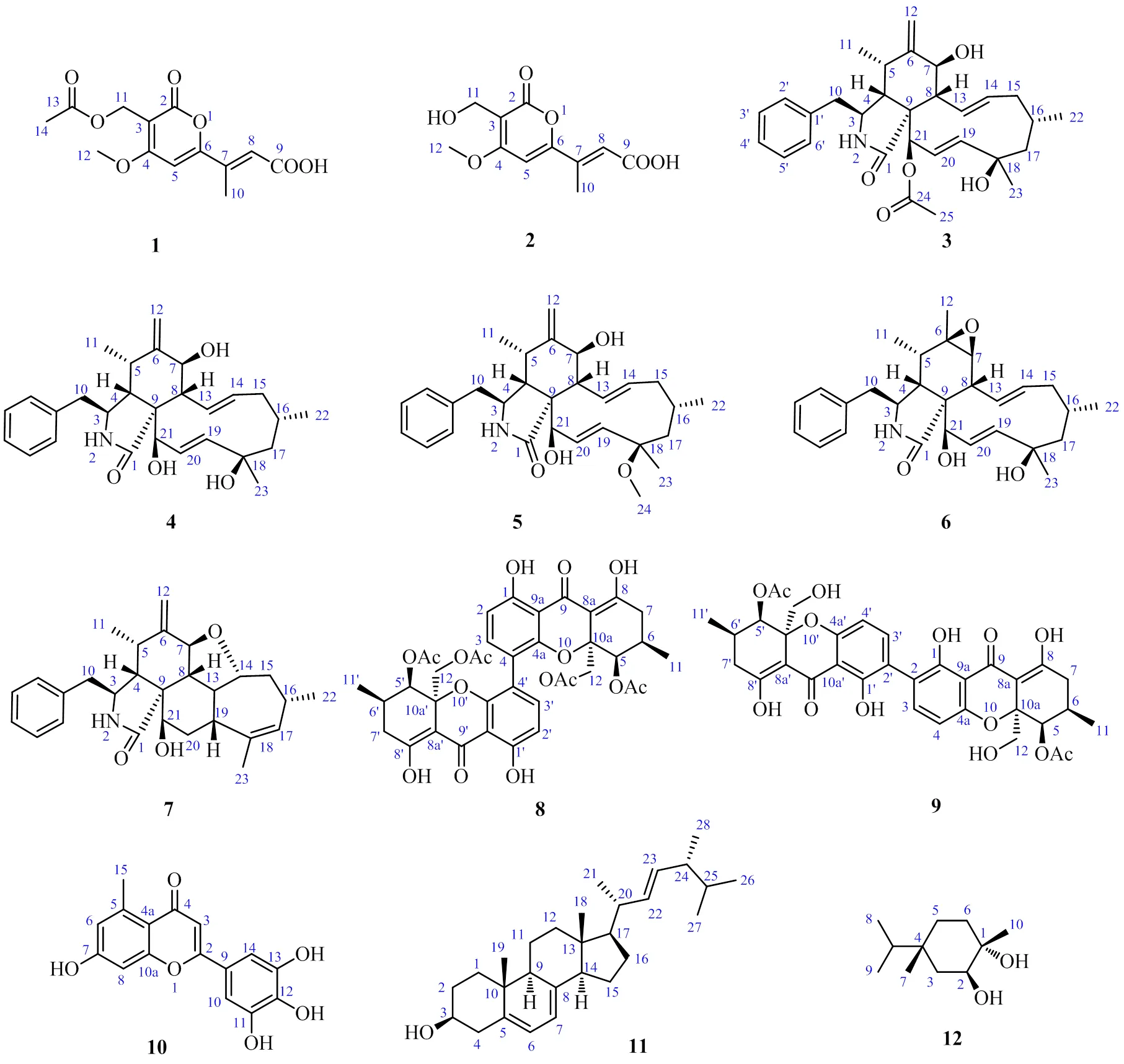
图1 化合物1~12的化学结构
阿霉素(Lot#H1421045)和吲哚美辛(Lot#F1905137)均购自阿拉丁生化科技股份有限公司。
2 方法
2.1 菌株发酵
将活化后的菌株撕裂耙齿菌A878均匀接入PDB培养基(马铃薯200 g/L、葡萄糖20 g/L、KH2PO43 g/L、MgSO4·7H2O 1.5 g/L、维生素B110 mg/L)中,在28 ℃、120 r/min振荡培养3 d,待长出明显的菌丝球转接到大米培养基(大米250 g、纯净水380 mL)的锥形瓶(3 L)中,在28 ℃静置培养33 d,发酵规模15瓶。
2.2 提取与分离
用适量的醋酸乙酯对发酵产物提取4次,减压浓缩后得到粗提物76.8 g。粗提物经硅胶柱色谱分离,首先用石油醚-醋酸乙酯(1∶0~0∶1)梯度洗脱,再以醋酸乙酯-甲醇(10∶1)等度洗脱,最后用纯甲醇冲洗硅胶柱。流分经TLC分析,合并主斑点相同的流分得到10个组分Fr. 1~10。当菌株A878粗提物过正相硅胶色谱柱,正己烷-醋酸乙酯(4∶1)洗脱时,流分中会析出白色针状晶体,用甲醇反复洗涤晶体纯化,即可得到化合物11(6.5 mg)。Fr. 3粗组分中则会析出大量淡黄色晶体,用甲醇溶剂反复洗涤纯化,进而得到化合物8(10.0 g)。
Fr. 4经反相硅胶柱色谱,用50%~100%甲醇水梯度洗脱,得8个亚组分Fr. 4-1~4-8;Fr. 4-1通过正相硅胶柱色谱,正己烷-醋酸乙酯(3∶1~1∶5)梯度洗脱,得到3个组分Fr. 4-1-1~4-1-3;Fr. 4-1-1经半制备HPLC(甲醇-水75∶25,2.0 mL/min)纯化,得到化合物7(6.8 mg)。Fr.4-1-3经正相硅胶柱色谱,用二氯甲烷-甲醇(100∶1~60∶1)梯度洗脱得到化合物12(17.4 mg)。Fr. 4-3通过凝胶柱色谱,用二氯甲烷-甲醇(1∶1)洗脱,得到化合物10(5.1 mg)。Fr. 4-4过正相硅胶柱色谱,正己烷-醋酸乙酯(3∶1~2∶1)洗脱,得到4个组分Fr. 4-4-1~4-4-4。Fr. 4-4-4再经HPLC(甲醇-水72∶28,2.0 mL/min)纯化得到化合物9(4.0 mg)。
Fr. 5经反相硅胶柱色谱,用50%~100%甲醇梯度洗4个亚组分Fr. 5-1~5-4,Fr. 5-2经正相硅胶柱色谱,石油醚-醋酸乙酯(2∶1)洗脱纯化,得到化合物3(4.3 mg)。Fr. 5-1通过凝胶柱色谱,甲醇洗脱得到3个组分Fr. 5-1-1~5-1-3。Fr. 5-1-1再经HPLC大量制备(乙腈-水35∶65,6.0 mL/min)纯化,得到化合物4(5.0 mg)。
Fr. 6经反相硅胶柱色谱,50%~100%甲醇梯度洗脱,得到8个亚组分Fr. 6-1~6-8;Fr. 6-6通过正相硅胶柱色谱,正己烷-醋酸乙酯(3∶2)洗脱得到2个组分Fr. 6-6-1~6-6-2;Fr. 6-6-1继续用正相硅胶柱色谱,正己烷-醋酸乙酯(1∶1)纯化,得到化合物5(15.0 mg)。Fr. 6-6-2通过凝胶柱色谱,甲醇洗脱,得到Fr. 6-6-2-1。Fr. 6-6-2-1再经半制备HPLC(甲醇-水65∶35,2.0 mL/min)纯化,得到化合物6(5.0 mg)。Fr. 6-8析出晶体,用甲醇反复洗涤晶体纯化,得到化合物1(2.9 mg)。
Fr. 7经反相硅胶柱色谱,甲醇-水(50∶50~100∶0)梯度洗脱,得到7个组分Fr. 7-1~7-7。Fr. 7-1经正相硅胶柱色谱,正己烷-醋酸乙酯(2∶1~1∶5)梯度洗脱,得到3个组分Fr. 7-1-1~7-1-3;Fr. 7-1-3经凝胶柱色谱(甲醇)洗脱得到Fr. 7-1-3-1~7-1-3-4;Fr. 7-1-3-1再通过正相硅胶柱色谱,二氯甲烷-甲醇(1∶0~30∶1)洗脱,得到Fr. 7-1-3-1-1~7-1-3-1-4。Fr. 7-1-3-1-3组分经反复重结晶得到化合物2(10.0 mg)。
3 结构鉴定
化合物1:白色针状晶体(甲醇),mp 219~220 ℃。紫外光谱显示化合物1在311 (4.48)、267 (4.47)、209 (5.12) nm处有最大吸收。红外光谱表明该化合物具有羰基(1703 cm−1)的吸收峰。HR-ESI-MS谱给出准分子离子峰/283.080 9(计算值为283.081 2),可以确定化合物的分子式为C12H15O7,氢谱(表1)显示2个双键质子信号H6.95 (1H, s, H-5) 和6.56 (1H, d,= 1.2 Hz, H-8)、1个亚甲基信号H4.82 (2H, s, H-12)、2个甲基信号H2.37 (3H, s, H-10) 和1.99 (3H, s, H-14)、1个甲氧基信号H4.05 (3H, s, H-11)。碳谱表明化合物1共有13个碳信号。根据HMBC谱(图2),H-5到C-3、C-4、C-6的相关信号以及1组碳信号(C162.3、101.3、169.3、97.8、167.3)表明化合物1为吡喃内酯类衍生物[10]。双键氢信号H-8到C-6、C-9和C-10的HMBC相关信号表明化合物1中有1个甲基丁烯酸片段连接在吡喃环的C-6位上。此外,由甲氧基信号H3-12与C-4的HMBC相关信号可以得出甲氧基位于吡喃环的C-4位上。同时,连氧亚甲基信号H2-11与C-2、C-4和C-13的HMBC相关,表明亚甲基连在吡喃环的C-3位,并且被1个乙酰基取代。化合物1在常温下静置于甲醇溶液中可析出白色针状结晶,因而获得了化合物1的单晶结构(图3),证实化合物1的结构如图1所示,为1个新的吡喃内酯类化合物,命名为撕裂耙齿菌内酯A(irpexone A)。

表1 化合物1和2的1H-和13C-NMR波谱数据(500/125 MHz, DMSO-d6)

图2 化合物1和2的HMBC相关图
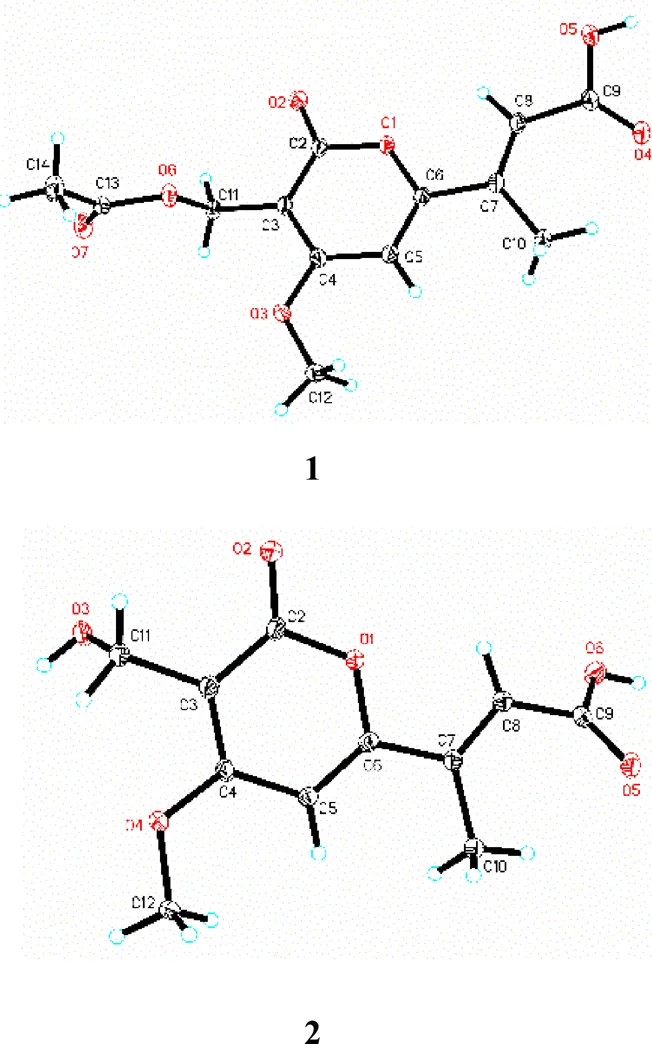
图3 化合物1和2的单晶结构图
化合物2:白色针状晶体(甲醇),mp 194~195 ℃,分子式为C10H10O5。紫外光谱显示化合物2在340 (4.89)、277 (4.49)、235 (5.38) nm处有最大吸收。化合物2的核磁谱图与化合物1的相似,推测其母核结构也为吡喃内酯类。化合物2比1缺少了2个碳信号(C170.8、21.1),它们正好是乙酰基的碳信号,从而推测化合物2为C-12位没有被乙酰基取代。这一推测被进一步的2D NMR谱图分析及单晶衍射实验得以证实(图3)。因而,化合物2的结构确定如图1所示,为1个新的吡喃内酯类化合物,命名为撕裂耙齿菌内酯B(irpexone B)。
化合物3:白色固体,ESI-MS/: 516.3 [M+Na]+,分子式为C30H39NO5;1H-NMR (500 MHz, CD3COCD3): 3.18 (1H, m, H-3), 2.13 (1H, t,= 4.2 Hz, H-4), 2.69 (1H, m, H-5), 3.81 (1H, d,= 10.8 Hz, H-7), 2.91 (1H, m, H-8), 2.78 (1H, m, H-10a), 2.27 (1H, m, H-10b), 1.00 (3H, d,= 6.9 Hz, H-11), 4.93 (1H, s, H-12a), 5.17 (1H, s, H-12b), 5.72 (1H, dd,= 15.6, 10.2 Hz, H-13), 5.26 (1H, m, H-14), 2.03 (1H, d,= 6.6 Hz, H-15a), 1.78 (1H, m, H-15b), 1.77 (1H, m, H-16), 1.53 (1H, m, H-17a), 1.86 (1H, m, H-17b), 5.86 (1H, dd,= 16.7, 2.2 Hz, H-19), 5.55 (1H, m, H-20), 5.50 (1H, s, H-21), 0.64 (3H, d,= 6.8 Hz, H-22),1.25 (3H, s, H-23) 2.27 (3H, s, H-25), 7.24 (2H, d,= 7.2 Hz, H-2′, 6′), 7.31 (2H, d,= 7.2 Hz, H-3′, 5′), 7.24 (1H, d,= 7.2 Hz, H-4′);13C-NMR (125 MHz, CD3COCD3): 174.2 (C-1), 53.3 (C-3), 48.9 (C-4), 32.3 (C-5), 150.6 (C-6), 70.8 (C-7), 47.0 (C-8), 52.0 (C-9), 44.7 (C-10), 13.0 (C-11), 111.4 (C-12), 128.5 (C-13), 136.0 (C-14), 43.1 (C-15), 28.1 (C-16), 54.0 (C-17), 72.8 (C-18), 126.0 (C-19), 138.2 (C-20), 77.1 (C-21), 25.7 (C-22), 30.6 (C-23), 137.7 (C-1), 129.7 (C-2), 128.4 (C-3), 126.5 (C-4), 128.4 (C-5), 129.7 (C-6), 170.0 (C-24), 19.9 (C-25)。上述数据与文献报道基本一致[11],故鉴定化合物3为细胞松弛素H。
化合物4:白色固体,ESI-MS/: 474.3 [M+Na]+,分子式为C28H37NO4;1H-NMR (500 MHz, CDCl3): 3.31 (1H, m, H-3), 2.60 (1H, t,= 4.2 Hz, H-4), 2.92 (1H, m, H-5), 3.83 (1H, d,= 10.8 Hz, H-7), 2.91 (1H, m, H-8), 2.92 (1H, dd,= 13.2, 4.2 Hz, H-10a), 2.60 (1H, m, H-10b), 1.11 (3H, d,= 6.6 Hz, H-11), 5.34 (1H, s, H-12a), 5.12 (1H, s, H-12b), 5.74 (1H, dd,= 15.6, 10.2 Hz, H-13), 5.35 (1H, m, H-14), 2.03 (1H, d,= 6.6 Hz, H-15a), 1.79 (1H, m, H-15b), 1.79 (1H, m, H-16), 1.89 (1H, dd,= 14.4, 3.0 Hz, H-17a), 1.58 (1H, m, H-17b), 5.74 (1H, dd,= 2.2, 16.8 Hz, H-19), 6.00 (1H, dd,= 16.8, 2.6 Hz, H-20), 4.08 (1H, dd,= 2.6, 2.2 Hz, H-21), 1.05 (3H, d,= 6.6 Hz, H-22), 1.36 (3H, s, H-23), 7.17 (2H, dd,= 8.0, 1.3 Hz, H-2′, 6′), 7.33 (2H, dd,= 8.0, 7.2 Hz, H-3′, 5′), 7.27 (1H, dd,= 7.2, 1.3 Hz, H-4′);13C-NMR (125 MHz, CDCl3): 176.1 (C-1), 54.0 (C-3), 50.1 (C-4), 33.0 (C-5), 148.5 (C-6), 69.9 (C-7), 45.9 (C-8), 53.1 (C-9), 45.5 (C-10), 14.1 (C-11), 113.8 (C-12), 127.8 (C-13), 137.8 (C-14), 42.8 (C-15), 28.5 (C-16), 53.8 (C-17), 74.6 (C-18), 137.1 (C-19), 130.9 (C-20), 76.6 (C-21), 26.5 (C-22), 31.0 (C-23), 137.6 (C-1), 129.2 (C-2), 128.8 (C-3), 127.8 (C-4), 128.8 (C-5), 129.2 (C-6)。上述数据与文献报道基本一致[12],故鉴定化合物4为细胞松弛素J。
化合物5:白色固体,ESI-MS/: 488.3 [M+Na]+,分子式为C29H39NO4;1H-NMR (500 MHz, CDCl3): 3.33 (1H, m, H-3), 2.61 (1H, t,= 4.2 Hz, H-4), 2.93 (1H, m, H-5), 3.84 (1H, d,= 10.8 Hz, H-7), 2.93 (1H, m, H-8),2.93 (1H, m, H-10a), 2.58 (1H, m, H-10b), 1.11 (3H, d,= 6.6 Hz, H-11), 5.35 (1H, s, H-12a), 5.13 (1H, s, H-12b), 5.75 (1H, dd,= 15.6, 10.2 Hz, H-13), 5.39 (1H, m, H-14), 2.07 (1H, m, H-15a), 1.77 (1H, m, H-15b), 1.77 (1H, m, H-16), 2.01 (1H, m, H-17a), 1.72 (1H, m, H-17b), 5.75 (1H, dd,= 2.4, 14.4 Hz, H-19), 6.01 (1H, dd,= 16.8, 2.4 Hz, H-20), 4.15 (1H, m, H-21), 0.99 (3H, d,= 6.0 Hz, H-22), 1.29 (3H, s, H-23), 3.23 (3H, s, H-24), 7.17 (2H, dd,= 8.0, 1.3 Hz, H-2′, 6′), 7.34 (2H, dd,= 8.0, 7.2 Hz, H-3′, 5′), 7.27 (1H, dd,= 7.2, 1.3 Hz, H-4′);13C-NMR (125 MHz, CDCl3): 175.5 (C-1), 53.8 (C-3), 50.6 (C-4), 33.0 (C-5), 148.4 (C-6), 70.0 (C-7), 45.9 (C-8), 52.8 (C-9), 45.7 (C-10), 13.9 (C-11), 113.8 (C-12), 127.9 (C-13), 137.6 (C-14), 42.9 (C-15), 27.8 (C-16), 51.5 (C-17), 78.9 (C-18), 136.8 (C-19), 131.3 (C-20), 77.1 (C-21), 26.1 (C-22), 24.4 (C-23), 50.5 (C-24), 137.6 (C-1), 129.1 (C-2), 128.9 (C-3), 127.1 (C-4), 128.9 (C-5), 129.1 (C-6)。上述数据与文献报道基本一致[11],故鉴定化合物5为18-甲氧基细胞松弛素J。
化合物6:白色固体,ESI-MS/: 474.3 [M+Na]+,分子式为C28H37NO4;1H-NMR (500 MHz, CDCl3): 3.56 (1H, m, H-3), 2.46 (1H, t,= 4.2 Hz, H-4), 1.60 (1H, m, H-5), 2.77 (1H, d,= 10.8 Hz, H-7), 2.41 (1H, m, H-8),2.88 (1H, m, H-10a), 3.50 (1H, m, H-10b), 1.09 (3H, d,= 7.7 Hz, H-11), 1.39 (3H, s, H-12), 5.86 (1H, dd,= 15.6, 10.2 Hz, H-13), 5.22 (1H, m, H-14), 1.91 (1H, d,= 6.6 Hz, H-15a), 1.60 (1H, m, H-15b), 1.77 (1H, m, H-16), 1.60 (1H, m, H-17a), 1.39 (1H, m, H-17b), 5.72 (1H, dd,= 2.2, 16.7 Hz, H-19), 5.95 (1H, m, H-20), 4.26 (1H, s, H-21), 1.06 (3H, d,= 6.8 Hz, H-22), 1.25 (3H, s, H-23), 7.20 (2H, d,= 7.4 Hz, H-2′, 6′), 7.36 (2H, d,= 7.4 Hz, H-3′, 5′), 7.29 (1H, d,= 7.4 Hz, H-4′);13C-NMR (125 MHz, CDCl3): 178.4 (C-1), 54.3 (C-3), 51.2 (C-4), 36.7 (C-5), 57.3 (C-6), 63.2 (C-7), 44.1 (C-8), 54.9 (C-9), 42.7 (C-10), 12.8 (C-11), 19.8 (C-12), 128.4 (C-13), 136.8 (C-14), 46.3 (C-15), 30.9 (C-16), 53.7 (C-17), 74.6 (C-18), 134.8 (C-19), 130.4 (C-20), 75.5 (C-21), 26.4 (C-22), 28.2 (C-23), 137.3 (C-1), 129.2 (C-2), 129.0 (C-3), 127.2 (C-4), 129.0 (C-5), 129.2 (C-6)。上述数据与文献报道基本一致[13],故鉴定化合物6为去乙酰基细胞松弛素H。
化合物7:白色固体,ESI-MS/: 456.3 [M+Na]+,分子式为C28H35NO3;1H-NMR (500 MHz, CDCl3): 3.36 (1H, m, H-3), 2.52 (1H, m, H-4), 3.02 (1H, m, H-5), 4.10 (1H, d,= 13.0, 2.0Hz, H-7), 2.48 (1H, m, H-8),2.99 (1H, m, H-10a), 2.52 (1H, m, H-10b), 1.26 (3H, d,= 6.6 Hz, H-11), 5.41 (1H, s, H-12a), 5.23 (1H, s, H-12b), 1.85 (1H, m, H-13), 3.71 (1H, m, H-14), 1.99 (1H, m, H-15a), 1.41 (1H, m, H-15b), 2.13 (1H, m, H-16), 5.26 (1H, m, H-17), 2.34 (1H, dd,= 14.4, 2.4 Hz, H-19), 2.64 (2H, m, H-20a), 1.96 (2H, m, H-20b), 3.80 (1H, m, H-21), 1.10 (3H, d,= 7.2 Hz, H-22), 1.74 (3H, s, H-23), 7.17 (2H, d,= 7.2 Hz, H-2′, 6′), 7.34 (2H, t,= 7.2 Hz, H-3′, 5′), 7.27 (1H, t,= 7.2 Hz, H-4′);13C-NMR (125 MHz, CDCl3): 175.7 (C-1), 53.4 (C-3), 47.4 (C-4), 30.4 (C-5), 149.2 (C-6), 77.0 (C-7), 43.9 (C-8), 48.4 (C-9), 45.5 (C-10), 15.0 (C-11), 113.8 (C-12), 45.0 (C-13), 88.0 (C-14), 40.0 (C-15), 30.4 (C-16), 133.4 (C-17), 138.3 (C-18), 35.4 (C-19), 34.7 (C-20), 71.1 (C-21), 24.7 (C-22), 23.7 (C-23), 137.2 (C-1), 129.3 (C-2), 128.9 (C-3), 127.1 (C-4), 128.9 (C-5), 129.3 (C-6)。上述数据与文献报道基本一致[12],故鉴定化合物7为细胞松弛素J3。

化合物9:黄色固体,ESI-MS/: 689.2 [M+Na]+,分子式为C34H34O14;1H-NMR (500 MHz, CDCl3): 14.0 (2H, s, 8, 8-OH), 12.0 (2H, s, 1, 1-OH), 7.42 (2H, d,= 8.4 Hz, H-3,3), 6.55 (2H, d,= 8.4 Hz, H-4, 4), 5.70 (2H, s, H-5, 5), 4.12 (2H, d,= 13.1 Hz, H-12a, 12a), 3.55 (2H, d,= 13.1 Hz, H-12b, 12b), 2.45 (2H, m, H-7b, 7b), 2.46 (2H, m, H-6, 6), 2.33 (2H, m, H-7, 7), 1.10 (6H, d,= 5.3 Hz, H-11, 11), 2.12 (6H, s, COOMe-12, 12);13C-NMR (125 MHz, CDCl3): 159.5 (C-1, 1), 117.9 (C-2, 2), 140.1 (C-3, 3), 107.9 (C-4, 4), 159.5 (C-4a, 4a), 70.2 (C-5, 5), 27.7 (C-6, 6), 33.4 (C-7, 7), 178.0 (C-8, 8), 100.8 (C-8a, 8a), 187.7 (C-9, 9), 106.4 (C-9a, 9a), 82.5 (C-10a, 10a), 17.6 (C-11, 11), 65.6 (C-12, 12), 170.6 (C-11, 11), 20.9 (COOMe-12, 12)。上述数据与文献报道基本一致[15],故鉴定化合物9为狄瑟酚A。
化合物10:黄色固体,ESI-MS/: 323.1 [M+Na]+,分子式为C16H12O6;1H-NMR (500 MHz, CD3OD): 6.24 (1H, s, H-3), 6.28 (1H, d,= 2.0 Hz, H-6), 6.26 (1H, d,= 2.0 Hz, H-8), 6.36 (1H, d,= 2.4 Hz, H-10), 6.24 (1H, d,= 2.4 Hz, H-14), 2.22 (3H, s, H-15);13C-NMR (125 MHz, CD3OD): 166.6 (C-1), 112.3 (C-2), 183.4 (C-3), 112.6 (C-4), 140.4 (C-4a), 109.5 (C-5), 160.7 (C-6), 99.4 (C-7), 157.9 (C-8), 100.7 (C-8a), 104.8 (C-9), 159.7 (C-9a), 162.7 (C-10a), 165.3 (C-11), 99.4 (C-12), 19.7 (Me-15)。上述数据与文献报道基本一致[16],故鉴定化合物10为桔青霉三酚A。
化合物11:白色针状晶体(甲醇),ESI-MS/: 419.3 [M+Na]+,分子式为C28H44O;1H-NMR (500 MHz, CDCl3): 3.66 (1H, m, H-3), 5.60 (1H, m, H-6), 5.41 (1H, m, H-7), 0.66 (3H, s, H-18), 0.97 (3H, m, H-19), 0.94 (3H, d,= 6.8 Hz, H-21), 5.22 (2H, m, H-22, 23), 0.78 (6H, d,= 6.4 Hz, H-26, 27), 1.06 (3H, d,= 6.6 Hz, H-28);13C-NMR (125 MHz, CDCl3): 39.2 (C-1), 32.1 (C-2), 70.5 (C-3), 37.0 (C-4), 139.8 (C-5), 119.6 (C-6), 116.3 (C-7), 141.4 (C-8), 46.3 (C-9), 38.4 (C-10), 21.1 (C-11, 12), 42.9 (C-13), 54.6 (C-14), 23.0 (C-15), 20.6 (C-16), 55.8 (C-17), 12.1 (C-18), 20.4 (C-19), 20.4 (C-20), 20.4 (C-21), 135.7 (C-22), 132.1 (C-23), 42.9 (C-24), 33.1 (C-25), 19.5 (C-26), 20.0 (C-27), 17.6 (C-28)。上述数据与文献报道基本一致[17],故鉴定化合物11为(22,24)-麦角甾-5,7,22-三烯-3β-醇。
化合物12:无色油状物,ESI-MS/: 209.2 [M+Na]+,分子式为C10H12O6;1H-NMR (500 MHz, CD3OD): 3.53 (1H, brs, H-2), 1.95 (1H, m, H-3a), 1.80 (1H, m, H-3b), 1.95 (1H, m, H-5a), 1.85 (1H, m, H-5b), 1.44 (2H, m, H-6), 1.63 (3H, s, H-7), 0.93 (3H, d,= 6.8 Hz, H-8), 0.93 (3H, d,= 6.8 Hz, H-9), 1.25 (3H, s, H-10);13C-NMR (125 MHz, CD3OD): 70.6 (C-1), 74.3 (C-2), 33.4 (C-3), 74.3 (C-4), 29.0 (C-5), 28.9 (C-6), 37.6 (C-7), 15.8 (C-8), 15.7 (C-9), 25.7 (C-10)。上述数据与文献报道基本一致[18],故鉴定化合物12为1,2,4-三羟基-对-薄荷烷。
4 活性测试结果
4.1 抗肿瘤活性
采用SRB法[19]对化合物1~12进行体外细胞毒活性评价,供试肿瘤细胞株为SF-268、MCF-7、HepG2、A549,阳性对照为阿霉素。测试表明(表2),化合物4、5和9对SF-268、MCF-7、HepG2和A549细胞株表现出中等的细胞毒活性,半数抑制浓度(median inhibition concentration,IC50)范围为24.83~69.63 μmol/L。
4.2 抗炎活性
采用Griess法[20]测定化合物1~12对细菌脂多糖(lipopolysaccharide,LPS)诱导RAW264.7巨噬细胞一氧化氮(NO)释放的影响,从而评估化合物的抗炎活性,阳性对照为吲哚美辛。结果(表2)显示化合物11表现出一定的抗炎活性。
5 讨论
本研究从广藿香内生真菌撕裂耙齿菌的固体发酵产物中分离得到2个新化合物和10个已知化合物,分离得到的化合物结构类型多样,其中1和2为吡喃内酯类化合物,3~7为10-苯基细胞松弛素类化合物,8和9为氧杂蒽酮二聚体,10为黄酮类化合物,11为甾体类化合物,12为萜类化合物。


表2 化合物1~12的细胞毒和抗炎活性()
通过文献调研发现,撕裂耙齿菌(异名撕裂蜡孔菌)的次级代谢产物类型主要是倍半萜和三萜类化合物。如Ying等[23]从蛇足石杉内生真菌液体发酵产物中分离出11个新的tremulane型倍半萜类化合物ceriponols A~K和1个新的单环tremulane型倍半萜ceriponol P,其中ceriponol B具有新颖的12-去甲肾上腺素骨架。Zhao等[24]从棘冠海星内生真菌的液体发酵产物中分离出3个新的羊毛脂烷型三萜化合物3β-乙酰氧基-15α-羟基羊毛脂-8,24-二烯-21-酸、1β,3β-二羟基羊毛脂-8,24-二烯-21-酸和15α-羟基-3-氧代羊毛脂-8,24-二烯-21-酸。本研究从菌株A878中分离获得的化合物类型主要为生物碱和聚酮类化合物,与前人研究结果有较大的差异,这可能是由于菌株来源和培养条件不同所导致。本研究结果丰富了耙齿菌属的化学成分,为进一步挖掘该类真菌的活性代谢产物奠定了基础。
利益冲突 所有作者均声明不存在利益冲突
[1] Hoffmeister D, Keller N P. Natural products of filamentous fungi: Enzymes, genes, and their regulation [J]., 2007, 24(2): 393-416.
[2] Zhang H W, Song Y C, Tan R X. Biology and chemistry of endophytes [J]., 2006, 23(5): 753-771.
[3] 曾松荣, 徐成东, 王海坤, 等. 药用植物内生真菌及其具宿主相同活性成分的机制初探[J]. 中草药, 2000, 31(4): 306-308.
[4] Kouipou Toghueo R M, Boyom F F. Endophytic fungi fromspecies: A comprehensive review [J].(), 2019, 5(2): 43.
[5] Swamy M, Sinniah U. A comprehensive review on the phytochemical constituents and pharmacological activities ofBenth.: An aromatic medicinal plant of industrial importance [J]., 2015, 20(5): 8521-8547.
[6] Chen J R, Xie X F, Li M T,. Pharmacological activities and mechanisms of action ofBenth: A review [J]., 2021, 16(1): 5.
[7] Kong F D, Yi T F, Ma Q Y,. Biphenyl metabolites from the patchouli endophytic fungussp. PfuH1 [J]., 2020, 146: 104708.
[8] Wang M, Sun Z H, Chen Y C,. Cytotoxic cochlioquinone derivatives from the endophytic fungusderived from[J]., 2016, 110: 77-82.
[9] Liu H X, Li H H, Chen Y C,. Cytotoxic secondary metabolites from an endophytic fungal strain of[J]., 2019, 39(5): 1475.
[10] Liu D, Li X M, Li C S,. Sesterterpenes and 2-pyran-2-ones (=-pyrones) from the mangrove- derived endophytic FungusMA-84 [J]., 2013, 96(3): 437-444.
[11] Shen L, Luo Q, Shen Z P,. A new cytochalasin from endophyticsp. IFB-E060 [J]., 2014, 12(7): 512-516.
[12] Shang Z, Raju R, Salim A A,. Cytochalasins from an Australian marine sediment-derivedsp. (CMB-M0042F): Acid-mediated intramolecular cycloadditions enhance chemical diversity [J]., 2017, 82(18): 9704-9709.
[13] Cole R J, Wilson D M, Harper J L,. Isolation and identification of two new [11]cytochalasins from[J]., 1982, 30(2): 301-304.
[14] Isaka M, Jaturapat A, Rukseree K,. Phomoxanthones A and B, novel xanthone dimers from the endophytic fungusspecies [J]., 2001, 64(8): 1015-1018.
[15] Wagenaar M M, Clardy J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungusisolated from an endangered mint [J]., 2001, 64(8): 1006-1009.
[16] Li X L, Zhang L, Liu Y H,. A new metabolite from the endophytic fungus[J]., 2013, 8(5): 1934578X1300800.
[17] 文小玲, 何承刚, 翁瑞旋, 等. 云南干巴菌次生代谢产物研究 [J]. 云南农业大学学报: 自然科学, 2016, 31(3): 571-574.
[18] Todorova M, Vogler B, Tsankova E. Terpenoids from[J]., 2000, 55(9/10): 840-842.
[19] Skehan P, Storeng R, Scudiero D,. New colorimetric cytotoxicity assay for anticancer-drug screening [J]., 1990, 82(13): 1107-1112.
[20] Miranda K M, Espey M G, Wink D A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite [J]., 2001, 5(1): 62-71.
[21] Lee J, Yi J M, Kim H,. Cytochalasin H, an active anti-angiogenic constituent of the ethanol extract ofthorns [J]., 2014, 37(1): 6-12.
[22] Elsässer B, Krohn K, Flörke U,. X-ray structure determination, absolute configuration and biological activity of phomoxanthone A [J]., 2005, 2005(21): 4563-4570.
[23] Ying Y M, Shan W G, Zhang L W,. Ceriponols A-K, tremulane sesquitepenes fromHS-ZJUT-C13A, a fungal endophyte of[J]., 2013, 95: 360-367.
[24] Zhao Y, Li S Q, Li H J,. Lanostane triterpenoids from the fungusassociated with[J]., 2013, 49(4): 653-656.
Study on secondary metabolites of endophytic fungusA878 from
WANG Nuo-yi1, 2, LIU Hong-xin2, CHEN Yu-chan2, ZHANG Wei-min2, GAO Xiao-xia1
1. School of Pharmacy, Guangdong Pharmaceutical University, Guangzhou 510006, China 2. State Key Laboratory of Applied Microbiology of Southern China, Guangdong Key Laboratory of Microbial Culture Collection and Application, Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou 510070, China
To investigate the secondary metabolites of endophytic fungusA878 from medicinal plant.The fermentation products were separated and purified by silica gel column, Sephadex LH-20, HPLC and recrystallization. The structures of the compounds were identified by analysis of their1H-,13C-NMR and UV spectra. The biological activities of the compounds were evaluated by SRB and Griess methods.Two new compounds and 10 known compounds were isolated and identified from the solid fermentation products of endophytic fungus A878, their structures were identified as irpexone A (1), irpexone B (2), cytochalasin H (3), cytochalasin J (4), 18-methoxy cytochalasin J (5), deacetylcytochalasin H (6), cytochalasin J3(7), phomoxanthone A (8), dicerandrol A (9), penicillocitrin A (10), (22,24)-ergosta-5,7,22-trien-3β-ol (11) and 1,2,4-trihydroxy--menthane (12).Compounds 1 and 2 were new pyranolactones. The bioactivity assay results showed that compounds 4, 5, and 9 demonstrated moderate cytotoxic activity against SF-268, MCF-7, HepG2, and A549 cell lines with IC50values ranging from 24.83 to 69.63 μmol/L. Besides, compound 11 showed weak anti-inflammatory activity.
(Blanco) Benth.; endophytic fungi;(N. Maek., Suhara & R. Kondo) C.C. Chen & Sheng H. Wu; secondary metabolites; bioactivity; irpexone A; irpexone B; cytochalasin J; dicerandrol A
R284.1
A
0253 - 2670(2023)04 - 1017 - 09
10.7501/j.issn.0253-2670.2023.04.001
2022-11-24
广东省特支计划项目(2019TQ05Y375)
王诺依(1993—),女,硕士,研究方向为天然药物化学。Tel: 15622311838 E-mail: wnykevin@163.com
高晓霞,教授,硕士生导师。Tel: 13828488103 E-mail: gaoxxia91@163.com
章卫民,研究员。Tel: (020)87682785 E-mail: wmzhang@gdim.cn
[责任编辑 王文倩]
