Root Endophyte Shift and Key Genera Discovery in Rice under Barnyardgrass Stress
2023-02-20LIShuyanYANQilingWANGJieyuJIANGHuidanLIZurenPENGQiong
LI Shuyan ,YAN Qiling ,WANG Jieyu ,JIANG Huidan ,LI Zuren,PENG Qiong
(1Long Ping Branch,Graduate School of Hunan University,Changsha 410125,China;2Hunan Agricultural Biotechnology Research Institute,Hunan Academy of Agricultural Sciences,Changsha 410125,China)
Abstract: Despite increasing knowledge of barnyardgrass (Echinochloa crus-galli) interference with rice,relatively little is known how endophytes improve the ability of rice against barnyardgrass stress.Here,we provided a detailed temporal characterization of rice root-associated microbiomes during co-cultivation with barnyardgrass and a comparison with the microbiomes of weed-free rice plants.Alpha diversity analysis indicated that barnyardgrass had the opposite effects on endophytic bacteria and fungi in rice roots,in terms of the community diversity,richness and coverage at the rice seedling stage.Principal coordinate analysis showed that barnyardgrass had only a minor effect on the community composition of endophytes in rice roots at the rice seedling stage,but showed a significant and maximum interference at the heading stage.Rice recruited many endophytes to resist biotic stress from barnyardgrass,especially for fungi.PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states) predictive analysis indicated that 23 metabolic pathways of bacteria were overrepresented in rice.In addition,the main trophic mode of fungi was pathotroph according to FUNGuild analysis.A positive correlation between bacteria and fungi in rice roots was found via network analysis.Anaeromyxobacter,Azospira and Pseudolabrys were the vital bacteria,Phaeosphaeria and Funneliformis were the dominant fungi in maintaining the stability of the ecological network.These results provided data and a theoretical basis for the in-depth understanding of what role endophytes play in rice resistance to barnyardgrass stress and will have implications on improving the resistance of rice against biotic stress using root microbiota.
Key words: rice;Echinochloa crus-galli;biotic stress;endophytic bacterium;endophytic fungus;phylogenetic investigation of communities by reconstruction of unobserved states;FUNGuild test
Barnyardgrass (Echinochloa crus-galli),with its broad ecological fitness and superior competitive ability,is one of the most dominant and noxious weed species in rice paddy (Peng et al,2019).Barnyardgrass can produce numerous seeds and grow better than crop plants in adverse environments.It may cause 21%-79%yield loss in rice (Bajwa et al,2015).The quality of rice is also significantly decreased when neighbored by barnyardgrass,in terms of leaf photosynthetic rate,root biomass,root oxidation activity and dry matter accumulation (Zhang et al,2017).
Plant roots are colonized by complex microbiota,especially endophytes,which are very important for the acquisition of nutrients,growth,development and enhancing tolerance to different stresses (Liu et al,2020).Endophytes include both bacteria and fungi that colonize the plant tissues either intracellularly or intercellularly without causing any harm to the plants(Wilson,1995).Various types of endophytes have been isolated from rice and characterized for their roles in promoting rice health and vigor (Amprayn et al,2012).Candida tropicalis,Phoma glomerataandPaecilomyces formosuscan improve shoot and root lengths,and fresh and dry weights of rice by producing auxins and gibberellins (Amprayn et al,2012;Waqas et al,2015).Burkholderia vietnamiensisandPantoeaagglomeransplay a vital role in fixing nitrogen (Feng et al,2006;Puri et al,2018).Lysinibacillus sphaericuscan solubilize inorganic phosphate via production of 1-aminocyclopropane-1-carboxylate deaminase (Shabanamol et al,2020).
Apart from that,endophytes can also help rice to overcome the adverse effects of abiotic and biotic stresses.The fungiP.formosusLWL1,Bacillus pumilusandArthrinium phaeospermumcan mitigate heat,high salt and drought stresses,respectively (Waqas et al,2015;Kumar et al,2021).Bacillus amyloliquefaciensRWL-1 enhances growth,photosynthesis and biomass of rice seedlings subjected to copper stress (Shahzad et al,2019).When it comes to biotic stress,Streptomyces endusOsiSh-2 causes hyphal swelling and growth suppression ofMagnaporthe oryzae,thereby reducing the severity of rice blast disease (Xu et al,2017).Piriformospora indicasuppresses the rice root herbivory byLissorhoptrus oryzophilus(Cosme et al,2016).Recently,the use of microbiome has been the most important consideration to think of innovative methods to increase rice stress reactions.In the case of mixed culture with barnyardgrass,no significant differences were observed in bacterial biomass and actinobacterial biomass of rice and barnyardgrass root zones,whereas fungal biomass of barnyardgrass and rice root zones were changed significantly (Wang et al,2013).Moreover,barnyardgrass infestation may have positive effects on soil microbial diversity and community composition and may facilitate rice growth(Kong et al,2013).However,it remains unknown how rice utilized endophytes to resist barnyardgrass stress.
As the barnyardgrass infestation problem has become more frequent,it is important to gain detailed knowledge of the consequences of invasive barnyardgrass on rice growth,especially the rice root microbiome.Here,we conducted a detailed temporal profiling of the endosphere communities of rice plants grown with barnyardgrass to investigate how endophytes help rice against biotic stress from barnyardgrass and what is the interrelationship between endophytic bacterial and fungal communities in the rice root system.The results provided insights into the impacts of barnyardgrass on rice root microbiota establishment and strengthened the defensive system of rice from the perspective of the interaction of microorganisms.
RESULTS
Sequencing results
A total of 10 568 642 high-quality sequences were retrieved after denoising,merging and chimera checking.After separating bacterial and fungal reads,4 970 657 bacterial sequences remained,and a total of 3 508 operational taxonomic units (OTUs) were obtained,belonging to 44 phyla,133 classes,281 orders,487 families,941 genera and 1 710 species.For fungal sequences (5 597 985),a total of 2 072 OTUs were obtained,belonging to 1 kingdom,13 phyla,50 classes,122 orders,282 families,546 genera and 855 species.
α-and β-diversity in rice root endophytic communities
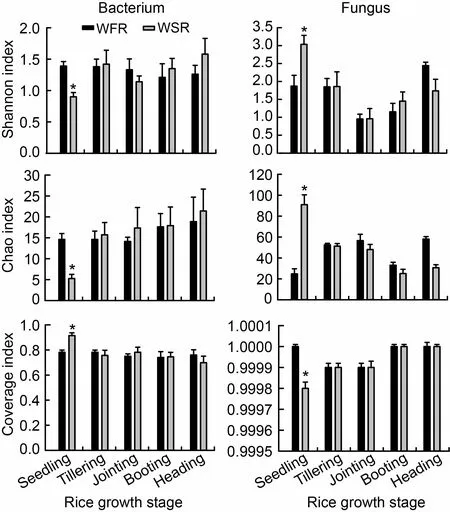
Fig.1.Species diversity,richness and coverage in rice roots at different growth stages for operational taxonomic units constructed at 0.03 dissimilarity for bacterial and fungal sequences.
Due to the disturbance of barnyardgrass,differences were observed in α-diversity indices for bacterial and fungal communities (Fig.1).At the seedling stage,the bacterial community diversity (Shannon index) and richness (Chao index) were significantly lower in libraries derived from weed-stress rice (WSR,rice was co-cultivated with barnyardgrass) than those from weed-free rice (WFR,rice grown alone without barnyardgrass).The bacterial community coverage of WSR was dramatically higher than that of WFR.By contrast,those parameters showed completely opposite trends in fungal libraries compared with bacterial libraries.However,no dramatic differences were observed at the remaining growth stages.The results showed that the effects of barnyardgrass on endophytic bacteria and fungi in rice roots were completely opposite.To be specific,the community diversity and richness of endophytic bacteria and the community coverage of endophytic fungi in roots of WSR were decreased significantly at the rice seedling stage,while the community diversity and richness of endophytic fungi and the community coverage of endophytic bacteria were increased significantly under the stress from barnyardgrass (Fig.1).
β-diversity analysis revealed the similarities or differences in the compositions of bacterial and fungal communities of rice samples.Principal coordinate analysis (PCoA) based on Bray-Curtis distance was used to study the effects of the stress from barnyardgrass on the composition of the endophytic community in the rice root system.In the PCoA of Bray-Curtis distance from all the samples,the distances in the ordination plot for WSR and WFR samples showed differences both in bacterial and fungal communities,indicating that the existence of barnyardgrass significantly affected the compositions of the bacterial and fungal communities of rice (Fig.2).For bacterial communities,the sample points of WFR and WSR at the seedling stage were close,indicating that the two samples were more similar in species composition (Fig.2).Distances between the WFR and WSR sample points gradually appeared at the tillering,jointing and booting stages,and peaked at the heading stage.Moreover,the bacterial community composition of rice at the seedling stage was significantly different from those at the other four stages.For the fungal communities,the sample points of WFR and WSR were also close at the seedling stage,while the largest distance between the samples occurred at the heading stage,which was similar to bacterial communities.The results demonstrated that barnyardgrass had the least disturbing effect on the endophytic bacteria and fungi in the rice root system at the seedling stage,while the highest disturbing effect was observed at the heading stage.
Endophytic bacterial and fungal species composition
Bacterial community composition

Fig.2.Principal coordinate analysis (PCoA) based on Bray-Curtis distances between weed-free rice (WFR) and weed-stress rice (WSR)for endophytic bacteria and fungi in rice roots at different growth stages.
At the genus level,61 genera were enriched in rice roots under the disturbance of barnyardgrass (Fig.3-A and Table S1).To be more specific,there were 6 genera [0319-6G20(o),Anaeromyxobacter,Azospira,Geothrix,LautropiaandStreptomyces] at the seedling stage,23 genera [Actinomadura,Azospira,Bacteroidales(o),Bradyrhizobium,Ciceribacter,Delftia,Dechloromonas,Ideonella,Lechevalieria,Methyloligellaceae(f),Methylosinus,Mycobacterium,Paenibacillus,Pleomorphomonadaceae(f),Pleomorphomonas,Rhizobiaceae(f),Rhizobiales(o),Rhodocyclaceae(f),Schlegelella,SC-I-84(f),Sideroxydans,SphingomonasandVogesella] at the tillering stage,17 genera [0319-6G20(o),Azospira,Bradyrhizobium,Bacillus,Burkholderiales(o),CPR2(c),Dechloromonas,Delftia,Gallionella,Ideonella,Kineosporiaceae(f),Methylotenera,Micrococcales(o),Microvirga,Ralstonia,Paenibacillusand Rhodocyclaceae(f)] at the jointing stage,21 genera [Acidovorax,Anaeromyxobacter,Azoarcus,Bacillus,Bradyrhizobium,Candidatus-Accumulibacter,Chlorobaculum,Christensenellaceae(f),Clostridium-sensu-stricto-1,Gallionella,Gallionellaceae(f),KD3-93(f),Methylosinus,Methylocaldum,Micrococcales(o),Noviherbaspirillum,Phreatobacter,Pseudactinotalea,Rhizobiales_Incertae_Sedis(f),Roseiflexaceae(f) and SB-5(f)] at the booting stage,and 18 genera [0319-6G20(o),Anaerovorax,Azospirillum,Ciceribacter,Fibrobacteraceae(f),Haliangium,Microtrichales(o),Ideonella,Phreatobacter,Pleomorphomonas,Ralstonia,Pseudolabrys,Ramlibacter,Rhodococcus,Sphingomonas,Rhodocyclaceae(f),Streptomycesand Xanthobacteraceae(f)]at the heading stage.To sum up,the bacterial diversity of rice was increased at the genus level at each stage after co-planting with barnyardgrass compared with the control groups.
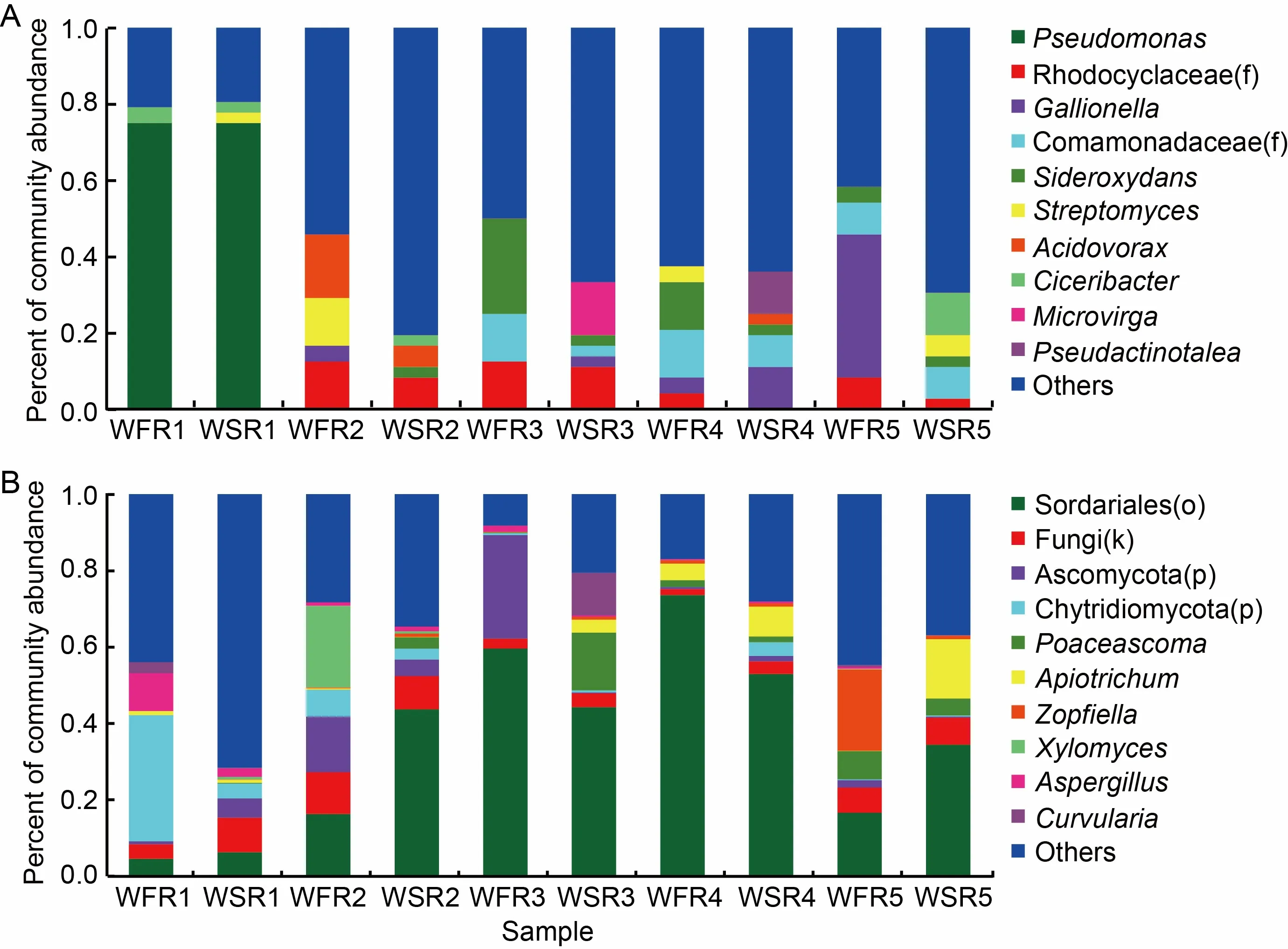
Fig.3.Endophytic bacterial (A) and fungal (B) community structures at genus level in weed-free rice (WFR) and weed-stress rice (WSR) roots among five different growth stages.
Fungal community composition
For fungi,at the genus level,47 genera were enriched in WSR (Fig.3-B and Table S2).There were 31 genera [Alternaria,Arthrobotrys,Ascomycota(p),Candida,Chaetomiaceae(f),Chaetomium,Cladosporium,Cordyceps,Cutaneotrichosporon,Epicoccum,Fusarium,Filobasidium,Gibberella,Gibellulopsis,Hannaella,Issatchenkia,Mortierella,Neocosmospora,Olpidium,Pezizella,Phaeosphaeriaceae(f),Phaeosphaeriopsis,Poaceascoma,Serendipita,Sordariaceae(f),Sordariales(o),Solicoccozyma,Sordariomycetes(c),Talaromyces,ThermomycesandXylomyces] at the seedling stage,19 genera [Acrophialophora,Arthrobotrys,Ascobolus,Aspergillus,Branch03(o),Candida,Chaetomiaceae(f),Cutaneotrichosporon,Issatchenkia,Phaeosphaeria,Phaeosphaeriaceae(f),Poaceascoma,Serendipita,Sordariaceae(f),Sordariales(o),Sordariomycetes(c),Talaromyces,VishniacozymaandZopfiella] at the tillering stage,23 genera[Alternaria,Chaetomiaceae(f),Branch03(o),Cephalotrichum,Apiotrichum,Chaetomium,Cordyceps,Chytridiomycota(p),Dokmaia,Sordariomycetes(c),Curvularia,Cutaneotrichosporon,Cladosporium,Gibellulopsis,Poaceascoma,Meyerozyma,Gibberella,Hannaella,Tausonia,Saccharomycetales_fam_Incertae_sedis(f),Zopfiella,TalaromycesandThermomyces] at the jointing stage,23 genera [Arthrobotrys,Ascomycota(p),Cladosporium,Apiotrichum,Ascobolus,Candida,Aspergillus,Chytridiomycota(p),Cordyceps,Cutaneotrichosporon,Dokmaia,Filobasidium,Gibberella,Epicoccum,Rozellomycota(p),Hannaella,Phaeosphaeria,Issatchenkia,Thermomyces,Vishniacozyma,Meyerozyma,TalaromycesandZopfiella] at the booting stage,19 genera [Acrophialophora,Alternaria,Apiotrichum,Branch03(o),Cladosporium,Cordyceps,Fusarium,Chytridiomycota(p),Gibellulopsis,Cutaneotrichosporon,Issatchenkia,Mortierella,Neocosmospora,Olpidium,Phaeosphaeria,Rozellomycota(p),Sordariomycetes(c),SerendipitaandSordariales] at the heading stage.Overall,the fungal diversity of WSR was increased at each stage after co-planting with barnyardgrass compared with the WFR groups.
Bacterial and fungal metabolic pathways differentially represented in rice under barnyardgrass stress
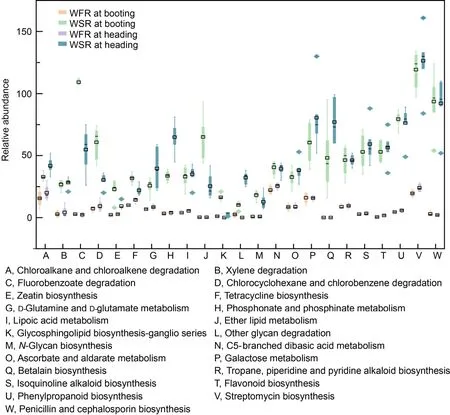
Fig.4.Relative abundance of PICRUSt1 (phylogenetic investigation of communities by reconstruction of unobserved states) inferred function in weed-free rice (WFR) and weed-stress rice (WSR) samples at booting and heading stages.
Possible bacterial metagenomes were estimated using PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states) with GreenGenes as the underlying database (Fig.4).This difference was significant at rice growth stages under the disturbance of barnyardgrass.At the reproductive stage (booting and heading),23 pathways were overrepresented in WSR compared with WFR groups.Namely,seven of them belonged to biosynthesis of secondary metabolites (betalain biosynthesis,tropane,piperidine and pyridine alkaloid biosynthesis,isoquinoline alkaloid biosynthesis,flavonoid biosynthesis,phenylpropanoid biosynthesis,streptomycin biosynthesis,and penicillin and cephalosporin biosynthesis);four belonged to xenobiotic biodegradation and metabolism(chloroalkane and chloroalkene degradation,xylene degradation,fluorobenzoate degradation,and chlorocyclohexane and chlorobenzene degradation);three came from carbohydrate metabolism (C5-branched dibasic acid metabolism,ascorbate and aldarate metabolism,and galactose metabolism);another three came from glycan biosynthesis and metabolism(glycosphingolipid biosynthesis-ganglio series,other glycan degradation,andN-glycan biosynthesis);and the remains came from metabolism of terpenoids and polyketides (zeatin biosynthesis,and tetracycline biosynthesis),metabolism of other amino acids(D-glutamine and D-glutamate metabolism,and phosphonate and phosphinate metabolism),metabolism of cofactors and vitamins (lipoic acid metabolism),and lipid metabolism (ether lipid metabolism).
FUNGuild was used to predict the nutritional and functional groups of the fungal communities.Eight trophic mode groups can be classified,with saprotrophs,symbiotrophs and pathotrophs being the major components (Fig.5-A).The saprotroph composition analysis showed that the relative abundance of soil saprotroph was higher in WSR with no dramatic difference (Fig.5-B).In addition,the symbiotrophic mode groups primarily consisted of arbuscular mycorrhizal,endophyte and ectomycorrhizal (Fig.5-C).As to pathotroph,there was more plant and animal pathogens in WSR,and plant pathogens showed differences at the different growth stages (Fig.5-D and Table S3).At the seedling stage,WSR held,by far,the most specific pathogens (163 species),followed by the tillering and jointing stages,with 81 and 88 species,respectively.There were only 47 and 45 specific species that showed pathogenicity in WSR at the booting and heading stages (Table S3).IssatchenkiaandParaphomashowed pathogenicity over the whole growth period.About 40 species of fungi,such asPoaceascoma,PeniophoraandClonostachys,merely exhibited pathogenicity at the vegetative growth stage of rice.
In conclusion,rice utilized bacterial metabolic pathways to resist barnyardgrass stress at the reproductive stage (booting and heading),and the main trophic mode of fungi was pathotroph.
Interactions between endophytic bacterial and fungal communities
To unravel the relationship between endophytic fungi and bacteria,we constructed a co-occurrence network for each species.Network analysis indicated that there was a significant influence of the bacterial community on the fungal one and vice versa.In other words,the fungal community might be predicted based on the bacterial one.The relationship between most bacterial and fungal communities was positive,regardless of WFR or WSR groups.
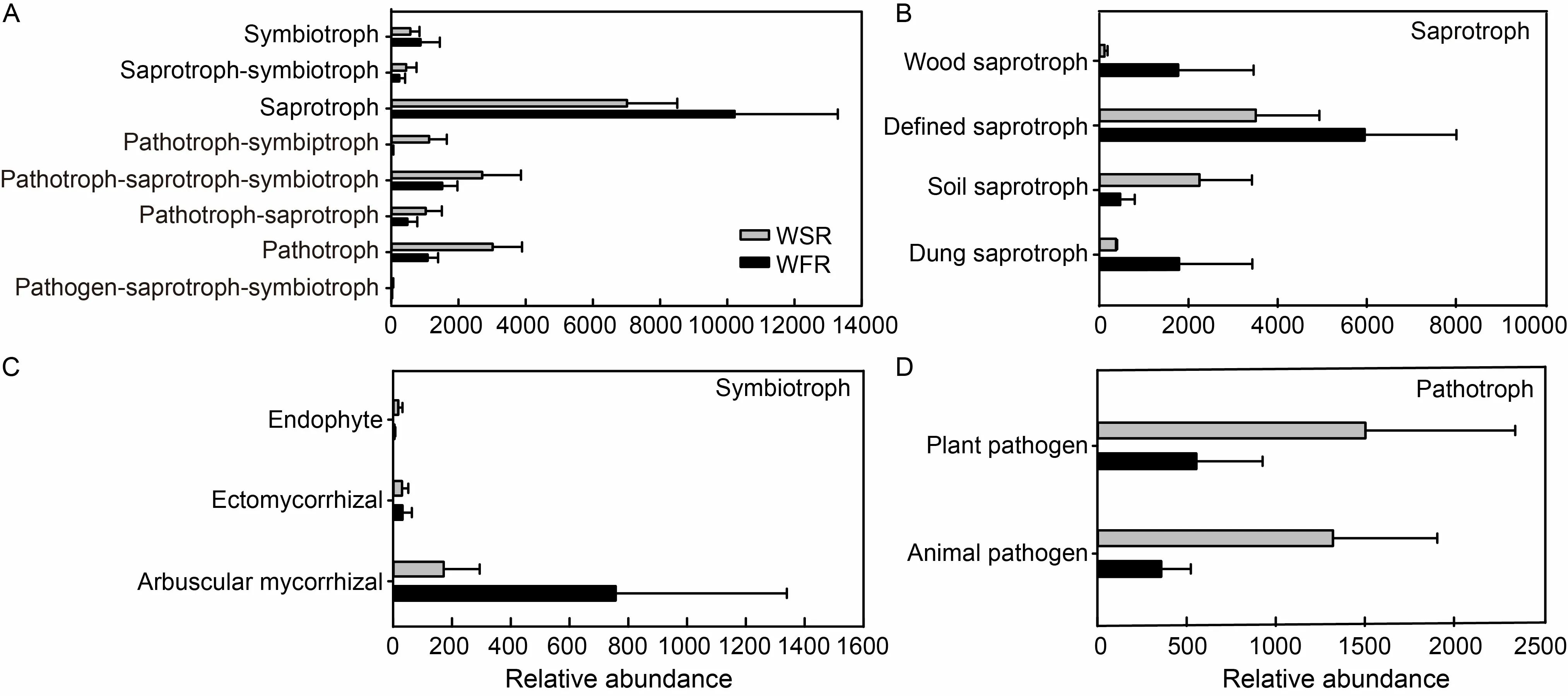
Fig.5.Relative abundance of trophic modes (A) and mainly guilds (B-D) assigned by FUNGuild for fungal community.
To reveal the key microbes in the networks,we looked into the nodes with max degree,max closeness and max betweenness centrality.Tables S4 and S5 show the key bacteria and fungi in WFR and WSR and their taxa in the networks.The key bacteria and fungi were different between the WSR and WFR groups.For the WSR groups,the key bacterial genera wereAnaeromyxobacter,AzospiraandPseudolabrys,and the dominant fungal genera werePhaeosphaeriaandFunneliformis(Fig.S1).For the WFR groups,GallionellaandBradyrhizobiumwere the dominant bacteria,andEpicoccumandMortierellawere the main fungi (Fig.S2).
DISCUSSION
Barnyardgrass-induced changes in rice endophytic community structure
Exudate-mediated root behavior is particularly interesting in crop-weed competition,and the weeds respond to the presence of rice through the asymmetric root distribution.Exudate-mediated root-placement patterns affect dormant soil microbes,which account for more than 95% of the total microbial biomass (Fierer,2017).In this study,the community diversity,richness and coverage of endophytic bacteria and fungi in rice roots were significantly affected by barnyardgrass at the seedling stage.Wang et al (2013) found that the ratio of bacteria to fungi in soil was significantly higher and Shannon Wiener diversity index was significantly increased in the presence of barnyardgrass.
The richness of endophytic fungi classified to the genus level in this study was higher compared with the richness in endophytic bacterial communities at each stage.The reason may be the pH and soil texture correlated with the soil bacterial community composition,while the fungal community was rather associated with the changes in soil nutrient status (Furtado et al,2019).When rice grown with barnyardgrass,they secrete a series of root exudates against each other.Therefore,the internal micro-ecological environment of the root was changed,which is the habitat of endophytes (Bacilio-Jiménez et al,2003;Bais et al,2006).
In particular,the effect of barnyardgrass on endophytic bacteria in rice roots was completely opposite to that on fungi.This differential response may be attributed to a closer interaction of bacteria and fungi under the barnyardgrass stress.Therefore,when one of them was affected by barnyardgrass,the other can take compensatory action against the stress.
Most abundant taxa in endosphere communities were identified under co-planted condition
Biological nitrogen fixation via microorganisms by utilizing complex procedures with the help of enzymes,such as nitrogenase,can effectively convert atmospheric nitrogen into ammonia.Nitrogenase is a highly conserved protein,which is commonly found in all nitrogenfixing bacteria,and a series of diazotrophic bacteria were detected in rice roots (Ji et al,2014).In this study,the relative abundance of bacteria with nitrogen-fixing and nitrogen-cycling effects was increased in WSR,such asAllorhizobium-Neorhizobium-Pararhizobium-Rhizobium,Microvirga,Anaeromyxobacter,Ciceribacter,Ensifer,AzospirillumandBradyrhizobium.Previous studies also showed that a strong shift appears in the rice diazotrophic under abiotic stress (Gholamalizadeh et al,2017),and the richness estimator and Shannon diversity index of diazotrophic communities from flooded rice roots are more diverse than those from unflooded rice roots (Ferrando and Fernández Scavino,2015).
Moreover,rice has rich methane emissions by providing emission pathways (aerenchyma) and methanogenetic substrates (Oda and Chiem,2018).Methylosinus,MethylocaldumandMethylobactercan utilize methane and methanol as carbon and energy sources to promote plant growth (Chu et al,2020).In this study,all these bacteria increased in rice roots.A previous study showed that the relative abundance ofMethylosinusis significantly increased in paddy field with inadequate nutrients (Ikeda et al,2014).Apart from those associated with nitrogen fixation and energy production,the invasion of barnyardgrass also positively regulates other bacteria that have a positive effect on plant growth especially in extreme environments (Asaf et al,2020;Siani et al,2021).The reason may be that rice needs to recruit more beneficial microbes to resist barnyardgrass stress,because total absorbing surface area,active absorption surface area,oxidation activity in rice roots are dramatically reduced by barnyardgrass (Zhang et al,2021).The positive effects of barnyardgrass on rice can also be found in fungi.Most of the fungi that increased under barnyardgrass stress are those that have something to do with carbon and nitrogen cycles,such asAscomycetesandPenicillium(Koul and Singh,2017;Challacombe et al,2019;Oses-Pedraza et al,2020).Likewise,a series of fungi which can exude auxin,phenolics and flavonoids are also increased(Jan et al,2019).These results are consistent to the results that the relative abundance of beneficial fungi is increased after diclofop-methyl exposure (Qian et al,2018),with this shift potentially influencing the nitrogen cycle.
Therefore,we further suggested that increasing the relative abundance of endophytes with nitrogen fixation and methane oxidation functions in rice artificially can be labelled as an effective way to magnify the competitiveness of rice.Coupled with the previous study (Hala,2020),it was found that inoculation of nitrogen-fixing bacteria to upland rice can increase tiller formation,shoot and root dry weights,and plant total nitrogen production.
Rice utilized metabolism pathways and different trophic modes to improve its defensive system
In this study,23 metabolic pathways were up-regulated in WSR at the reproductive stage (booting and heading).Namely,pathways related to carbohydrate metabolism,e.g.,C5-branched dibasic acid metabolism,which can produce energy,carbon source and various compounds for bacterial metabolism.This may be caused by the great diversity of complex carbohydrates in the WSR roots,which need various enzymes to be utilized by bacteria (Reid and Abratt,2005).Carbohydrate metabolism is a complex progress which associated with a series of enzymes.Zhang et al (2021) found that the activities of key enzymes involved in sucroseto-starch conversion in rice roots are dramatically reduced when grown with barnyardgrass.Therefore,we deduced that stimulating the carbohydrate metabolism via enzymes can be seen as a novel way to improve the resistance of rice.Apart from that,flavonoid and betalain pigments have been demonstrated to assist in tolerance to a wide range of stresses,such as pest and pathogen attacks (Davies et al,2018;Liu et al,2020).Researchers have found the key structural genes in rice flavonoid biosynthesis (Shih et al,2008).Hence,controlling flavonoid production through genes may become an ideal way for rice to resist barnyardgrass stress.Rice can also utilize bacteria to degrade xenobiotics produced by barnyardgrass.However,why the genes involved in the rest of pathways are apparently more common in rice roots still remains to be elucidated.
Additionally,trophic status was used to further explain how rice utilized the fungal community to resist barnyardgrass stress.The majority of the predicted fungi observed in rice belonged to saprotroph,pathotroph-saprotroph-symbiotroph and pathotroph(Fig.5-A).Gu et al (2020) found that when infested by fusarium wilt disease,the relative abundance of pathotroph-saprotroph-symbiotroph in cucumber is up-regulated.Saprotrophic fungal is a key microbial group mediating nutrient cycling in the terrestrial ecosystem by decomposing complex organic materials via the release of lignocellulolytic enzymes (Crowther et al,2012).The higher fungus proportions of pathotroph and pathotroph-saprotroph-symbiotroph indicated that rice resisted the infestation of barnyardgrass by enhancing pathotrophic growth.Fungal plant pathogen species fall into two main categories: one involving biotrophic pathogens,which form close interactions with host plants and can persistently utilize living tissue (biotrophs),and the other including necrotrophic pathogens,which kill tissues to extract nutrients (necrotrophs) (Barna et al,2012;Doehlemann et al,2017).These pathogenic species are commonly found in the phyla Ascomycota and Basidiomycota (Doehlemann et al,2017).Most species showing pathogenicity in this study were primarily in the phyla Ascomycota and Basidiomycota,and very few species belonged to other phyla.Among Ascomycota,plant pathogens were in various classes such as Dothideomycetes,Sordariomycetes,Leotiomycetes,Eurotiomycetes and Taphrinomycetes.Four classes in Basidiomycota were pathogens,including Tremellomycetes,Agaricomycetes,Microbotryomycetes and Ustilaginomycetes,which indicated that endophytic pathotrophic fungi may play a vital role in inhibiting the competition of barnyardgrass.
Fungal community may be predicted based on bacterial one
Prior studies on the endophytic community in rice focused either on bacteria or fungi.However,as no creature thrives in isolation,the interactions of bacterial and fungal communities in different plant species can be of great importance (Furtado et al,2019).Bacterial and fungal communities play different roles in regulating plant growth and health.Bacteria may play a positive role in fungal activity through producing cellulases and pectinases,which increase available substrates for fungi.In addition,bacteria may also break down solutes that are toxic to certain fungi (de Boer et al,2008) and may increase the amount of nitrogen available to them (Hendrickson,1991).However,bacteria also exert antifungal strategies by secreting a series of inhibitory factors such as hydrogen cyanide,antibiotics,lytic enzymes and volatiles,as well as nutrient-sequestering factors such as ironchelating siderophores (Whipps,2001;Weller et al,2002;Wheatley,2002).When the density of saprophytic fungi in the rhizosphere increases,the enrichment of bacteria with antifungal properties,such as secreting siderophores,cyanide and lyase,also increases (de Boer et al,2008).Fungi also influence bacteria by releasing beneficial nutrients and using fungal hyphae in helping bacterial transport to sites that cannot be reached by bacterial cells alone (Leveau and Preston,2008).The influence of the rice bacterial community on the fungal community was found by network analysis,when its habitat was invaded by barnyardgrass(Figs.S1 and S2).The results showed that the dominant endophytes in WSR were totally different from WFR.We deduced that rice utilized bacterial generaAnaeromyxobacter,AzospiraandPseudolabrys,and fungal generaPhaeosphaeriaandFunneliformisto stabilize the ecological network to resist barnyardgrass stress.The exact function of the above genera on rice resistance still remains to be elucidated and would require further studies through inoculation.
METHODS
Plant growth
The experiments were conducted at the experimental farm of Hunan Academy of Agricultural Sciences,Changsha,Hunan Province,China,during the summer of 2020 to study the variations in root microbiota of rice under the condition of rice-barnyardgrass co-culture at different rice growth stages.Rice variety Tianyouhuazhan and barnyardgrass were cultivated together at a ratio of 1:1 (two rice plants and two barnyardgrass plants were grown together at each pot).A pot with four rice plants only (weed-free) was used as the control.At each stage,the treatment and control were repeated six times.Tianyouhuazhan was provided by the Hunan Rice Research Institute,and barnyardgrass was collected from paddy fields in Hunan Province,China.
Really her one idea was to let the Princess be seen by as few people as possible; so, throwing a veil over her head, she led her away and locked her up securely
To avoid seed endophytes and surface-related microorganisms,rice and barnyardgrass seeds were dehulled,surface sterilized in 75% ethanol for 30 s,in 2.5% sodium hypochlorite for 15 min with three times,and then germinated in Murashige and Skoog agar media.After germination,rice and barnyardgrass seedlings were transplanted to plastic pots.The pots with the dimensions of 46 cm (length) × 35 cm (width) × 17 cm (height)were filled with 15.0 kg of sieved (2 mm) dry paddy soil.The soil was derived randomly from three spots in the same field block that was only used for rice cultivation.The contents of soil organic matter,total nitrogen,total phosphorus,total potassium,available nitrogen,available phosphorus and available potassium were 28.37 g/kg,1.67 g/kg,0.85 g/kg,17.40 g/kg,131.00 mg/kg,2.30 mg/kg and 150.00 mg/kg,respectively,and soil pH was 6.2.Cation exchange capacity was 14.40 cmol/kg and electroconductivity was 9.88 mS/m.In addition,the soil texture was sandy loam.Seedlings were watered and fertilized as required according to conventional field management practices.Other weeds were manually pulled out to prevent additional competition.
Sample collection
The soil samples were collected randomly in rice fields (0-15 cm depth) from three different spots.The above-ground part and root samples were collected at the seedling,tillering,jointing,booting and heading stages.Ten rice plants selected from each growth stage were sampled for root endophyte analysis.After gently shaken off excess soil from the roots,and washed with flowing water,the roots were cleaned up with a sterilized scissor and transferred to a sterile tube with 50 mL of chilled (4 °C) sterile phosphate buffered saline (PBS) solution and shook vigorously until no soil remained on the root surface.Then,the samples were surface sterilized in 75% ethanol for 90 s and washed in sterile water for three times.After washing,roots were dried on sterile filter paper,wrapped in sterile aluminum foil,flash froze in liquid N2,and stored at -80 °C.
DNA extraction and PCR amplification
Microbial genomic DNA was extracted from rice root samples using E.Z.N.A.®Soil DNA Kit (Omega Bio-tek,Norcross,GA,USA) according to the manufacturer’s protocols.The DNA extraction quality was analyzed using 1% agarose gel,and DNA concentration and purity were determined using a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific,Wilmington,USA).
The V3-V4 region of the bacteria 16S ribosomal RNA gene from rice root samples was amplified with primers 779F(5'-AACMGGATTAGATACCCKG-3') and 1193R (5'-ACGT CATCCCCACCTTCC-3').The hypervariable region of ITS was amplified with primers ITS1F (5'-CTTGGTCATTTAGA GGAAGTAA-3') and ITS2R (5'-GCTGCGTTCTTCATCGAT GC-3') using an ABI GeneAmp®9700 PCR thermocycler(Applied Biosystems,CA,USA).The PCR thermal cycle was as follows: initial denaturation at 95 °C for 3 min,followed by 27 cycles of denaturing at 95 °C for 30 s,annealing at 55 °C for 30 s and extension at 72 °C for 45 s,and single extension at 72 °C for 10 min and end at 4 °C.
For bacteria,the formal PCR test was performed using the TransStart Fastpfu DNA Polymerase (TransGen,Beijing,China).PCR reactions were performed in triplicate with 20 μL mixtures containing 4 μL of 5× FastPfu buffer,2 μL of 2.5 mmol/L dNTPs,0.8 μL of each primer (5 μmol/L),0.4 μL of FastPfu polymerase,0.2 μL of bovine serum albumin (BSA),and 10 ng of template DNA.PCR was performed with following procedure: 95 °C for 3 min,followed by 27 cycles at 95 °C for 30 s,55 °C for 30 s and 72 °C for 45 s,and a final extension at 72 °C for 10 min,and end at 10 °C.For fungi,PCR was performed in triplicate with 20 μL mixtures containing 2 µL of 10× buffer,2 µL of 2.5 mmol/L dNTPs,0.8 μL of each primer (5 μmol/L),0.2 µL of rTaq polymerase(TaKaRa,Beijing,China),0.2 μL of BSA,and 10 ng of template DNA.PCR was performed with following procedure:95 °C for 3 min,followed by 27 cycles at 95 °C for 30 s,55 °C for 30 s,and 72 °C for 45 s,and a final extension at 72 °C for 10 min,and end at 10 °C.The PCR products were evaluated using 2% agarose gel electrophoresis.
Sequence analysis
Sequencing and library construction were performed by Beijing Biomarker Technologies Co.,China.The raw 16S rRNA gene sequencing reads were demultiplexed,quality-filtered using the fastp version 0.20.0 (Chen et al,2018),and merged using FLASH version 1.2.7 (Magoč and Salzberg,2011) with the following criteria: (i) The 300-bp reads were truncated at any site receiving an average quality score of < 20 over a 50-bp sliding window,and the truncated reads shorter than 50 bp or containing ambiguous characters were discarded.(ii) Only overlapping sequences longer than 10 bp were assembled according to their overlapped sequences.The maximum mismatch ratio of overlap region was 0.2.Reads that could not be assembled were discarded.(iii) Samples were distinguished according to the barcodes and primers,the allowed mismatch number of barcode was 0,and the maximum primer mismatch number was 2.OTUs with 97% similarity cutoff were clustered using UPARSE version 7.1 (Edgar,2013),and chimeric sequences were identified and removed.The taxonomy of each OTU representative sequence was analyzed using the RDP Classifier version 2.2 (Wang et al,2007) against the 16S rRNA database (Silva v138) and the ITS database (unite 8.0) using a confidence threshold of 0.7.
Statistics analysis
The α-diversity estimators consisted of the Chao1 estimator and Shannon and Coverage species diversity indices,were carried out using Mothur Version 1.30 (http://www.mothur.org/).Additionally,β-diversity estimators were used to analyze the differences among all the samples.PCoA was performed based on the Bray-Curtis distances among the bacterial and fungal communities.Bacterial and fungal taxonomic distributions of sample communities were visualized using the R package software.The Mann-Whitney U test was used to examine differences in bacterial and fungal compositions between the two groups.The differences in diversity and richness among the treatments were determined using one-way ANOVA (SPSS 22.0).The bacterial functions were predicted by PICRUSt Version 1.0 (http://picrust.github.io/picrust/).The fungal functions were predicted by Funguild Version 2.2.0 (http://www.funguild.org/).The influence of communities on each other was assessed using the network analysis (Python2.7,Networkx,and Gephi).All data were denoted as Mean ± SE (n=6).
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No.31701803),Changsha Natural Science Foundation,China (Grant No.kq2202336),and the Special Project of Hunan Innovative Province Construction,China (Grant No.S2021ZCKPZT0004).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science;http://www.ricescience.org.
Fig.S1.Correlation network diagram of bacteria and fungi in weed-stress rice at genus level.
Fig.S2.Correlation network diagram of bacteria and fungi in weed-free rice at genus level.
Table S1.Bacteria enriched in weed-stress rice at different rice growth stages.
Table S2.Fungi enriched in weed-stress rice at different rice growth stages.
Table S3.Plant pathogens in weed-stress rice groups at different growth stages.
Table S4.Vital bacteria in weed-stress rice and weed-free rice groups.
Table S5.Vital fungi in weed-stress rice and weed-free rice groups.
杂志排行
Rice Science的其它文章
- Antioxidant Activities and Characterization of Polyphenols from Selected Northern Thai Rice Husks: Relation with Seed Attributes
- NaCl Facilitates Cell Wall Phosphorus Reutilization in Abscisic Acid Dependent Manner in Phosphorus Deficient Rice Root
- A Pleiotropic Drug Resistance Family Protein Gene Is Required for Rice Growth,Seed Development and Zinc Homeostasis
- Peptide Transporter OsNPF8.1 Contributes to Sustainable Growth under Salt and Drought Stresses,and Grain Yield under Nitrogen Deficiency in Rice
- Novel Deletion in Exon 7 of Betaine Aldehyde Dehydrogenase 2(BADH2)
- Transfer Learning-Based Image Recognition of Nitrogen and Potassium Nutrient Stress in Rice
