A Pleiotropic Drug Resistance Family Protein Gene Is Required for Rice Growth,Seed Development and Zinc Homeostasis
2023-02-20LIChaoLIHeZHANGXianduoYANGZhimin
LI Chao,LI He,ZHANG Xianduo,YANG Zhimin
(Department of Biochemistry and Molecular Biology,College of Life Sciences,Nanjing Agricultural University,Nanjing 210095,China)
Abstract: Zinc (Zn) is an essential mineral element for plant growth and development.Zn deficiency in crops frequently occurs in many types of soils.It is therefore crucial to identify genetic resources linking Zn acquisition traits and development of crops with improved Zn-use efficiency for sustainable crop production.In this study,we functionally identified a rice uncharacterized ABCG (ATP-binding cassette G-subfamily) gene encoding a PDR20 (pleiotropic drug resistance 20) metal transporter for mediation of rice growth,seed development and Zn accumulation.OsPDR20 was localized to the plasma membrane,but it was not transcriptionally induced under Zn deficiency,rather was sufficiently up-regulated under high level of Zn stress.Yeast (Saccharomyces cerevisiae) transformed with OsPDR20 displayed a relatively lower Zn accumulation with attenuated cellular growth,suggesting that OsPDR20 had an activity for Zn transport.Knocking-down OsPDR20 by RNA interference (RNAi) compromised rice growth with shorter plant height and decreased biomass in rice plantlets grown under hydroponic media.Zn concentration in the roots of OsPDR20 knocked-down rice lines declined under Zn deficiency,while they remained unchanged compared with the wild type under normal Zn supply.A rice lifelong field trial demonstrated that OsPDR20 mutation impaired the capacity of seed development,with shortened panicle and seed length,compromised spikelet fertility,and reduced grain number per plant or grain weight per unit area.Interestingly,OsPDR20 mutation elevated the accumulation of Zn in husk and brown rice over the wild type.Overall,this study pointed out that OsPDR20 is fundamentally required for rice growth and seed development through Zn transport and homeostasis.
Key words: OsPDR20;zinc transport;rice;seed development;ABCG53;pleiotropic drug resistance
Zinc (Zn) is required for plant growth and seed development owning to its active participation in a variety of molecular regulations such as gene transcription,protein synthesis,signaling transduction and activation of many proteins or enzymes (Amini et al,2022).As a cofactor,Zn-containing enzymes or proteins are involved in massive physiological processes like photosynthesis,nitrogen metabolism,carbohydrate or tryptophan synthesis and so forth (Bashir et al,2012).Zinc deficiency is commonly occurring in most upland crops due to unfavorable soil texture and chemical composition,which thereby leads to abnormal growth,seed development and quality or yield (Marschner,1995).Understanding regularity of Zn acquisition to maintain an optimal window of Zn concentrations is critical for health crop and high-quality seed productivity (Wessells and Brown,2012).
Great efforts have been made recently to identify Zn transporters from diverse protein families such as Zn-regulated transporter and iron-regulated transporterlike protein (ZIP),cation diffusion facilitator protein or metal tolerance/transport protein (MTP),heavy metal ATPase (HMA),yellow stripe-like (YSL)proteins,and natural resistance-associated macrophage proteins (Andresen et al,2018;Amini et al,2022).The major Zn transporters belong to the ZIP protein family.In rice,most of the ZIP members (OsIRT1,OsZIP1-9 and OsZIP11) have been functionally characterized though they have marked disparities in metal specificity,subcellular localization,tissue allocation or direction of metal delivery (Ramesh et al,2003;Lee and An,2009;Lee et al,2010a,b;Kavitha et al,2015;Yang et al,2020;Zhao et al,2022).Most of the transporters are responsible for Zn acquisition and mobility from soil to root and translocation from root to the above-ground (Liu et al,2019).These processes may be also accompanied by organic substances such as peptides like glutathione,phytosiderophore,metallothionein or phytochelatin (Milner et al,2013;Rono et al,2022).Several other important types of Zn transporters like HMAs such as AtHMA1-4 fromArabidopsisand OsHMA1-3 from rice have also been reported for Zn acquisition and mobility (Takahashi et al,2012;Andresen et al,2018).Additionally,MTP and YSL family members are similarly engaged in Zn allocation through phloem to seed organs (Olsen and Palmgren,2014).
ATP-binding cassette (ABC) transporter is one of the largest super-family proteins that take advantage of ATP hydrolysis-released energy to drive internal metabolites and external chemicals across cellular membrane networks (Garcia et al,2004).The substrates of ABCs cover a broad range of molecules and compounds such as metabolites (sugars,fatty acids,amino acids or peptides),xenobiotics (drugs or other artificial compounds),and inorganic compounds like metal ions (Krämer et al,2007;Liu,2019).It is apparent that ABC proteins participate in numerous molecular,biochemical and physiological metabolic processes during the plant growth,development and environmental stress responses (Hwang et al,2016;Lefèvre and Boutry,2018;Zhang et al,2018a).Based on the sequence and domain arrangement,the plant ABC transporters are divided into a couple of subfamilies(A-G and I) (Verrier et al,2008).Several subfamily members are given additional names like MDR(multidrug resistance for ABCB),MRP (multidrug resistance associated protein for ABCC) or PDR(pleiotropic drug resistance for ABCG),due to the fact that they are originally assigned for their specific functions in resistance to drugs and xenobiotics (Dahuja et al,2021).Recent studies demonstrate that some members in the above three subfamilies can transport mineral elements (Lefèvre and Boutry,2018).For example,rice ABCI8 is a chloroplast-targeted transporter andOsABCI8mutation leads to a phenotype with overload of iron (Zeng et al,2017).Of the ABC families,the PDR group members are the focus of research interest as most of them so far identified are associated with the response to biotic and environmental stresses(Moons,2008;Oda et al,2011;Nuruzzaman et al,2014;Wang et al,2019).Knocking-outAtPDR8increases cadmium (Cd) accumulation inArabidopsis(Kim et al,2007).AtPDR9is transcriptionally up-regulated inArabidopsisunder Fe deficiency,playing a role in iron homeostasis (Ziegler et al,2017).In rice,OsPDR9is strongly induced by Cd and Zn stresses,and mutation ofOsPDR9increases plant sensitivity to Cd and accumulates more Cd in the plant (Fu et al,2019).So far,approximately 50 ABCG members are available in rice genome,but only a handful of numbers have been reported for metal transport.
In this study,we investigated an uncharacterized rice ABCG53 family gene (OsPDR20) which was previously isolated from one of our metal stressresponsive libraries (Feng et al,2016).OsPDR20is preferentially localized to the plasma membrane.Yeast complement assay revealed that OsPDR20 is able to transport Zn across the cells.Knocking-downOsPDR20by RNAi led to compromised rice growth under Zn deprivation at the vegetative and seed development stages under natural conditions.The knocked-down rice lines showed a lower Zn accumulation in rice straw but a higher level of Zn in rice grains,suggesting that OsPDR20 plays an essential role in rice growth and seed development through Zn acquisition or distribution.The purpose of this study is to figure out the role of OsPDR20 in Zn acquisition and homeostasis in rice plants,which is implicated in a possible scheme for breeding rice varieties with high accumulation of Zn.
RESULTS
Expression pattern of OsPDR20
The genomic sequence ofOsPDR20comprises seven exons and six introns (Fig.S1-A).The length ofOsPDR20mRNA coding sequence is 1 272 bp and encodes 423 amino acids.The OsPDR20 sequences encompass two conserved domains: ATP binding domain and ABC2_memtran domain with seven transmembrane structures,which is most likely involved in the transport of its substrates (Fig.S1-B).Using the MEGA7.0 software,the phylogenetic trees were created within the rice andArabidopsisPDR subfamily members,which turned out that the close member is OsPDR13(LOC_Os10g13830.1) with the domain identity being 42.65% and the protein sequence identity of 37.12% (Fig.S2).Their functions of both genes have not yet been reported.
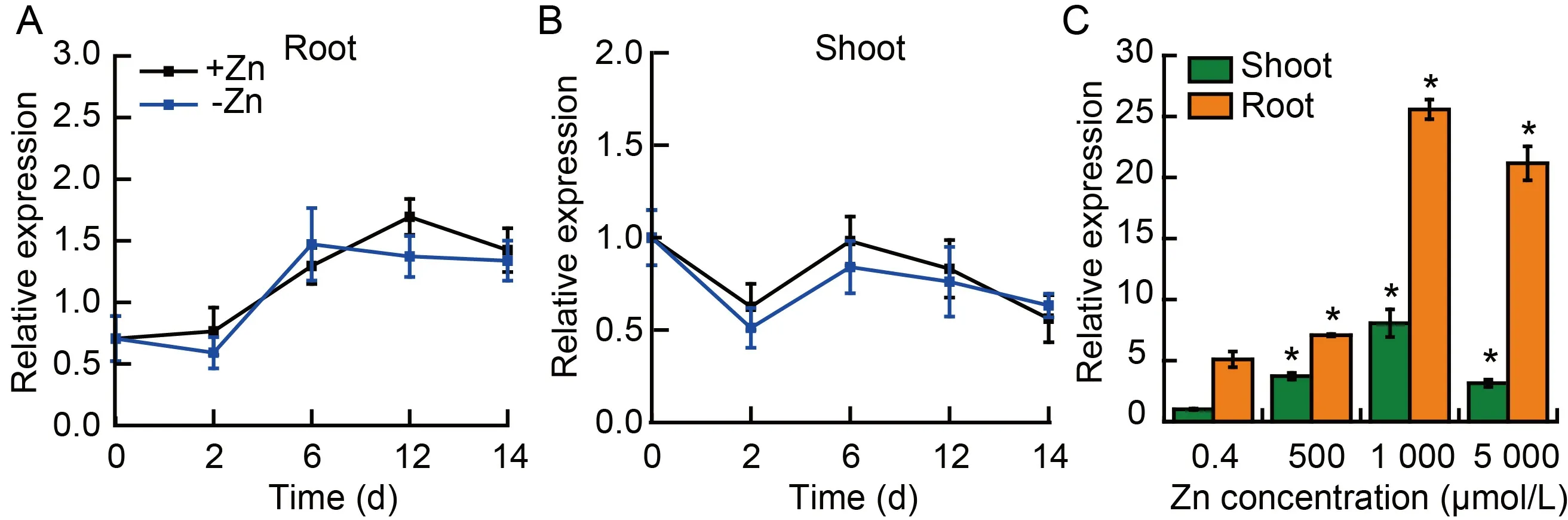
Fig.1.Expression patterns of OsPDR20 in wild type rice under Zn deficient or overloaded stress by qRT-PCR.
To investigate whetherOsPDR20is associated with Zn stress,we first analyzed the transcriptional expression under Zn deficiency.Two-week-old rice plants were hydroponically grown under Zn deprivation for 14 d,and the transcripts in rice roots and shoots were measured by qRT-PCR.In the time-dependent experiment,no significant changes inOsPDR20transcripts were detected between the Zn treatments (Fig.1-A and -B).The response ofOsPDR20to excessive Zn stress was further investigated and showed thatOsPDR20in roots and shoots could be sufficiently induced at 500,1 000 and 5 000 μmol/L (Fig.1-C).The induction ofOsPDR20in roots was seemingly more pronounced than that in shoots.
We then constructed an OsPDR20-pAN580-GFP vector and allowed it to be transformed into rice mesophyll protoplasts to detect the subcellular localization.Under observation using a confocal laser scanning microscopy,the green fluorescent protein (GFP) signal was strongly visualized in the plasma membrane (Fig.2-A and -B).To affirm the residing of OsPDR20 in the plasma membrane,we additionally transformed the OsPDR20-pAN580-GFP fusion into tobacco epidermal cells,with a red fluorescence of PIP2A-RFP as a plasma membrane biomarker (Fig.2-C).The fusions of green fluorescence of OsPDR20-pAN580-GFP and PIP2ARFP were dominantly overlapped in the cell plasma membrane,confirming that OsPDR20 was localized to the plasma membrane in rice cells.
Disruption of OsPDR20 impaired rice growth and Zn accumulation at early vegetative stage
To identify the regulatory role ofOsPDR20in rice development,growth and Zn accumulation,a set ofOsPDR20knocked-down mutant lines were constructed by the RNAi protocol.Plants of T3generation were bred,and three of them (RNAi-1,RNAi-2 and RNAi-6) were randomly selected for this study.The DNA identification in transgenic lines was performed (Fig.S3).The expression levels ofOsPDR20in the three lines were dropped to 25.0%-38.4% of the wild type (Fig.S3),confirming that the RNAi lines were reliable for further investigation.
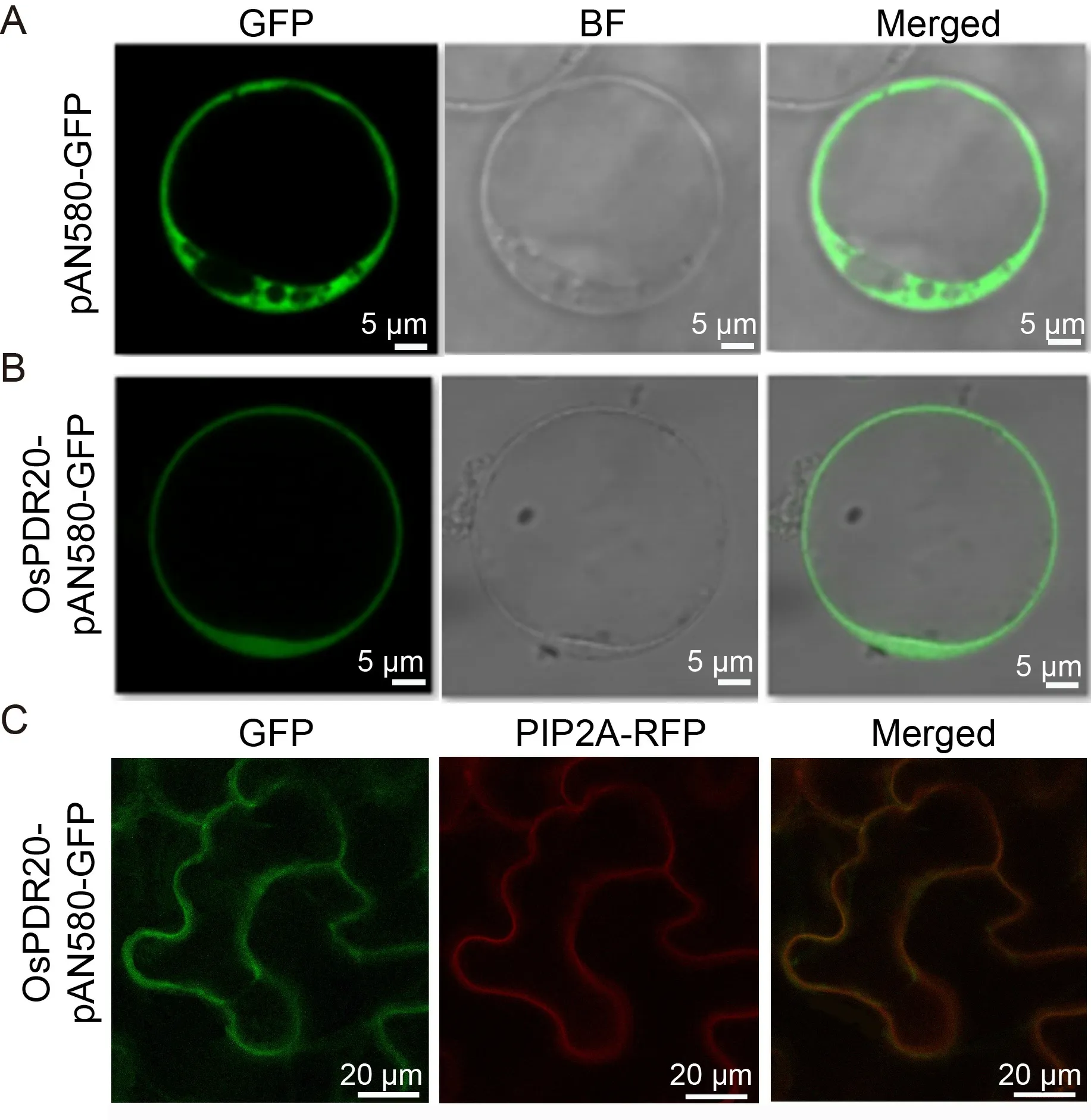
Fig.2.Assessment of subcellular localization of OsPDR20 by expression in rice mesophyll protoplasts and tobacco epidermal cells.
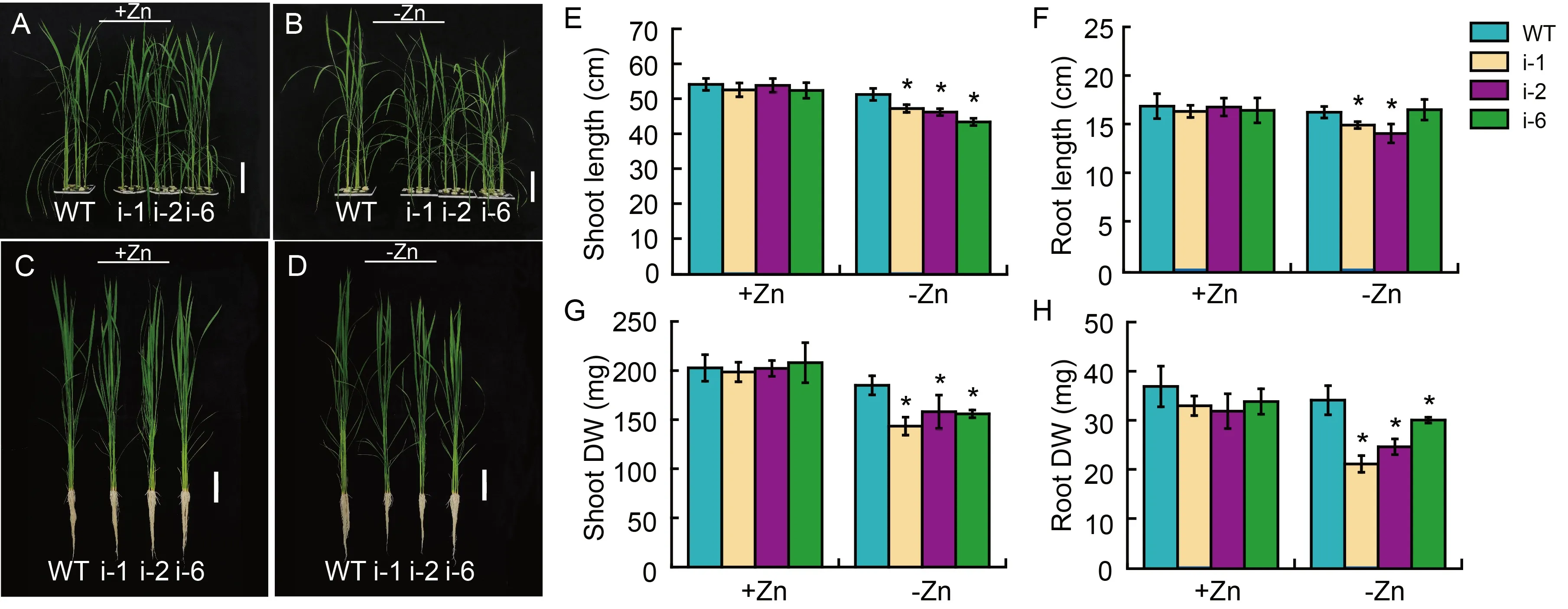
Fig.3.Growth phenotypes of wild type (WT) rice and its RNAi lines (i-1,i-2 and i-6) under Zn-normal supply (+Zn) and Zn-deficiency (-Zn)conditions.
We first performed a hydroponic experiment with the wild type plants and RNAi lines during the early growth stage under Zn deficiency.Two-week-old rice seedlings were grown with normal Zn supply (+Zn)and Zn deficiency (-Zn) for four weeks.During the course of studies,no evident difference in growth was observed between the wild type and RNAi plants grown under supply of +Zn condition (Fig.3-A and-C).However,when under -Zn condition Zn was deprived,the growth of RNAi lines appeared weaker than that of the wild type (Fig.3-B and -D).Assessment of plant height showed that the shoot lengths of RNAi lines decreased by 7.81%-15.31%and the root lengths decreased by 8.14%-13.69%compared with those of the wild type (Fig.3-E and -F).Similarly,the shoot dry weight of RNAi lines declined by 14.52%-22.47%,and the root dry weight dropped by 11.91%-38.10% relative to those of the wild type(Fig.3-G and -H),pointing out that disruption ofOsPDR20led to more vulnerability to Zn starvation during the early stage of rice growth.
Further,the samples indicated above were used to measure Zn concentrations.For plants grown under+Zn condition,the average Zn concentrations in the roots of the three RNAi lines were slightly but insignificantly higher than those of the wild type (Fig.4-A).Under -Zn conditions,both the wild type and RNAi lines lost more than half of Zn accumulation in rice plants,and the Zn concentrations in the roots of RNAi plants drastically increased by 30.10%-45.31%compared with the wild type.With regards to shoots,the Zn concentrations in the RNAi lines were significantly decreased by 9.79%-18.08% under +Zn condition,while no difference was observed between the wild type and RNAi lines under -Zn condition (Fig.4-B).Assessment of root-to-shoot Zn translocation showed that under +Zn condition,the wild type had a translocation rate of 48.91%,while the RNAi lines had the range of 42.61%-44.93%,which was significantly lower than the wild type (Fig.4-C).A similar result was detected under -Zn condition,where the translocation rates of RNAi lines (34.71%-39.91%) were much lower than that of the wild type (44.02%) (Fig.4-C).These results suggested that disruption ofOsPDR20restricted Zn translocation from roots to shoots and as a consequent led to Zn accumulation in roots.
Ectopic expression of OsPDR20 showed Zn transport activity in yeast cells
To examine whetherOsPDR20can transport zinc ions,OsPDR20was transformed into the yeast strain BY4741 (wild type) and its correspondingzrclmutant defective of Zn transport (Khan et al,2019).Omitting or supplying Zn at the lower levels in growth media had an impact on the cellular growth of the control cellszrcl+pYES2(empty vector) andzrcl+OsPDR20(constructed in the vector of pYES2) (Fig.S4-A).When the cells were exposed to 2 and 4 μmol/L Zn,the transformants (zrcl+OsPDR20) displayed Zn sensitive phenotypes compared with its control cells.However,when exposed to higher levels of Zn (8 μmol/L),the transformed cells appeared better growth.The Zn concentration was measured and showed that thezrclcells expressingOsPDR20accumulated less Zn than the control cells although there was no significant difference of Zn concentrations between them (Fig.S4-B).This result suggested thatOsPDR20had the ability to transport zinc ions.
Knocking-down OsPDR20 impacted rice growth under field conditions
To clarify the functional role ofOsPDR20in rice growth and development,field trials on the wild type and RNAi rice lines were conducted under natural conditions for two independent years.When rice ripened,the plants were randomly sampled and taken back to the laboratory for measurement and statistical analysis.First,the plant heights of RNAi lines and wild type plants were measured and comparatively analyzed.The plant heights of three RNAi lines were slightly but significantly reduced (Fig.5-A).Compared with the wild type control,the plant heights of RNAi-1,RNAi-2 and RNAi-6 were reduced by 23.08%,21.17%and 23.85%,respectively (Fig.5-B).Then,the lengths of internodes were measured after removing the leaf sheath of intact plants.The internode elongation of the RNAi lines was also significantly lower than that of the wild type,where the total length of the first three internodes decreased by 32.08%,34.50% and 42.28%,respectively (Fig.5-C and -D).Further analysis of tiller number per plant showed that the RNAi plants had an overall higher level than the wild type,although RNAi-2 was not statistically different (Fig.5-E).Similarly,the effective panicle number per plant in the RNAi lines was general higher than the wild type,although RNAi-2 and RNAi-6 plants did not appear significant difference (Fig.5-F).
Knocking-down OsPDR20 impacted seed development

Fig.5.Morphological differences between wild type (WT) and RNAi lines (i-1,i-2 and i-6) at the maturity stage.
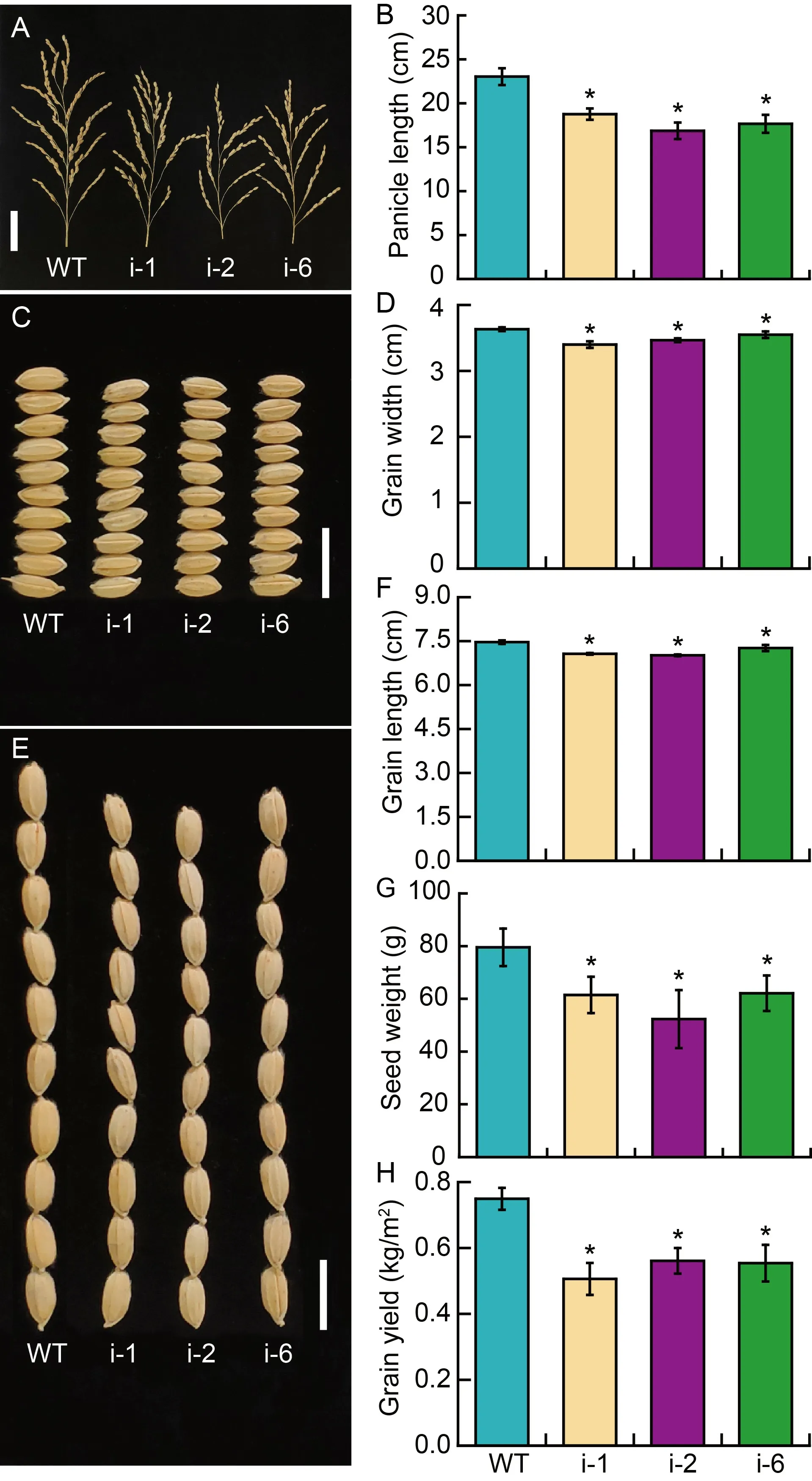
Fig.6.Agronomic traits relevant to seed development of wild type(WT) and RNAi lines (i-1,i-2 and i-6).
To figure out whether knocking-downOsPDR20affected the seed development,dominant agronomic traits such as yield makeup and grain yield were surveyed.Analyzing the phenotype of grains revealed that the panicle lengths of the three RNAi lines were significantly lower than that of the wild type (Fig.6-A),which was shortened by 18.53%,26.75% and 23.31%,respectively (Fig.6-B).The grain sizes of RNAi lines were also compromised (Fig.6-C to -F).The ten-grain widths and lengths of RNAi lines decreased by 2.29%-6.42% and 2.68%-6.03%,respectively.The seed weight per plant of RNAi lines was also found to be significantly lower than that of wild type and decreased by 21.90%-34.20% (Fig.6-G).Furthermore,spikelet fertilities were reduced by 8.54%,7.59% and 5.91% for RNAi-1,RNAi-2 and RNAi-6,respectively(Fig.S5-A).With regards to 1000-grain weight,the RNAi-1,RNAi-2 and RNAi-6 plants had 8.44%,9.64%and 10.18% reductions,respectively compared with the wild type (Fig.S5-B).Finally,grain yield per square was reduced by 32.44%,25.10% and 26.03% for RNAi-1,RNAi-2 and RNAi-6 plants,respectively (Fig.6-H).These results signified that the mutation ofOsPDR20repressed the panicle and seed development of rice,which eventually led to the decrease of rice yields.
RNAi rice decreased Zn concentrations in rice leaves but increased Zn concentrations in grains
Given that the attenuated rice growth and development caused by dysfunction ofOsPDR20,the Zn concentrations were investigated in the wild type and RNAi plants.Several organs of tissues including upper leaf,lower leaf,stem,rice husk and brown rice for Zn concentrations were quantified by an Inductively Coupled Plasma-Mass Spectrometry (ICP-MS).Compared with the wild type,the concentrations of Zn in the upper and lower leaves of the RNAi lines were drastically reduced,especially in the lower leaves (Fig.7-A).The Zn concentrations in the upper leaves of RNAi-1,RNAi-2 and RNAi-6 plants were decreased by 37.11%,34.52% and 26.19% and those in the lower leaves of RNAi-1,RNAi-2 and RNAi-6 were decreased by 57.47%,34.05% and 52.97%,respectively(Fig.7-A).In contrast,the Zn concentrations in husk and brown rice of RNAi plants were remarkably higher than those of the wild type,with the Zn concentrations being increased by 61.59%-129.91%and 47.88%-54.54%,respectively (Fig.7-A).We also found that Zn concentrations in husk and brown rice increased but no change in stem (Fig.7-A).Translocation of Zn from leaves to husk and brown rice for RNAi plants were increased by 14.23-17.82 percent point and 17.31-20.11 percent point compared with those of wild type (6.59% and 9.54%),respectively (Fig.7-B).These results implied that the mutation ofOsPDR20resulted in overall reduction of Zn accumulation in leaves but increase in rice grains.
DISCUSSION
Zinc is a primary micronutrient required for plant growth and development,but in environmental reality,its bioavailability to plants is constantly changing(Broadley et al,2007;Theodoulou and Kerr,2015).Excess or deficiency of Zn disorders biochemical and physiological processes (Wessells and Brown,2012).In this regard,understanding the mechanism for Zn uptake and allocation in plants is critically important.Although a set of metal transporters for Zn mobility in plants have been identified (Amini et al,2022),they are not enough to allow us confidently to formulate a complete network of Zn uptake,transport and distribution.This study functionally identified an uncharacterized ABCG family geneOsPDR20,which turned out that OsPDR20 is necessary for rice growth and seed development.Our work sheds light on a new layer of Zn homeostasis and provides a genetic resource that may come into apply for improvement of Zn nutrition-related agronomic traits.
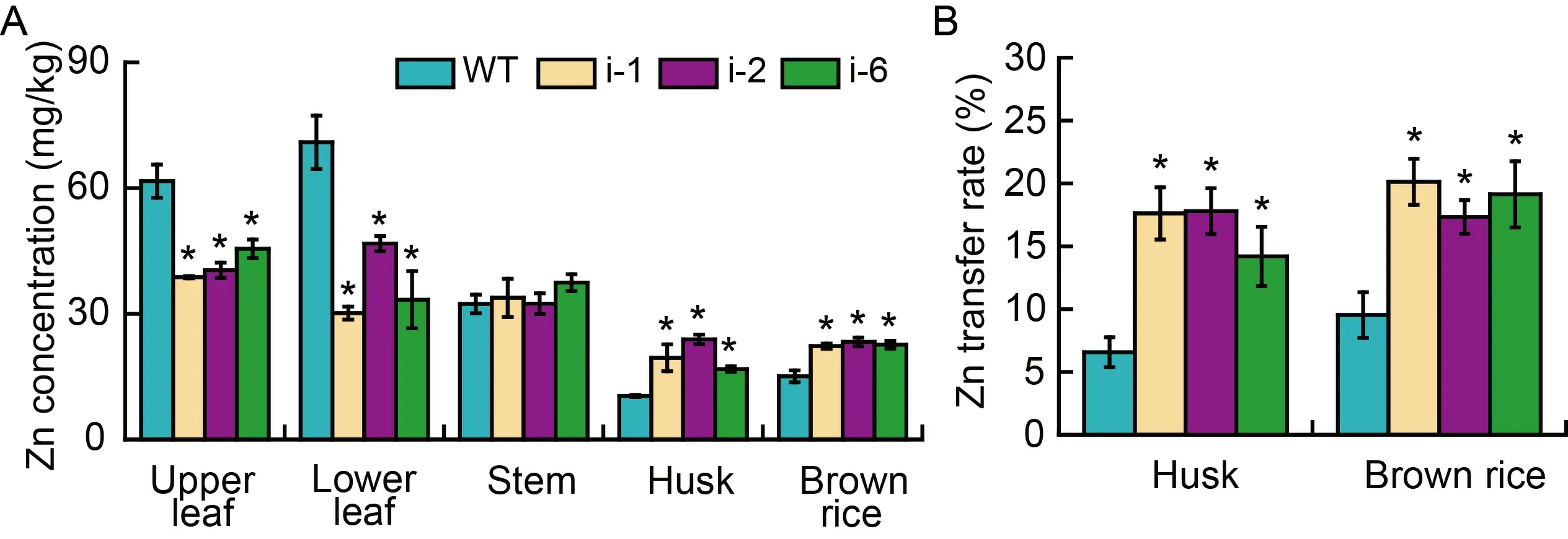
Fig.7.Zn concentrations in different tissues or organs of wild type (WT) and RNAi lines(i-1,i-2 and i-6) at the maturity stage.
OsPDR20 is required for proper rice growth and development
OsPDR20 is endowed with seven transmembrane domains,which most likely involves in the transport of its substrates inside and outside cells.Subcellular localization analysis showed that OsPDR20 is located to the cytoplasmic membrane.Some Zn transporters like OsPDR9 can be induced under Zn stress (Moons,2008;Fu et al,2019).In this study,two-week-old rice seedlings were subjected to Zn deficiency for four weeks,but expression level ofOsPDR20remained unchanged.This phenotype is similar to the report of OsHMA2,a heavy metal ATPase (HMA) subset of the P1b-type ATPase family,which appears no change in response to Zn deficiency (Satoh-Nagasawa et al,2012).Unexpectedly,treatment with excess Zn can induce a higher level ofOsPDR20transcripts.This observation is in line with the studies on OsABCG36 and OsABCG43,whose expression levels were highly up-regulated upon exposure to excessive Zn stress(Oda et al,2011;Fu et al,2019).To affirm whether expression ofOsPDR20can affect the Zn transport and consequence of rice growth and development,a set ofOsPDR20knock-down mutants were built up.The short-term hydroponic studies without Zn supply showed that the growth of RNAi plants was repressed and their biomass decreased significantly under Zn deficiency.However,under normal conditions,the growth phenotypes between the RNAi mutants and the wild type were unchanged,suggesting that expression ofOsPDR20is responsible for the rice growth.
We further examined the agronomic characters relevant to rice seed development and grain yield.Compared with wild type,the RNAi plants had an overall weaker growth status,shorter panicle length,smaller seed size,and reduced seed-setting rate and 1000-grain weight,all of which are corresponding to the reduction of rice grain yield(Zhang et al,2021;Cao et al,2022;Li et al,2022).Furthermore,Zn accumulations in the leaves of RNAi lines were significantly lower than that of the wild type,while there was no difference in internodes.The poor growth phenotype of RNAi plants is seemingly correlated with Zn starvation in rice.Zn-starved rice plants usually have special symptoms such as dwarfing plants,yellowing leaves,shorter internodes,followed by compromised seed development and grain yield(Broadley et al,2007).Many genes regulating plant height,panicle length and yield of rice have been identified,such asSD1regulating plant height of rice(Zhang et al,2020).OsSPL14(souamosa promoter binging proteins-like 14) encoded by a quantitative trait locus namedIPA1is regulated by miR156 in rice,and a point mutation inOsSPL14prevents the cleavage by miR156,creates desirable rice plant architecture (a reduced tiller number),and thus promotes grain yield(Jiao et al,2010).Furthermore,expression ofGAD1improves the number of grains by promoting the development of grain length and width (Jin et al,2016).Similarly,OsHIPP33encoding a metal chaperone is shown to actively participate in the dynamic process of Zn and Fe accumulation in rice,and mutation inOsHIPP33can not only affect rice growth,but also inhibit grain development and seed development (Cao et al,2022).Knocking-downOsZIP11is also involved in defective phenotypes of rice development and growth (Zhao et al,2022).
OsPDR20 is required for Zn homeostasis throughout lifespan of rice
Measurements of Zn concentrations revealed that under Zn-deficiency,the RNAi lines accumulated more Zn in roots than the wild type,while the Zn concentration remained unchanged under +Zn condition.Inversely,less Zn ions accumulated in the shoots of RNAi plants than the wild type under normal Zn supply,while those in shoots of the RNAi lines and wild type were similar under -Zn condition (Fig.4).The observations suggested that OsPDR20 played an important role in Zn efflux from rice root cells that may contribute to Zn translocation from roots to shoots.In other words,disruption of OsPDR20 most likely prevented Zn transport from roots to shoots.This was particularly evident at the Zn-deficient state(Fig.4).Calculation of Zn transfer rate also signified that Zn deficiency caused less Zn transfer from root to shoot than normal Zn-supplied conditions (Fig.4-C).Such phenotype was previously reported for some specific metal transporters that prevent translocation of metals such as Fe,Zn,and Cd from roots to shoots(Andresen et al,2018).OsHMA2,for example,is a critical transporter of Zn/Cd,and knock-out ofOsHMA2by a T-DNA insertion leads to the loss of transport function and consequently impairs translocation of the metals from roots to the above-ground(Satoh-Nagasawa et al,2012).Dysfunction ofOsZIP7also increases accumulation of Zn in roots but decreases in leaves (Tan et al,2019;Gindri et al,2020).In contrast,transgenicArabidopsisover-expressingOsZIP7was reported to decrease Zn accumulation in roots but increase Zn accumulation in leaves (Ricachenevsky et al,2018).
The increased Zn concentration in the knock-down rice grains was not positively associated with the Zn concentration in other parts of the plants,suggesting that OsPDR20 repression would jeopardize the proper deployment of Zn within the tissues and organs,and eventually lead to more Zn efflux from the upper leaves to grains.However,the mechanism for the process is unknown.The transport rate of Zn to grains depends on the well-developed vascular system at the nodes of rice,which is mainly composed of enlarged vascular bundles (EVB) and diffuse vascular bundles(DVB) (Yamaji and Ma,2017).OsZIP3 is responsible for unloading Zn to the xylem of EVB,and then OsHMA2 gets Zn into phloem to transport it to grains(Yamaji et al,2013;Sasaki et al,2015;Yamaji and Ma,2017).OsZIP4 is also reported to involve in the transport of Zn into phloem of the DVB in the node,which eventually dispatches Zn to tiller shoots or other developing tissues or organs (Ishimaru et al,2005).Although the pathway for Zn transport from the phloem to seeds is proposed based on the current knowledge on identified Zn transporters (Yoneyama et al,2015;Amini et al,2022),the accurate regulation of Zn transport to grains is largely unknown.Also,whether OsPDR20 is independently or coordinately involved in regulation of Zn transport and deposition during the seed development is an open question to address.
To sum up,this study demonstrated that the knock-down ofOsPDR20repressed the rice growth,biomass and other agronomic traits.The stunt growth and seed development can be due to the dysfunction of Zn transport,and the over-accumulation of Zn in the rice grains could be also the result of impaired regulation of Zn distribution between the upper leaves or panicles and seeds.These results suggest that OsPDR20 would play a primary role in proper Zn allocation and homeostasis,which may be utilized for building up higher Zn-accumulating crops.
METHODS
Rice materials and experimental conditions
The wild type (Nipponbare) rice seeds were sterilized with 75%ethanol for 2 min and 10% NaClO for 10 min.Following rinsing in water,the seeds were germinated at 30 °C for 2-3 d.The growing seedlings were transferred to a plastic board with a mesh floating on Kimura B nutrient solution for 3 d under darkness and then moved to a growth incubator under the condition of alternating light for 16 h at (30 ± 1) °C and darkness for 8 h at (26 ± 1) °C.For hydroponic experiment,two-week-old seedlings were grewn under Zn-deficient stress for 14 or 28 d or Zn excess (500,1 000 and 5 000 μmol/L) stress for 6 h.The nutrient solution was renewed daily (Liu et al,2019).
Field trials were conducted at the Research Station of Nanjing Agricultural University,Nanjing,China,where the local paddy farmland for rice cropping contains a typical yellow brown soil with the physical and chemical properties described previously (Zhang et al,2018b).Three-week-old rice seedlings growing in the hydroponic media were transplanted in the open paddy soil.The field trials were set up with three independent plots (in triplicate).Each plot contained two brocks for planting wild type and knock-down mutant plants,respectively.Each block accommodated 300 rice plants,with a plant distance being 20 cm × 20 cm.There were ditches between the neighborhoods and protective lines around them.The irrigation and fertilization of the field trials were consistent with the local farmland management.The field experiment was carried out for two independent years (2020-05-09 and 2021-05-09).When harvested,the mature plants were separately sampled according to the special tissues or organs like leaves,panicles and grains (husk and brown rice),and measured for agronomic traits and metal concentrations (Cao et al,2022).
Analysis of OsPDR20 structural sequences
TheOsPDR20(LOC_Os09g16330.1) gene and protein sequences were downloaded from the Rice Genome Annotation database(http://rice.plantbiology.msu.edu).Specific domains of OsPDR20 protein were predicted on the Simple Modular Architecture Research Tool (SMART,http://smart.embl-heidelberg.de/).The transmembrane structure of OsPDR20 protein was predicted on the TMHMM2.0c website (http://www.mybiosoftware.com/tmhmm-2-0c-prediction-transmembrane-helices-proteins.html).A phylogenetic tree was created by MEGA7.0 for analyzing rice PDR subfamily (Kumar et al,2016).The amino acid sequences of OsPDR20 and similar proteins were compared by the DNAMAN software.Motif elements were analyzed by MEME(http://meme-suite.org/tools/meme) to detect the difference between the target genes.
qRT-PCR for transcript analysis
Total RNAs of rice tissues were extracted with Trizol reagent.The purity and concentration of RNA were detected by a Nano-400 ultraviolet microspectrophotometer (Allsheng,Hangzhou,China).DNA was removed by one-step procedure using DNase I (Khan et al,2020).The cDNA was synthesized by the reverse transcriptase SuperMix (Transgene,Beijing,China) using the specific primers (Table S1).qRT-PCR was carried on with the 7500 Real-Time PCR System (Applied Biosystems,California,USA).The reaction was run as follows: 1.0 μL cDNA template,0.8 μL primers,10.0 μL 2× Hieff qPCR SYBR Green Master Mix (No Rox) and 8.2 μL RNase-free water.The reaction procedure was set to 40 cycles: 94 °C 30 s,94 °C 5 s,60 °C 30 s.Experiments were repeated in triplicates (Khan et al,2020).
Subcellular localization of OsPDR20
The coding DNA sequence ofOsPDR20was isolated by PCR.The sequences of either N-terminal or C-terminal were separately connected with the GFP,and each of them was inserted into the pAN580 vector,leading to the fusion of OsPDR20-GFP driven by a 35S promoter.The pAN580-GFP vector without OsPDR20 was used as a control.The 35S::OsPDR20-GFP vectors were transformed into rice mesophyll protoplasts.In addition,the OsPDR20-GFP fusions were simultaneously transferred and co-expressed with a plasma membrane-localization marker PIP2A (with red fluorescence) in the epidermal cells of tobacco (Nicotiana benthamiana) leaves (Liu et al,2019).Expression of the fusions was visualized by a confocal laser scanning microscopy(LSM710,Zeiss,the Netherlands).
Generation and analysis of OsPDR20 knock-down plants
A specific fragment ofOsPDR20sequence with a length of 261 bp was designed and isolated to build up RNAi lines.The forward primers withKpnI andSacI as the restriction sites and the reverse primers withBamHI andPstI restriction sites were used to amplify the positive and reverse fragments (Table S1)(Wang et al,2015).The forward and reverse fragments were ligated to pCAMBIA1390-RNAi vector and transformed intoEscherichia colito gain 1390-OsPDR20-RNAi plasmid.The RNAi positive clone plasmid was then transformed into calli by theAgrobacterium tumefaciensinfection.At least 20 RNAi lines were selected,and homozygotes were obtained after three generations of breeding (T3).Three RNAi lines (RNAi-1,RNAi-2 and RNAi-6) were randomly selected for following study.
Yeast cell compensation assay for OsPDR20 transport activity
Full-lengthOsPDR20sequence was amplified by PCR using specific primers (Table S1).The cloned sequences were introduced into theyeast (Saccharomyces cerevisiae) expression vector pYES2/NTC.The recombinant plasmid and empty vector were separately transferred into the Zn-sensitive mutantΔzrc1strains with the wild type strain BY4741 as background (Miyabe et al,2001).To examine the activity of Zn transport,the wild type and mutant strain expressingOsPDR20or empty vector were incubated on the SD-Ura (Solarbio,Nanjing,China) solid medium supplemented with 2,4 and 8 mmol/L Zn at 28 °C for 3 d.For measurement of Zn concentration in the treated cells,the transformed yeast was cultured in a liquid nutritional solution with 0.2 mmol/L Zn at 28 °C for 2 d.Following the treatment,the yeast cells were collected by centrifugation and rinsed by deionized water for metal measurement.
Determination of Zn concentrations
Rice samples were rinsed with deionized water and separated into different tissues.The collected samples were dried at 78 °C for 3 d.Each sample was digested in a tube containing superior pure nitric acid for 40 min in a microwave.The concentrations of metal ions in the sample were determined by an ICP-MS(NexION 2000,PerkinElmer,MA,USA) (Khan et al,2019).
Statistical analysis
All experimental treatments were set up with biological triplicate.Each treatment comprised 18-24 plants.The significant difference between treatments was statistically valuated by the analysis of variance post hoc tests (Tukey’s test,P< 0.05) using the statistics package SPSS 20.0 (IBM Crop,Armonk,NY,USA).
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China (Grant No.21777072).We thank WU Xuechun for participating in part of the technical research work.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science;http://www.ricescience.org.
Fig.S1.Structural analysis ofOsPDR20gene and prediction of transmembrane structure.
Fig.S2.Phylogenetic,multiple alignments and motif analysis of OsPDR20.
Fig.S3.Identification of transgenic RNAi lines and expression ofOsPDR20.
Fig.S4.Transport activity of OsPDR20 to Zn and its concentrations in mutant yeast.
Fig.S5.Analysis of agronomic traits relevant to seed development of wild type and RNAi lines.
Table S1.Primer sequences used in this study.
杂志排行
Rice Science的其它文章
- Root Endophyte Shift and Key Genera Discovery in Rice under Barnyardgrass Stress
- Antioxidant Activities and Characterization of Polyphenols from Selected Northern Thai Rice Husks: Relation with Seed Attributes
- NaCl Facilitates Cell Wall Phosphorus Reutilization in Abscisic Acid Dependent Manner in Phosphorus Deficient Rice Root
- Peptide Transporter OsNPF8.1 Contributes to Sustainable Growth under Salt and Drought Stresses,and Grain Yield under Nitrogen Deficiency in Rice
- Novel Deletion in Exon 7 of Betaine Aldehyde Dehydrogenase 2(BADH2)
- Transfer Learning-Based Image Recognition of Nitrogen and Potassium Nutrient Stress in Rice
