脂质响应型探针用于动脉粥样硬化成像及治疗的研究进展
2023-01-25徐心昱张乐天刘柳卉宋国胜张晓兵
徐心昱,张乐天,曹 晖,马 原,刘柳卉,宋国胜,张晓兵
(湖南大学化学化工学院,化学生物传感与计量学国家重点实验室,长沙 410082)
1 Introduction
As the aging population increases,cardiovascular disease(CVD)strikes as a major public health problem.It turns out to be a leading cause of death globally,accounting for 44%of deaths from non-communicable disease(NCD)[1].Atherosclerosis(AS)is an immune-mediated chronic progressive inflammatory process feature in the formation of atherosclerotic plaque beneath the endothelium which is regarded as a principal and potential risk factor for CVDs[2,3].Long-term irritation of endothelial cells in the endotheliumviavarious risk factors(e.g.,obesity,hyperlipidemia,hypertension,alcohol)can lead to endothelial cell damage and disruption of normal physiological functions,such as abnormal secretion and increased endothelial permeability[4].In response to injury,endothelial cells secrete large amounts of inflammatory factors as well as chemokines that recruit monocytes from the circulating blood to the endothelial injury[5,6].These monocytes further migrate,infiltrate,and differentiate into macrophages towards the subendothelium[7].Macrophages phagocytose lipoproteins and form foam cells.As the foam cells continue to accumulate under the vascular endothelium,it has the possibility to eventually evolve into fibrous plaques[8—10].Over time,the lipid accumulation in the plaque also induces apoptosis and necrosis of foam cells,macrophages,and vascular smooth muscle cells,eventually forming a necrotic core rich in liposomes within the plaque[11].The inflammation generated by this necrotic core makes the plaque fragile and vulnerable to fragmentation,leading to acute clinical events such as heart attack and cerebral infarction[12—14].
Lipid droplets(LDs)are the dominating organelles for the storage of lipids[15].A single lipid droplet consists of a neutral lipid core,cholesterol esters,and triglycerides with a phospholipid monolayer wrapped in the outer layer[16,17].The overexpression and overexpansion of LDs in cells are closely connected with diseases like fatty liver,typeⅡdiabetes,obesity,AS,and cancer[18].Since the formation of vulnerable atherosclerotic plaques is associated with the expansion of lipid-rich necrotic cores[3],the development of lipid-specific imaging probes is expected to provide new ideas for the diagnosis as well as the treatment of atherosclerosis.The principle of LDs imaging is mainly considered from the structure of lipid droplets,such as their polarity,viscosity,pH,and water content[19].
2 Common Probes in LDs Fluorescence Bioimaging
2.1 Nile Red
Nile Red is a wide commercially fluorogenic dye that is applied to stain LDs in cells[20](Fig.1).It shows solvatochromism in most organic solvents and lipid environments,but its fluorescence intensity reduces in aqueous media.Despite many advantages of Nile Red as one of the LD markers,there are still some limitations to ponder:(i)non-specific labeling ability toward intracellular membranes and organelles;(ii)the partial overlap of the absorption and emission spectra of Nile red,resulting in cross-talk in the red channel,which makes it unsuitable for multicolor imaging[21].Based on Nile Red ligands,a series of polarity-sensitive chromogenic probes has been designed,for example,Danylchuket al.[22]designed Nile Red-based probes specifically targeting the endoplasmic reticulum,mitochondria,lysosomes,Golgi apparatus,plasma membrane,and lipid droplets which proposed an approach for the quantitative comparison of the local lipid order in organelles.

Fig.1 Chemical structures of Nile Red and BODIPY 493/503
2.2 Boron Dipyrromethene(BODIPY)493/503
Boron dipyrromethene(BODIPY)493/503 is another commercially popular fluorescent dye showing weak water solvation[23](Fig.1).It can effectively and quickly label LDs with bright green fluorescence and it is more selective for LDs than Nile red.
BODIPY fluorophores exhibit the superiorities of hydrophobicity and high brightness which are suitable for labeling LD[24].Nevertheless,these fluorophores such as BODIPY 493/503 usually have symmetrical configurations,which are unfavorable for intramolecular charge transfer(ICT).It results in a relatively short wavelength and small Stokes shift,leading to interference between the excitation source and fluorescence emission,which ultimately displays a strong background fluorescence[25].
3 Design Strategies for Fluorescent Probes of LDs
Even though Nile Red and BODIPY 493/503 are the most extensively applied probes for LDs,both molecules have a certain extent of low specificity.Therefore,it is necessary to continue developing new fluorescent probes for monitoring LDs.
Hydrophobicity is one of the most important factors to be considered when designing new LD probes[26].Lipophile micromolecule probes can penetrate the biofilm and target LDs specifically through hydrophobic interactions because of the neutral lipid core.In general,most hydrophobic fluorophores are appropriate for the development of LD probes.BODIPY,coumarin,1,8-naphthimide,etc.are classic hydrophobic fluorophores that are commonly used in designing LD probes.
3.1 Rhodamine-based Probes
Rhodamine fluorescent dyes have the advantages of good photostability,wide wavelength ranges,and high fluorescence quantum yield[27].At present,the synthesis of rhodamine fluorescent probes become a more extensive topic in the field of chemical and biological analysis.
A novel single fluorescent probe was designed to label LDs and mitochondria in legibly separated dual-emission channels[28].In their work,a“biform”fluorescent probe called MT-LD was developed using spiralization of rhodamine dye,firstly displaying mitochondria and LD separately in dual-emission channels[Fig.2(A)].MT-LD was synthesized by connecting the rhodamine moiety to coumarin derivative,with declined emission in polar solvents.MT-LD could exist in a dual form due to the invertible helocycle reaction of rhodamine dye:the cationic form enabled to target the mitochondria with red fluorescence whereas the neutral lipophilic form enabled to mark the LDs with blue fluorescence[Fig.2(B)].The newly devised probe was used to study the dynamic behavior of mitochondria and LDs and to investigate the interaction of the two organelles in living cells and tissues.The results of co-localization experiments confirmed the good subcellular localization ability of MT-LD.
Baiet al.[29]developed a multifunctional fluorescent probe that selectively recognized mitochondria and LDs at defined pH values through dual emission channels[Fig.2(C)].This type of design is often complex because mitochondrial targeting requires cationic structures,while LDs targeting requires lipophilic molecules.Thus,it is often difficult to achieve simultaneously in a single probe.The Hcy-Rh probe developed in this work showed different chemical structures in acidic and alkaline environments.In the acidic environment,rhodamine dye in Hcy-Rh is partially activated,and the probe shows rhodamine structure’s red fluorescence and hydrophilicity,thus could target mitochondria.Under alkaline conditions,a spirocyclization reaction that disrupts the molecular conjugate system is conducted by Hcy-Rh.Additionally,because of the quinone configuration of cyanine fluorophores,Hcy-Rh showed strong blue emission with lipophilic form under alkaline conditions and could target LDs.Hcy-Rh,as a ratio pH sensor,was used to study the interaction of LD-mitochondria at different pH values under starvation-induced autophagy.

Fig.2 Chemical structure and the targeting mechanism of the probe MT-LD(A)[28],chemical structure of Hcy-Rh and the proposed polarity-reversible and ratiometric pH-sensing mechanism(B)and targeting mechanism of Hcy-Rh(C)[29]
3.2 Coumarin-based Probes
Coumarin derivatives have been extensively used to develop fluorescent biological imaging probes because of their great capabilities,i.e.,high molar absorption coefficient and high fluorescence quantum yield[30].Nonetheless,most coumarin-based probes have short-wavelength fluorescent emission in solution and are susceptible to interference from background fluorescent noisein vivoimaging.
Xuet al.[31]designed and synthesized CM2P and CM4P based on coumarin structure,which could realize the super-resolution visualization of LDs in Hela cells[Fig.3(A)].In the water phase,probes were aggregated into nanoparticles,and then fluorescence quenching occurred.Upon addition of liposomes,the interaction between the probe and liposome would break up the agglomeration and turn on the fluorescence signal[Fig.3(B)].CM2P was performed as a biological probe to image LDs under stimulated emission depletion(STED)microscopy,which helped to reveal details of specific interactions between LDs and other organelles and cellular mechanisms[Fig.3(C)—(G)].

Fig.3 Synthetic route for CM2P and CM4P(A),simulation of interaction between CM2P and liposome,O/Wemulsions(B),and fluorescent images of LDs stained with CM2P in live HeLa cells(C—G)[31]
The overexpression of LDs in cells is tightly associated with fatty liver.In that case,imaging LDs in the livers of living mice is useful for monitoring the progression of the disease.Yoshiharaet al.[32]developed a probe called PC6S for imaging LDs of both normal and fatty livers in living mice.PC6S has aπ-extended fluorescent coumarin structure which provides it the advantage of high fluorescence quantum yield and allows it to vary the emission wavelength with the polarity of the solvent[Fig.4(A)].Researchers used confocal fluorescence lifetime imaging microscopy(FLIM)to image the livers of mice injected with PC6S through the tail vein and observed different sizes of LDs[Fig.4(B)].PC6S’s FLIM images can be used to assess not only the distribution and formation of LDs due to lipid overaccumulation in fatty liver in live mice but also progressive liver fibrosis.
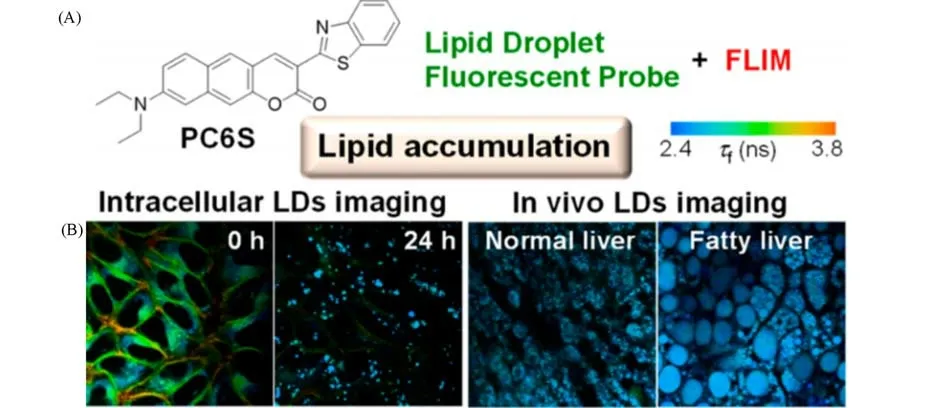
Fig.4 Chemical structure of PC6S(A)and FLIM images of HeLa cells stained with PC6S(2μmol/L)for 30 min at 37℃,then treated with oleic acid(400μmol/L)and FLIM images of the livers of living mice(B)[32]
Liuet al.[33]developed the first ratio fluorescent probe,CQPP,which could target LDs and the nucleus and monitor changes in the polarity of the microenvironment.A lipophilic coumarin donor and a cationic quinolinium acceptor were combined to form the D-π-A structure,permitting it to show polarity-sensitive properties to solvent.The absorption peaks of CQPP in 1,4-dioxane or DMSO are similar.Comparatively,under the excitation(λex=405 nm),enormously different fluorescence spectra are displayed(λem=467 nm for 1,4-dioxane,whileλem=671 nm for DMSO).The long-wavelength absorption is mainly due to the strong ICT effect from the electronic donor of coumarin to the cationic quinolinium receptor.In addition,CQPP demonstrated a brilliant affinity and excellent fluorescence lifetime response to nucleic acid.
HT-1080 cells were incubated with CQPP after suffering from ferroptosis inducers.The decreased fluorescence ratio signal indicated that the polarity of LDs was increasing during iron death.By FLIM measurement,CQPP has the potential to separate the ferroptosis process and cell apoptosis(Fig.5)[33].During the ferroptosis process,LDs’polarity increased tremendously,which was proved by the fluorescence lifetime of CQPP in the cytoplasm decreased to 0.79 s.Comparatively,the fluorescence lifetime of CQPP in the nucleus of apoptotic cells decreased yet it did not change greatly in the cytoplasm.
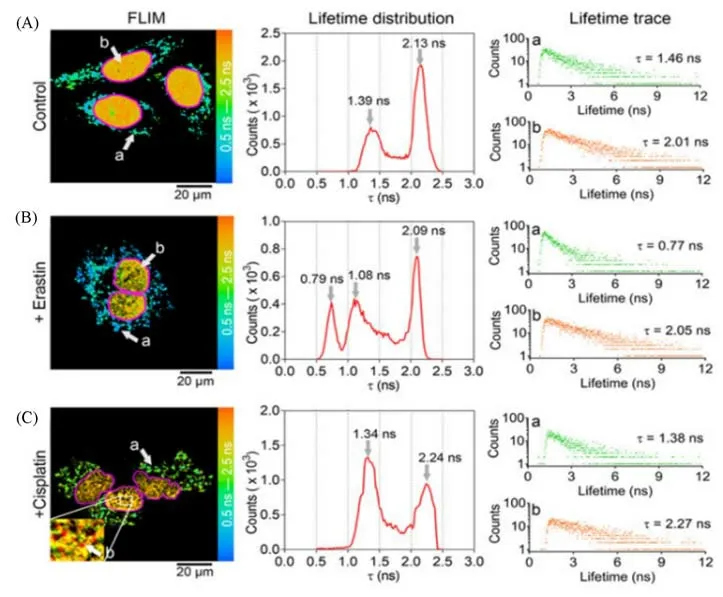
Fig.5 Application of CQPP(5μmol/L)to monitoring the polarity changes of LDs and nucleus in living cells under different conditions by fluorescence lifetime imaging(FLIM)[33]
3.3 BODIPY-based Probes
Embellishing BODIPY which has a hydrophobic structure into optical probes is already a relatively successful method for labeling and studying LDs.These probes often have the advantages of high fluorescence quantum yield,good photostability,and are highly flexible in terms of molecular design.However,the excellent symmetrical structure of BODIPY is not that friendly for ICT.Thus,quite a few analogs of BODIPY with the asymmetric structure were exploited to enhance the ICT effect of the probes[34].
At present,there are many methods for the design of highly fluorescent efficient BODIPY probes(Fig.6).Greeneet al.[35]reported an optical probe H4BPMHC that monitors the generation of lipid peroxyl radicals by compounding 2,2,5,7,8-pentamethyl-6-hydroxy-chromanol(PMHC)with BODIPY without methyl groups at the C1 and C7 positions.The chromanol head was oxidized after scavenging two lipid peroxyl radicals which led to the deactivation of the PET effect and the restoration of BODIPY fluorescent emission.The oxidized probe H4BPMHCoxshowed strong absorption at 509 nm and maximum fluorescence wavelength at 520 nm with exceptionally high quantum yields(0.7—1.0).In addition,the coloration of lipid membranes in various organelle was viewed in three different buffer solutions,which proved the lipophilicity of the probe.

Fig.6 BODIPY-based LD probes
Tianet al.[36]designed a new BODIPY-based probe BoCz-Lip for targeting LDs in a living cell.4-Diethylamino-2-hydroxybenzaldehyde was applied as a strong electron donor whereas a six-membered ring N—B—O BODIPY performed as the electron receptor.The interaction between the probe and lipid structure in LDs was enhanced by introducing long-chain alkyl into the carbazole ring.The probe’s fluorescence quantum yields reach 25.91%.It is worth mentioning that the probe can specifically target LDs under super-resolution nanoscopy.
Taberoet al.[37]found that the optical toxicity of some BODIPY-based probes with LDs detection ability increases,revealing that LD is one of the targets of photodynamic therapy(PDT).They reported BODIPYs 9 with many advantages,including high-enough red fluorescence(quantum yields 0.6),high photostability,low dark toxicity,high PDT activity,and clear biological imaging ability.Interestingly,the author reported two different cell-death mechanisms induced by LD/BODIPY/light system at different dye concentrations:necrosis when at higher concentrations while apoptosis at the lower ones.
In addition to monitoring lipid droplets in cells,Panet al.[38]developed a BODIPY-based glycosyl probe(HNB)capable of simultaneously imaging NO under hypoxic conditions.The probe was designed to investigate the correlation between HIF-1 upregulation and NO.This provides a powerful tool for elucidating the biological pathways involved in changes in NO and LD levels.
3.4 1,8-Naphthalimide-based Probes
One D-π-A type probe called LD-Lys was synthesized by combining morpholine as a lysosomal targeting unit with LD targeting part based on 1,8-naphthalimide derivative[Fig.7(A)][39].Owing to the twisted intramolecular charge transfer(TICT)effect,LD-Lys would acquire greater stability in a high polar environment.LD-Lys exhibited strong emission at the LD region(580 nm)and weak emission at the lysosome region(600 nm)[Fig.7(B)—(F)].

Fig.7 Molecular design and structure of LD-Lys(A)and fluorescent images(B—E)and spectra(F)of live SiHa cells stained with LD-Lys(5μmol/L,30 min)[39]
Xuet al.[40]designed a buffering fluorogenic probe called LD-FG based on naphthalimide[Fig.8(A)].When LD-FG is outside the LDs,it could form hydrogen bonds with the proton of the medium,and then quench the fluorescence.When LD-FG enters the LDs,the fluorescence turns on[Fig.8(B)].The reversible fluorescence switch controlled by a hydrogen bond could effectively reduce background fluorescence interference and improve imaging resolution.The imaging effects of different dye concentrations on LDs in Hela cells were compared by structured illumination microscopy(SIM)imaging.LD-FG showed significantly higher resolution variability than BODIPY493 and Nile red,due to the buffering fluorogenic design strategy that reduced background fluorescence emitted by excess probes outside the LDs[Fig.8(C)].Based on the brilliant dynamic recognition characteristics and stable imaging capability of LDs by LD-FG,they conducted long-term dynamic fluorescence imaging of LD fusion,LD-mitochondria,and LD-lysosome interaction,which was of great significance for revealing the function of LDs in living cells.

Fig.8 Super-resolution lipid droplet imaging probe LD-FG[40]
3.5 Other Frameworks for LD Probes
Cyanines are one of the most well-known and extensively used fluorophores[41].The emission wavelengths of these molecules range from the visible range to the near-infrared(NIR)and constitute a platform for biomolecule marking.Collotet al.[42]developed a series of merocyanine fluorophores with fluorescence ranging from yellow to NIR called StatoMerocyanines(SMCy)[Fig.9(A)].The SMCy fluorophores were synthesizedvialinking an indolenine moiety to a dioxaborine barbiturate derivative and they showed narrow absorption and emission spectra with absorption wavelengths ranging from 526 nm to 770 nm as well as emission wavelengths ranging from 550 nm to 794 nm.The SMCy fluorophores have the advantages of sharp absorption bands and transmitting bands thus showing no crosstalk between multiple channels.Therefore,they have the potential to be good multicolor imaging dyes.
SMCy5.5 is one of the brightest NIR two-photon excitation(TPE)fluorophores.After incubating SMCy5.5 and mouse liver slice together,a clear multi-color 3D image of TPE was obtained,which was beneficial to reveal the distribution of lipid vesicles in liver tissue[Fig.9(B)].

Fig.9 The six members of the StatoMerocyanines family(A)and two-photon excitation 3D imaging(52μm depth)of a mouse liver slice,incubated with Hoechst(5μg/mL)and SMCy5.5(5μmol/L)(B)[42]
3.6 Metal Complex Probes
In addition to probes based on organic compounds,metal complex probes play a critical role in LDs imaging.Tanget al.[43]chose Zn2+as the metal center supported by luminescent Salen ligan as fluorescent metal probes for cell imaging.To stain the hydrophobic interior of LD,they used aliphatic chain modified N-substituent salicylaldehyde,and synthesized a luminescent ZnSalen complex(LD-TPZn)as shown[Fig.10(A)].In recent years,phosphorescent cyclometalated iridium(Ⅲ)complexes that exhibit favorable photophysical properties,such as large Stokes shifts,high photostability,and long luminescence lifetimes,have been emerging as interesting alternatives to conventional organic fluorophores in imaging applications.Heet al.[44]developed two lipophilic neutral phosphorescent Ir(Ⅲ)complexes(Ir1 and Ir2)as one-and two-photon LD-specific probes[Fig.10(B)].Based on this work,Panet al.[45]developed two new neutral cyclometalated iridium(Ⅲ)complexes[Fig.10(C)and(D)]by improving the water solubility of the dyes to some extent without altering their LD targeting properties.And the potential application forin vivoimaging of larval zebrafish and the phototherapy potential of these two phosphorescent iridium complexes have been investigated in detail in their work.

Fig.10 Chemical structures of some metal complex LD probes
4 Applications of Lipid-responsive Probes in Imaging and Treatment of Atherosclerosis
4.1 Application of Lipid-responsive Probes in Atherosclerosis Imaging
Atherosclerosis(AS)is an immune-mediated chronic progressive inflammatory process characterized by the formation of atherosclerotic plaques beneath endothelial cells and is one of the major threats to cardiovascular disease.The transformation of macrophage intake of excess lipoprotein into foam cells is a hallmark of early atherosclerosis.With the development of the disease,a necrotic core containing a large number of free cholesterol crystallization is gradually formed.And this is a key feature of vulnerable plaques.Therefore,the development of probes that are responsive to LDs in plaques is important for the diagnosis and treatment of AS.
4.1.1 Imaging of Atherosclerotic Plaque Imaging the water phase and lipid accumulation in atherosclerotic tissues at the same time can help to distinguish normal and pathological tissues more accurately.Zhanet al.[46]synthesized a fluorescent probe called LDs-DM with the ICT effect through a simple one-step reaction,which showed green fluorescence(535 nm)in oil,and the wavelength was red-shifted to the NIR region(707 nm)in water.Nile Red was used with LDs-DM to observe the main aorta of atherosclerosis model micein vitro[Fig.11(A)].The results showed that the combination of LDs-DM and Nile Red had good correspondence in diverse depths and 3D reconstructed images[Fig.11(B)—(D)],and LDs-DM exhibited bright fluorescence in both local lipid regions and water regions with barely any fluorescence crosstalk,which confirmed the potential of LDs-DM in studying the development of atherosclerosis.
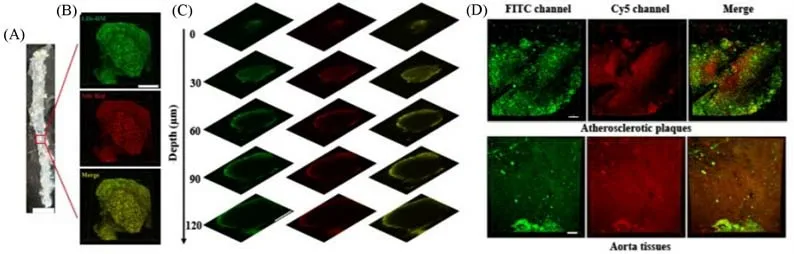
Fig.11 Simultaneous dual-color 3D imaging in atherosclerosis[46]
In addition to imaging aortic plaque areas in mice,MeTTI,an LDs-specific probe developed by Liet al.[47],also showed excellent LDs imaging ability in markedly thickened and calcified bicuspid aortic valves from patients who had valvular heart diseases(VHD)(Fig.12).

Fig.12 Colocalization imaging of aortic valve leaflets[47]
Most lipid-specific fluorophores have strong hydrophobicity.However,Wanget al.[48]developed fluorophores with aggregation-induced emission(AIEgen)(TPACN and TPAPhCN)that could perform three-photon imaging of lipids under NIR-Ⅱlaser excitation.TPAPhCN could not only specifically label LDs in foam cells,but also perform three-photon imaging of carotid artery plaque and cerebral artery plaque in mice under femtosecond laser excitation(Fig.13).The researchers found that the results of the dissection of the mice corresponded well with the imaging results.
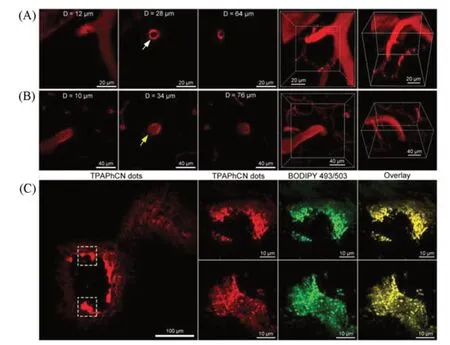
Fig.13 Intravital imaging of atherosclerotic plaques in the brain[48]
4.1.2 Imaging-guided Surgery When atherosclerotic plaque reaches a certain level,it can lead to vascular stenosis,occlusion,and even tissue ischemic necrosis.For example,plaque shedding in the carotid artery will greatly increase the risk of cerebral embolism.Drug therapy alone is of limited effectiveness,and surgical treatment should be considered to remove the“garbage”blocking the blood vessels so that they can be opened.One of the most common and effective treatments for atherosclerosis is surgical excision.The use of surgical real-time imaging technology allows doctors to see the location of the operation at a glance and accurately map the lesion structure,which will make surgical operations faster,more accurate,and safer.
Zhenget al.[49]developed four lipid-activated small-molecule fluorescent probes based on the structure of coumarin(CN,CN-N1,CN-N2,and CN-N3).These lipid-activated small molecule fluorescent probes have a higher signal-to-noise ratio in applications than“always on”probes.On the one hand,the emission wavelengths in DMSO were in the visible region(469,520,520,and 534 nm),which did not have the same deep tissue penetration capability as the NIR probes but were convenient for doctors to directly observe the lesion during the surgery.On the other hand,most fluorescent probes need a long time to reach the lesion after circulating in the blood and may be absorbed by other tissues passing through.To solve these problems,the researchers applied the CN-N2 probe as a patch to the surgical site directly[Fig.14(A)].Surprisingly,CN-N2 could penetrate from the outer to the intima of the human coronary artery in 5 min,and the patch soaked with CN-N2 probe increased the fluorescence intensity of adipocytesin vitroby 36.06 times after 5 min.They selected ApoE-/-C57BL/6 mice that had been treated with a high-fat diet as atherosclerosis models,and the left carotid artery ligation was done before that[Fig.14(B)].CN-N2 patch was applied to the carotid artery of atherosclerotic mice after the successful establishment of the model,and a plaque with a diameter of 0.5 mm could be displayed 5 min later[Fig.14(C)].
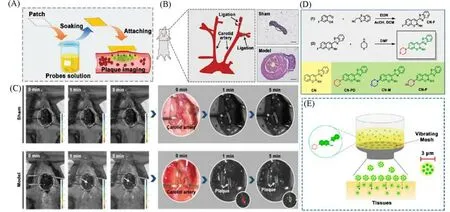
Fig.14 Lipid-activatable fluorescent probes for intraoperative imaging of atherosclerotic plaque
In addition to being fast,imaging-guided surgery also requires images that are stable for a long time.Sanget al.[50]proposed anin situspray method for rapidly atomizing the fluorescent probe CN-PD which is specific for lipid droplets.The synthesis of CN-PD is divided into two simple steps.Firstly,4-fluorosalicylaldehyde was cyclized with phenylpropithiazole-2-acetonitrile in the presence of triethylamine,after that,piperidine was used to attack the 7-position fluorine to get the final product[Fig.14(D)].Compared with BODIPY 493/503(10 nm),CN-PD has a larger Stokes shift(71 nm)and a higher fluorescence quantum yield.The fluorescence intensity of CN-PD was enhanced 30.38 times in 200μg/mL liposome solution,and the signal of CN-PD was 13.4 times stronger in foam cells than that in macrophages.Endarterectomy was simulated in atherosclerotic model mice after carotid ligation.The researchers used vibrating mesh to improve thein situspraying method[Fig.14(E)],allowing rapid and uniform spraying of CN-PD to achieve the objective of imaging atherosclerotic plaques.If the operation takes longer than expected,rapid imaging can be restored by repeated injection of the CN-PD probe.
4.2 Application of Lipid-responsive Probes in Atherosclerosis Treatment
An important manifestation of atherosclerosis is an abnormal accumulation of lipids[51].Statins,glucocorticoids,steroids,and other drugs are often used to inhibit cholesterol secretion and inflammation reactions.However,these conventional treatment methods are characterized by non-specificity,fast metabolism,and serious side effects[52].The development of new nanotherapy platforms promises to address these issues[53].
Maet al.[54]engineered a biomimetic nanoplatform(RBC/LFP@PMMP)consisting of a lipid-specific fluorophore(LFP)and a ROS-responsive prednisolone prodrug copolymer(PMMP)[Fig.15(A)].When LFP was dissolved in oil,the fluorescence shifted from 580 nm to 525 nm with the change from orange to green.When RBC/LFP@PMMP accumulated in atherosclerotic lesions and responded to overexpressed ROS by local inflammation,the nanoparticles would split and release LFP and prednisolone(Pred).LFP is specifically bound to lipids accumulated in plaques and emitted green fluorescence,whereas the release of Pred could achieve effective anti-inflammatory activity,simultaneously enabling targeted diagnostic imaging of atherosclerosis and accurate anti-inflammatory mediated therapy[Fig.14(B)—(D)].
Maet al.[55]also developed another nanoplatform called TPCDP with the aim of diagnosis and treatment of AS.TPCDP@PMM was able to accumulate in atherosclerotic tissuesviaworn vascular endothelium and could be activated by locally overexpressed ROS and abundant lipids[Fig.15(E)].Then the micelles would be destroyed and releasedβ-cyclodextrin(CD)and Pred.CD is beneficial for the removal of lipids in atherosclerotic plaques,and Pred helps to eliminate inflammation.This synergistic treatment approach is beneficial in inhibiting the development of atherosclerosis.
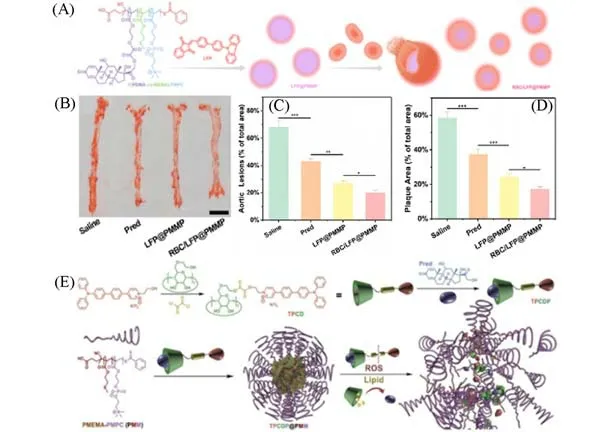
Fig.15 Nanoplatform with lipid-specific imaging for atherosclerosis-targeted therapy
5 Summary and Outlook
The aortic arch,carotid,and coronary arteries,which are closely associated with cardiovascular disease,are mainly located deep in the body.How to achieve effective diagnosis and treatment of AS has become an urgent challenge in the field of cardiovascular research.Visualization of intra-arterial lipids is of great scientific value for the study of AS.This review provides a categorical summary of lipid-responsive optical probes and their applications in the imaging and treatment of AS in recent years.Firstly,common LDs imaging strategies are summarized,and the principles of Nile Red as well as BODIPY493/503 for LDs staining and their characteristics are presented.Secondly,to further understand the design strategies of lipid-specific imaging probes,various types of optical probes developed based on common small organic molecule frameworks as well as metal complexes are analyzed.Finally,the value of lipid-responsive probes for AS imaging as well as therapeutic applications is highlighted,demonstrating their potential for biomedical and molecular medicine.
Despite several signs of progress in the field,the following challenges in the development of lipid-responsive probes for AS imaging and therapy exist.Firstly,the tissue penetration ability of probes needs to be further improved,and more suitable design approaches need to be found.The reason this shortcoming exists is that current commercial probes usually emit at 400—600 nm.Although some probes for NIR imaging have been mentioned in this paper,there is still a need to develop more stable NIR probes with excellent penetration capability to improve the possibility of imaging deeper tissues in living organisms.Secondly,the complex environment within the organism can also have an impact on lipid-activated optical probes.On the one hand,there is a need to explore methods that can improve the specificity of optical probes under different microenvironmental conditions such as polarity,viscosity,and pH.At the same time,more endogenous stimuli(e.g.,proteins)and multi-factor external stimuli(e.g.,light modulation)tools need to be explored for further accurate imaging in lesions.Finally,studies based on the application of lipid-activated optical probes in AS are currently focused on the validation ofin vitromodels,with limited application toin vivoimaging.Therefore,there is a need to further expand the scope of applications in this field and provide new tools and methods for relevant biomedical application studies.
