Reduction of N2O emissions by DMPP depends on the interactions of nitrogen sources (digestate vs.urea) with soil properties
2023-01-06LIHaoruoSONGXiaotongLarsBAKKENJUXiaotang
LI Hao-ruo ,SONG Xiao-tong ,Lars R.BAKKEN ,JU Xiao-tang,
1 College of Resources and Environmental Sciences,China Agricultural University,Beijing 100193,P.R.China
2 State Key Laboratory of Urban and Regional Ecology,Research Center for Eco-Environmental Sciences,Chinese Academy of Sciences,Beijing 100085,P.R.China
3 College of Tropical Crops,Hainan University,Haikou 570228,P.R.China
4 Faculty of Chemistry,Biotechnology and Food Science,Norwegian University of Life Science (NMBU),Ås 1430,Norway
Abstract The inhibition of nitrification by mixing nitrification inhibitors (NI) with fertilizers is emerging as an effective method to reduce fertilizer-induced nitrous oxide (N2O) emissions.The additive 3,4-dimethylpyrazole phosphate (DMPP)apparently inhibits ammonia oxidizing bacteria (AOB) more than ammonia oxidizing archaea (AOA),which dominate the nitrification in alkaline and acid soil,respectively.However,the efficacy of DMPP in terms of nitrogen sources interacting with soil properties remains unclear.We therefore conducted a microcosm experiment using three typical Chinese agricultural soils with contrasting pH values (fluvo-aquic soil,black soil and red soil),which were fertilized with either digestate or urea in conjunction with a range of DMPP concentrations.In the alkaline fluvo-aquic soil,fertilization with either urea or digestate induced a peak in N2O emission (60 μg N kg–1 d–1) coinciding with the rapid nitrification within 3 d following fertilization.DMPP almost eliminated this peak in N2O emission,reducing it by nearly 90%,despite the fact that the nitrification rate was only reduced by 50%.In the acid black soil,only the digestate induced an N2O emission that increased gradually,reaching its maximum (20 μg N kg–1 d–1) after 5–7 d.The nitrification rate and N2O emission were both marginally reduced by DMPP in the black soil,and the N2O yield (N2O-N per NO2–+NO3–-N produced) was exceptionally high at 3.5%,suggesting that the digestate induced heterotrophic denitrification.In the acid red soil,the N2O emission spiked in the digestate and urea treatments at 50 and 10 μg N kg–1 d–1,respectively,and DMPP reduced the rates substantially by nearly 70%.Compared with 0.5% DMPP,the higher concentrations of DMPP (1.0 to 1.5%) did not exert a significantly(P<0.05) better inhibition effect on the N2O emissions in these soils (either with digestate or urea).This study highlights the importance of matching the nitrogen sources,soil properties and NIs to achieve a high efficiency of N2O emission reduction.
Keywords: nitrous oxide,digestate,urea,nitrification inhibitors,DMPP,alkaline soils,acid soils
1.Introduction
Nitrous oxide (N2O),a long-lived potent greenhouse gas,has a global warming potential that is 265 times stronger than carbon dioxide (CO2) over a 100-yr timescale,and it is the most significant destroyer of the ozone layer in the 21st century (Ravishankaraet al.2009;IPCC 2014).Human activities have led to a 20% increase in atmospheric N2O concentration since pre-industrial periods (WMO 2018).Agriculture is the largest source(60%) of anthropogenic N2O emissions,mainly due to the intensification sustained by synthetic nitrogen (N)fertilizers and manure (UNEP 2013).
Anaerobic digestion is increasingly used to produce biogas from animal manure,prior to fertilization (Mirandaet al.2015).The products of anaerobic digestion(digestates) are valuable biofertilizers due to their high contents of plant nutrients (N,P and K) and organic matter.However,applying digestate inevitably induces N2O emissions from soils by providing ammonium (NH4+)for nitrification and organic carbon (C) for heterotrophic denitrification (Senbayramet al.2009).Further,the presence of both NH4+and available organic C in the digestate has a synergistic effect on N2O emissions by nitrification-coupled denitrification (NCD) due to accelerated oxygen consumption (Shiet al.2017).Although the available C in digestate effectively induces N2O production by denitrification,its effect on N2O emission could be marginal if it induces a more complete denitrification (more N2O converted to N2) than in soil without digestate (Senbayramet al.2012).Prior research has provided conflicting results regarding the effects of digestate on N2O emissions from soils (Möller and Stinner 2009;Collinset al.2011),demonstrating the need for a deeper understanding of how digestate affects N2O emissions from soilsvianitrification and denitrification.
In the last decade,nitrification inhibitors (NIs) are proved to be effective for reducing fertilizer-induced N2O emissions (Ruser and Schulz 2015;Lamet al.2017;Wuet al.2021).One of the most effective nitrification inhibitors,3,4-dimethylpyrazole phosphate (DMPP),is a Cu chelator that competes with ammonia monooxygenase(AMO) for Cu2+as a co-factor and so it can delay the first rate-limiting step of nitrification (oxidation of NH4+to hydroxylamine (NH2OH)) (Ruser and Schulz 2015).DMPP has been widely tested for improving the efficiency of N fertilizers,decreasing N2O emissions,and reducing nitrate leaching in agricultural systems (Qiaoet al.2015),given its ease of application,high persistence and stability in soils,long-lasting inhibitory effects,and lack of adverse effects on plants,earthworms,and non-target soil microbes (Fanet al.2019).
Previous studies have indicated that the inhibitory effects of DMPP on nitrification and N2O emissions are contingent on soil pH (Shiet al.2017;Guardiaet al.2018),fertilizer type (Menéndezet al.2009;Florioet al.2016),and ammonia oxidizer community structure (Duanet al.2017;Luchibiaet al.2020).Zhuet al.(2019)reported that the application of 1% DMPP (of applied N)could significantly decrease autotrophic nitrification rates and total N2O emissions in alkaline soils.However,this inhibitory effect was not observed in acidic soils (Friedlet al.2017).As for the performance of DMPP with different fertilizer types,recent studies have indicated a higher inhibitory effect on N2O emissions when DMPP was applied with digestate in comparison with urea in acidic soils (ten Hufet al.2020;Donget al.2021).In alkaline soils,DMPP almost completely inhibited ureainduced N2O emissions,while the effect on digestateinduced emissions was only moderate (Florioet al.2014;Liuet al.2021).The DMPP effect in alkaline soils was accompanied by a decrease in ammonia-oxidizing bacteria (AOB) abundance (Fanet al.2019),whereas a 6-year field experiment showed no significant longterm effects on the soil bacterial communities in acidic soils (Donget al.2021).A comparison of the effect of DMPP on the growth of AOB and ammonia-oxidizing archaea (AOA) in soils has strongly suggested that DMPP selectively inhibits the activity and growth of AOB,whereas AOA appears to be unaffected (Lanet al.2018).Therefore,DMPP has clear potential to reduce N2O emissions in either digestate-or urea-amended soils,but its effect at the process level (i.e.,nitrification or denitrification) in terms of nitrogen sources and interactions with soil properties remains unclear,which is critical for improving its mitigation efficacy.
In this study,we carried out a microcosm incubation experiment to systematically study the effects of DMPP on nitrification and N2O emissions induced by digestate and urea in three typical Chinese agricultural soils with contrasting pH,organic C,and total N.Our objectives were to (1) evaluate the impacts of digestatevs.urea on nitrification rates and N2O emissions in soils with different properties,and (2) assess the effectiveness of DMPP in mitigating N2O emissions under digestatevs.urea application through interactions with different soil pH conditions.
2.Materials and methods
2.1.Site description and soil sampling
Samples were collected from three typical agricultural soils in northern and southern China,namely,the fluvo-aquic soil in Hebei Province (36°52´N,115°10´E),the black soil in Jilin Province (43°37´N,124°36´E) and the red soil in Hunan Province (28°32´N,113°19´E).In April 2019,soil samples were taken from the top layer (0–20 cm) at nine random points per plot using a shovel.Samples were mixed thoroughly to obtain a composite sample from each site.The collected soils were air-dried in the laboratory for 2 wk and sieved to 2 mm after manually removing all of the stones,roots,and residues.Most samples were stored at room temperature prior to the incubation experiment,while some others were prepared for soil property measurements.The physiochemical properties of the three tested agricultural soils and site information are shown in Table 1.
2.2.Experimental design
The incubation experiment was designed to test the inhibitory efficacy of DMPP at a range of application concentrations in the three collected soils that were amended with two different N sources (digestate or urea,100 mg N kg–1soil).The DMPP levels were 0,0.5,1.0,and 1.5% of added N (in the digestate or urea).Nine treatments were conducted: a control (C)without the application of fertilizers and DMPP to check background nitrification rates and N2O emissions,a set of four treatments amended with digestate (D,D+DMPP 0.5%,D+DMPP 1.0%,D+DMPP 1.5%),and a parallel set of four with urea (U,U+DMPP 0.5%,U+DMPP 1.0%,U+DMPP 1.5%) (Table 2).Each treatment was replicated four times,using five jars for destructive sampling at each timepoint for the chemical analyses and one extra jar for gas monitoring until the soil sampling at the endpoint,yielding a total of 216 glass jars (9 treatments×4 replicates×6 sampling times).The digestate was sampled directly after the anaerobic digestion of pig manure from a pig farm in Zhejiang Province,China,and stored at 4°C prior to analysis and soil amendment.The digestate had a pH of 8.15,total organic C of 237.3 g kg–1,TN of 20.5 g kg-1,C/N ratio of 11.6,NH4+-N of 2.53 g kg-1,and gravimetric water content of 42.7%.
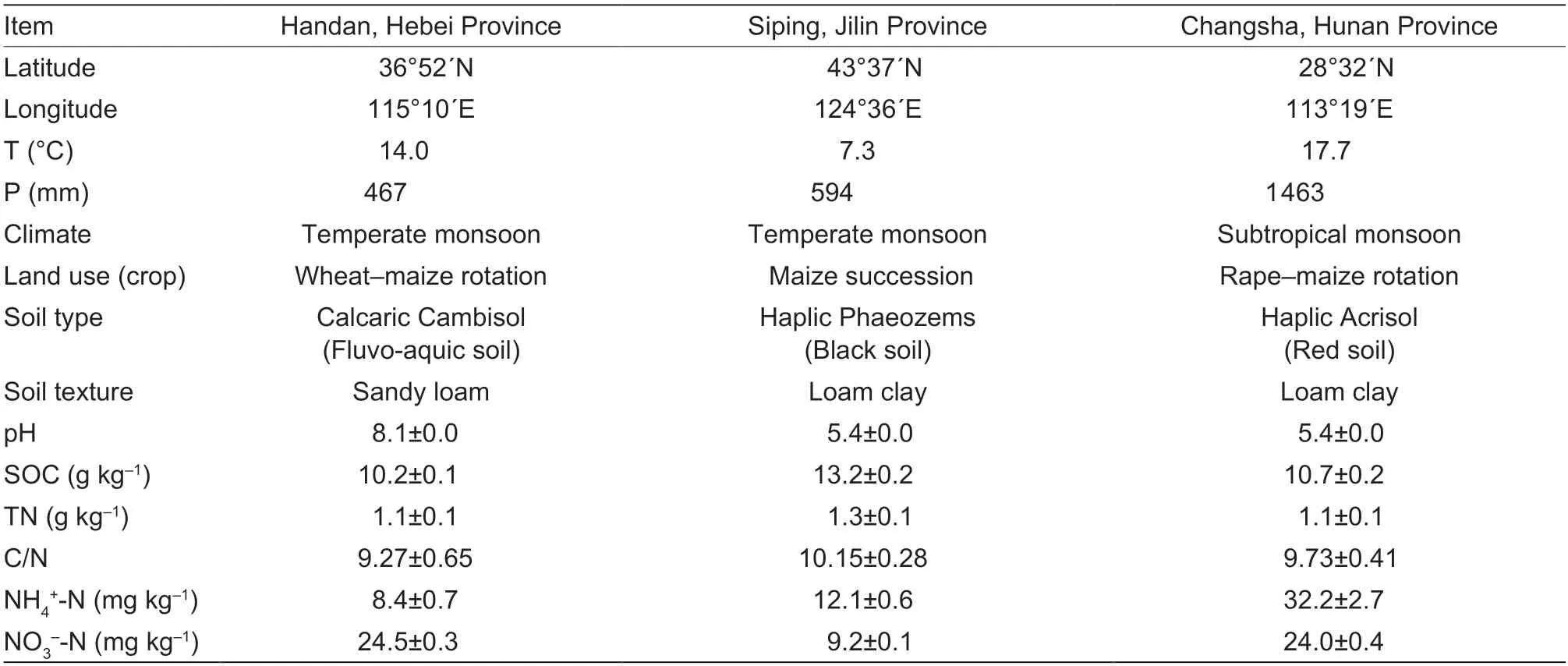
Table 1 Information on soil sampling sites and soil properties of the top layer (0–20 cm)
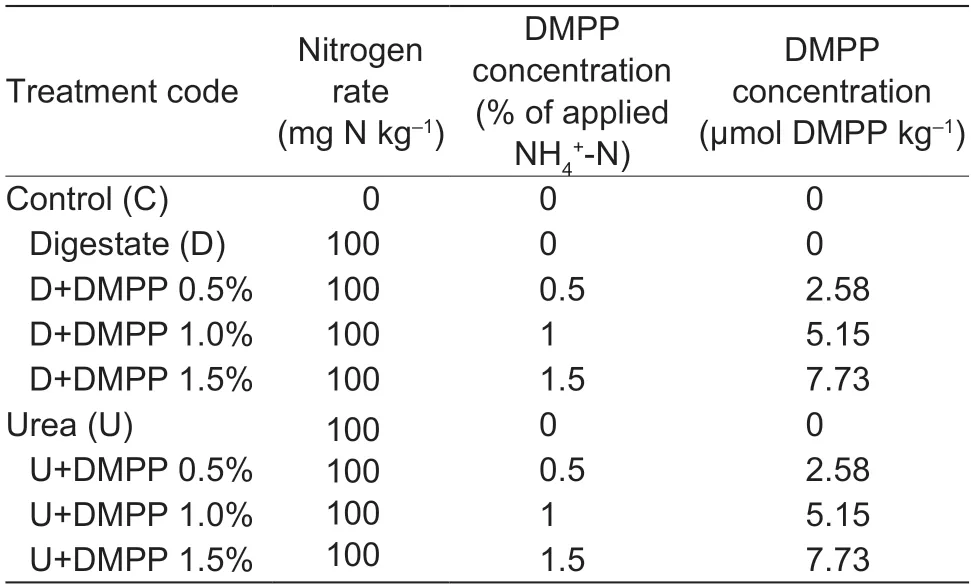
Table 2 Experimental design1)
2.3.Microcosm incubation
Dried soil samples were re-wetted to~15% gravimetric water content and preincubated at 20°C in the dark for a week to activate the microbial activity and avoid the priming effects of soil respiration.After preincubation,150 g(fresh weight) of the re-wetted soil was weighed into a 550-mL glass jar for each replicate of the treatments.The digestate was thoroughly mixed with the soil using a spatula.Syringes were used to apply the urea and DMPP as solutions.The concentrations were adjusted to ensure that the target soil moisture level of 19% gravimetric water content was reached for each treatment.After solution addition,a sheet of parafilm with six pinholes was used to cover the top of each jar,securing the aerobic conditions while minimizing the loss of water by evaporation.To maintain the soil moisture level at 19% gravimetric water content over the entire incubation period,distilled water was added every 4 d,with the amount depending on the weight loss for the jar.The jars were placed randomly in the incubation room at a constant temperature of (20±2)°C in the dark for 21 d.
2.4.Measurement of gas flux
Flux measurements of N2O and CO2were conducted between 8:30 a.m.and 10:30 a.m.on days 1,2,3,5,7,10,14,and 21 during incubation.Emissions weremeasured by removing the parafilm and allowing the air inside the jars to exchange with ambient air for 10 min,and then covering the jars with rubber-sealed lids equipped with a three-way valve (Yanget al.2020).The gas samples were taken from each jar at 0,10,20,and 30 min after closure (with 40 mL sampled each time)using a 50-mL plastic gas-tight syringe.The sampled gas was injected into pre-evacuated Exetainer vials with gray silicon septas (Labco,UK) and immediately analyzed for N2O and CO2concentrations using a gas chromatograph(GC,Agilent 7890,USA) fitted with an ECD detector for N2O at 350°C and an FID detector for CO2(converted to CH4by methanator) at 250°C (Zhenget al.2008;Huanget al.2017;Songet al.2019).
2.5.Soil sampling and measurements
Destructive soil samplings for soil mineral N (NH4+,NO2–,and NO3–) measurements were carried out on days 0,1,3,7,14,and 21.Each soil sampling was accompanied by gas sampling,except for the one on day 0.Soil mineral N was extracted using 1 mol L–1potassium chloride (KCl) solution (with 24 g soil to 100 mL KCl solution) on a shaker at a speed of 180 r min–1for 1 h,and then centrifuged (10 000 r min–1for 10 min).The supernatants were immediately analyzed for NO2–using the sulfanilamide colorimetric method with an ultraviolet spectrophotometer (UV mini-1240,Shimadzu,Japan).The NH4+and NO3–concentrations were analyzed by the sodium salicylate-nitroprusside and cadmium reduction methods,respectively,using a continuous flow analyzer(Auto Analyzer 3 system,Bran and Luebbe,Norderstedt,Germany).The soil samples on days 0 and 21 were also analyzed for pH.Soil pH measurements were performed by mixing the soils in water (1:2.5 soil–water ratio) and shaking for 30 min,after which the soil solution was left for 10 min and analyzed using a pH meter (S220 Seven Compact,Mettler Toledo,Switzerland).Soil organic carbon (SOC) and total nitrogen (TN) were measured using an elemental analyzer (Vario MARCRO CN,Elementar,Germany).Historical mean air temperature and precipitation data for the sampling sites were sourced from the National Meteorological Information Center(http://data.cma.cn/).
2.6.Calculations
N2O and CO2fluxes were calculated as:
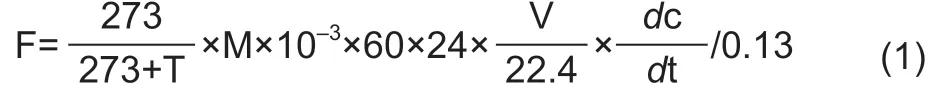
where F is the N2O (μg N2O-N kg-1day-1) or CO2(mg CO2-C kg-1d-1) flux,T is the air temperature (°C) in the jar during incubation,M is the molecular weight of either N in molecular N2O (28 g mol-1) or C in molecular CO2(12 g mol-1),60 is the number of minutes in an hour,24 is the number of hours in a day,V is the volume (L) of the headspace,22.4 (L mol-1) is the mole volume of gas at 101 kPa and 273 K,c is the concentration of N2O (nL L-1) or CO2(μL L-1),t is the time (min) of jar sealing,dc/dt is the rate of change in the N2O (nL L-1min-1) or CO2(μL L-1min-1) concentration after jar sealing,and 0.13 is the dry weight (kg) of soil in the jar.Accumulated N2O and CO2emissions during the full incubation period were calculated using the sums of measurements on sampling and non-sampling days as estimated by linear interpolation (Mosieret al.2006).
The N2O yield (Y) was calculated as follows (Hinket al.2017):
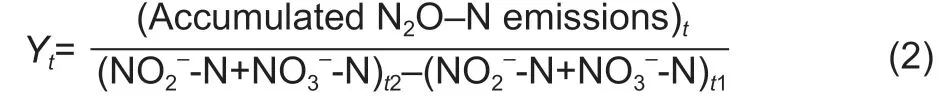
where (accumulated N2O-N emissions)tis the cumulative N2O emissions from days 0 to 3,7,and 21,respectively.(NO2–-N+NO3–-N)trepresents the concentrations of NO3–-N and NO2–-N in the soil on days 3,7,and 21,respectively,and (NO2–-N+NO3–-N)0represents the concentrations of NO3–-N and NO2–-N on day 0.
The nitrification rate (n) for a certain period during the incubation was calculated as follows (Nadeemet al.2020):

2.7.Statistical analyses
The differences in cumulative N2O and CO2emissions between treatments and pH levels between days 0 and 21 were assessed by one-way ANOVA with the least significant difference (LSD) test atP<0.05,using IBM SPSS Statistics 25 (SPSS Inc.,USA).The effects of soil,fertilizer type,and DMPP concentration on N2O emissions were analyzed by three-way ANOVA with LSD atP<0.01.The correlations of cumulative N2O emissions either with changes in pH (ΔpH=pH (end,day 21)–pH(beginning,day 0)) or with cumulative CO2emissions were fitted by linear regression using Sigmaplot 14 (Systat Software Inc.,Germany).All results are reported here as mean±standard errors,on a soil dry weight basis.
3.Results
3.1.N2O and CO2 emissions
N2O measurements showed significant effects of soil type,fertilizer type,and DMPP on the kinetics of N2O emissions (P<0.01;Fig.1;Appendix A).In fluvo-aquic soil amended with digestate and urea without DMPP,N2O emissions peaked on day 2 (up to 50–60 μg N kg–1d–1) and declined to very low values after 5–7 d.This early N2O peak was essentially eliminated by DMPP(<4 μg N kg–1d–1),independent of the DMPP level.Although DMPP effectively eliminated the initial peak of N2O emission from the fluvo-aquic soil with digestate,in the treatment with both digestate and DMPP,the N2O emission increased again after the initial peak toward the end of the incubation,and more so for 0.5% than for 1% or 1.5% DMPP.No such increase was observed for the urea treatments with DMPP.
Compared to the fluvo-aquic soil,the black soil emitted much less N2O,and there was a profound difference between the digestate and urea treatments.The former induced substantial emissions (peaking on day 5 at around 20 μg N kg–1d–1),which were depressed but not eliminated by DMPP,whereas the latter induced marginal N2O emissions that were only slightly higher than in the control treatment (Fig.1-E).
Emissions from the red soil were higher than those from the black soil but showed similar patterns regarding the effects of the N source and DMPP.The digestateinduced emissions were substantial (compared to the control treatment,peaking at 50 μg N kg–1d–1),but were reduced by DMPP,while the emissions in the ureaamended soil (10 μg N kg–1d–1) were no higher than those in the control treatment.In fact,treatments with U+DMPP emitted less N2O than the control (Fig.1-F).

Fig.1 N2O fluxes from the soils throughout the incubation.The results for soils amended with digestate (D) and urea (U) are shown in A–C and D–F,respectively.The insert panels show zoom-in results of all treatments in A and D–F.C,control;DMPP,3,4-dimethylpyrazole phosphate.Error bars are the standard errors of four replicates.
The CO2emissions in the digestate treatments were significantly higher than those in the urea treatments(Appendices B and C).In the digestate-amended group,the CO2fluxes overall ranged from 30–40 mg C kg–1d–1in the fluvo-aquic soil,but were always below 30 mg C kg–1d–1in the black and red soils.For the urea treatments,the CO2fluxes ranged from 10–20 mg C kg–1d–1in all three soils,except for a notable CO2peak of 40 mg C kg–1d–1in the fluvo-aquic soil without DMPP addition.Overall,the CO2fluxes peaked on the first or second day,then declined slightly,and fluctuated until the end of the incubation period in all soils and treatments.DMPP addition decreased CO2emissions only in the urea treatments of the fluvo-aquic soil,while it exerted no significant effects in the other cases.
3.2.Responses of N2O and CO2 emissions to different DMPP concentrations
To assess the overall effect of DMPP on N2O emissions,we plotted the cumulative emissions for each treatment against the DMPP concentration (Fig.2).DMPP reduced N2O emissions by over 50% in both the fluvo-aquic and red soils (for both digestate and urea),while the effect on emissions from the black soil was marginal.The 0.5%DMPP concentration effectively lowered N2O emissions.In the digestate-amended group,adding 0.5% DMPP decreased the cumulative N2O emissions by~50% in all three soils (Appendix C).For the urea-amended group,the addition of 0.5% DMPP reduced the cumulative N2O emissions by~90,~70,and~20% in the fluvo-aquic,red,and black soils,respectively.Compared with 0.5% DMPP,higher concentrations of DMPP (1.0–1.5%) did not exert a greater inhibitory effect on N2O emissions,based on the significance test for treatment effects (Appendix C).Overall,the effect of DMPP on N2O emissions was most significant in the fluvo-aquic soil amended with urea.
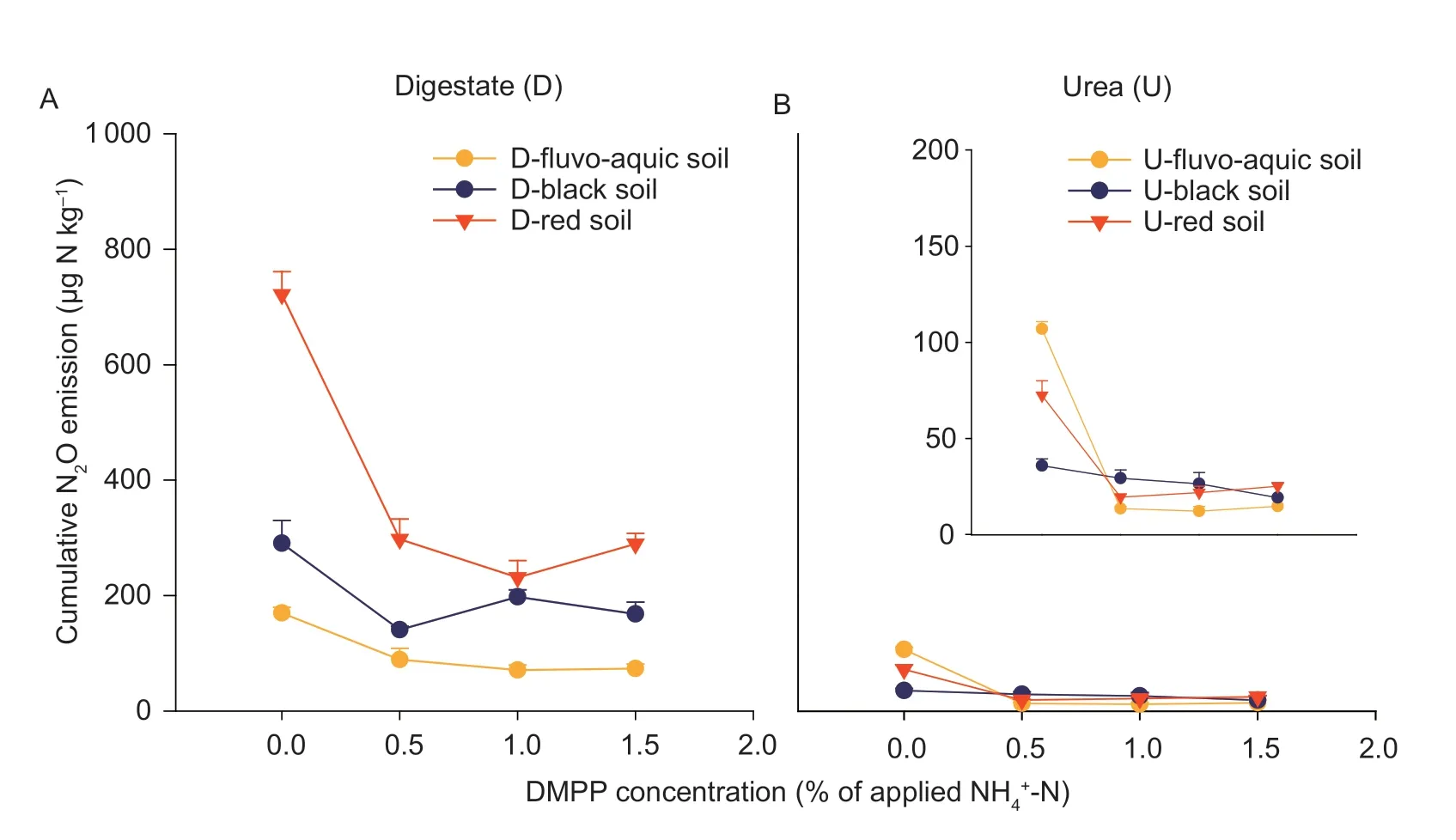
Fig.2 Cumulative N2O emissions in response to the 3,4-dimethylpyrazole phosphate (DMPP) concentrations in each of the studied soils during the incubation period.The results for soils amended with digestate and urea are shown in A and B,respectively.The insert panel shows zoom-in results of the soils with urea application.Error bars are the standard errors of four replicates.Detailed data for the points in this figure are given in Appendix C.
In the digestate amended group of the fluvo-aquic soil,there were no significant differences in CO2emissions between treatments with and without DMPP.In the black and red soils,0.5% DMPP reduced CO2emissions by~20%,while the 1.5% DMPP concentration had a weaker inhibitory effect (Appendices C and D).In the urea amended group,0.5 and 1% DMPP decreased CO2emissions by~50% in the fluvo-aquic soil (Appendices C and D),but DMPP did not have an obvious influence in the black or red soils.
We were also interested in the effect of DMPP on CO2emissions because (1) lowered CO2emissions due to increased DMPP concentrations could suggest a general toxic effect on soil microbiota,and (2) CO2emissions largely reflect the rate of heterotrophic metabolism,which could be a driver of N2O emissionsviaheterotrophic denitrification.However,our results provided no evidence for a general toxic (or stimulating) effect of DMPP on heterotrophic metabolism,as the CO2emissions did not increase or decrease consistently with increasing DMPP concentration in any of the soils (Appendices B and D).Overall,the N2O emissions were correlated significantly with CO2emissions,and more strongly in the black and red soils than in the fluvo-aquic soil (Appendices E and F),suggesting a stronger heterotrophic source of N2O emissions from the black and red soils.
3.3.NH4+,NO2-,and NO3- kinetics,nitrification rates,and mineral-N balance
When amended with digestate,a rapid nitrification process occurred in the fluvo-aquic soil,during which almost all of the NH4+(~150 mg N kg–1) was oxidized to NO3–,accompanied by a transient accumulation of NO2–(~5 mg N kg–1) within 3 d (Fig.3-A,D and G).There was a rebound in the NH4+concentration on day 3,probably due to the mineralization of organic N in the digestate.The nitrification in the fluvo-aquic soil was partially (~2/3)inhibited by DMPP,with no significant differences among the different concentrations of DMPP.In all the DMPP treatments of fluvo-aquic soil,there was a slight decrease in the NH4+concentration and a corresponding increase in NO3–(~50 mg N kg–1),but with no accumulation of NO2–.
In the digestate-amended black soil without DMPP,there was a slight and gradual decline in NH4+throughout the incubation period,and a similar gradual increase in NO3–,which equaled the rates observed in the unamended control treatment (Fig.3-B,E and H).DMPP apparently retarded the decline in NH4+but not the accumulation of NO3–.Thus,nitrification in the black soil was not stimulated by the digestate and was marginally inhibited by DMPP.
In the red soil,the NH4+concentrations in the digestate-amended soil declined gradually throughout the incubation period,apparently unaffected by DMPP,while the concentrations in the control treatment remained essentially constant (Fig.3-C,F and I).No accumulation of NO3–was detected in any of the red soil treatments.Thus,nitrification was essentially undetectable in this soil.

Fig.3 Mineral N kinetics in soils with digestate (D) addition during the incubation period.The insert panels show zoom-in results of nitrate in the black soil and red soil.C,control;DMPP,3,4-dimethylpyrazole phosphate.Error bars are the standard errors of four replicates.
Similar nitrification patterns were recorded for soils amended with urea (Fig.4),although the hydrolysis of urea by urease was an important influencing factor.In the fluvo-aquic soil,NH4+was high since the beginning,probably reflecting a very fast urease reaction in this soil.The subsequent nitrification was partially inhibited to 2/3 of its original value by DMPP.In the black soil,urea hydrolysis was evidently slower,resulting in a gradual increase in the NH4+concentration,and NO3–accumulation was slow and unaffected by urea or DMPP.In the red soil,urea hydrolysis was similarly slow and apparently inhibited slightly by DMPP,while no accumulation of NO3–was detected.

Fig.4 Mineral N kinetics in soils with urea (U) addition during the incubation period.The insert panels show zoom-in results of nitrate in the black soil and red soil.C,control;DMPP,3,4-dimethylpyrazole phosphate.Error bars are the standard errors of four replicates.
A significant positive mineral-N balance was observed with urea addition in all soils (Appendix G),attributable to net N mineralization.In the fluvo-aquic soil,applying digestate led to a positive mineral-N balance,which evidently increased in the DMPP treatments,likely reflecting the significant mineralization of organic N provided by the digestate.In contrast,a large negative mineral-N balance was observed in the digestate treatments in the red soil,indicating gaseous N loss by denitrification.In general,DMPP did not affect the mineral-N balance in the black or red soils.
3.4.N2O yield
In the fluvo-aquic soil,the N2O yield was~0.1% for uninhibited nitrification (i.e.,without DMPP),for the treatments with either digestate or urea (Fig.5-A and B).In this soil,DMPP treatments resulted in much lower yields(0.02%) for the period with active nitrification (0–7 d).In the urea treatment,the yield remained low throughout,but it increased toward the end in the digestate treatments because the N2O emissions increased substantially(Fig.1-A).In the black soil,uninhibited nitrification in the digestate treatment induced significantly higher N2O yields (0.5–3.5%) than in the urea treatment (<0.3%),while the yield increased toward the end and was reduced substantially (30–50%) by DMPP for both treatments.Because nitrification rates in the red soil were not detected,the N2O yield of this soil could not be calculated.
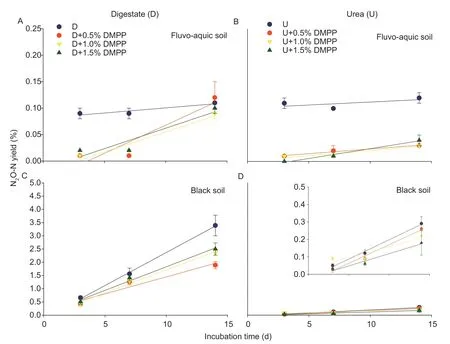
Fig.5 N2O-N yield per (NO2–+NO3–)-N produced (%) over the different incubation periods.Data for N2O-N yield in the red soil were not detected.The insert panel shows zoom-in results in the black soil with urea addition.DMPP,3,4-dimethylpyrazole phosphate.Error bars are the standard errors of four replicates.
3.5.Changes in soil pH
Considering that nitrification releases H+,we analyzed the correlation between the change in pH and nitrate,and found the expected correlation for the fluvo-aquic soil (a linear increase in acidification with increasing amounts of nitrate produced;Appendices H and I).Likewise,the cumulative N2O production in the fluvo-aquic soil correlated with the acidification,which is consistent with the dominant role of nitrification in N2O emissions.We found no correlations for the two other soils,most likely due to their very low/undetectable nitrification rates.
4.Discussion
4.1.Effects of digestate on N2O emissions in different soil types
Digestate is increasingly being used to fertilize soils in China.The large-scale use of digestate on agricultural soils is an integrated part of the Ministry of Agriculture of China’s action plan for improving soil quality and curbing the use of synthetic fertilizers (MOA 2015).Thus,our study provides important evidence for controlling N2O emissions when applying digestate on different soils.Our results showed that digestate (without DMPP) led to greater N2O emissions than urea (Fig.1;Appendix C).Senbayramet al.(2009) found that digestate-treated soils produced much higher N2O emissions than those receiving ammonium sulfate fertilizer,with 2.56 and 0.68% emitted as N2O,respectively.This difference was probably due to the organic C in the digestate,which could enhance heterotrophic microbial activity,resulting in oxygen depletion and denitrification (Heet al.2019).The contrasting patterns of N2O emissions exhibited between the different soils were noteworthy.The treatment of red soil with digestate resulted in prolonged high N2O emissions,and therefore the largest cumulative amount of N2O,while digestate induced much lower N2O emissions in the black soil.This contrast could possibly be due to the relatively high content of organic C in the black soil,allowing it to sustain a more complete denitrification (N2O reduction to N2) than the red soil (Senbayramet al.2012;Verdiet al.2019).Even though the fluvo-aquic soil had the highest peak of N2O flux after digestate application,the cumulative N2O emissions in this soil were lower than in the others owing to the transient nature of its emissions.
4.2.Effects of DMPP on nitrification
In the black soil,DMPP had no effect on the rate of NO3–production,while the rate of nitrification (during the first 7 d after fertilization,both digestate and urea) in the alkaline fluvo-aquic soil was reduced by~50%,indicating a stronger inhibitory effect of DMPP in alkaline soil than in acid soil (Figs.3 and 4;Appendix J).The differences between the alkaline (fluvo-aquic) and the two acidic soils (black and red soil),regarding the effect of DMPP,can be attributed to differences in the dominant ammonia oxidizers.For example,there is an evidence that DMPP inhibits the activity of AOB rather than AOA across a range of soil types (Donget al.2013;Duanet al.2017),soil pH and ammonia (NH3) levels are key to the ecological niche separation of AOB and AOA (Lehtovirta-Morley 2018),and generally,AOB dominates the nitrification in alkaline soils with high rates of NH3supply,whereas AOA are favored by low pH and low NH3availability (Prosser and Nicol 2012;Ouyanget al.2016;Nadeemet al.2020).Thus,DMPP is much more effective in the AOB-dominated fluvo-aquic soil than in the AOA-dominated black soil,which emphasizes the use of DMPP in soils with target nitrifiers.
Furthermore,our results are also helpful for understanding the side effects of DMPP on other N transformation-related processes besides nitrification.In the three soils,the increase in NH4+after urea application was virtually unaffected by DMPP (Fig.4-A–C),suggesting that DMPP does not inhibit urease activity in soil.Similarly,DMPP was not expected to affect the heterotrophic metabolism in soil (Zhuet al.2019).There was no consistent effect of DMPP on CO2evolution(Appendices B and D),or on the relationship between CO2and N2O emissions (Appendices E and F),indicating that the heterotrophic source (denitrification) of N2O was not directly affected by DMPP.Nevertheless,the mineral-N balance suggested enhanced mineralization of organic N by DMPP in the digestate,as judged by the content of mineral N at the end,which was clearly higher with DMPP than without it (Appendices G and K).This difference could be attributed to either the mineralization of DMPP itself,which is a nitrogen-rich organic compound(C5H8N2·H3PO4) (Vilaset al.2019),or the fact that DMPP treatment reduced the mineral N loss by denitrification(viainhibiting nitrification,thus lowering the overall O2consumption in the soil).In contrast,DMPP had an inconsistent effect on the endpoint mineral N in ureatreated soils.In the fluvo-aquic soil treated with urea,the endpoint mineral N was lower in the DMPP treatments compared with no DMPP treatment,whereas in the red and black soils the endpoint mineral N levels were higher with DMPP than without it.
4.3.Effects of DMPP on N2O emissions induced by digestate and urea
DMPP has been widely tested with diverse soils and fertilizers,but the effectiveness of DMPP in retarding nitrification and N2O emissions has been distinctly variable (Juet al.2011;Naueret al.2018;Fanet al.2019).DMPP mitigates N2O emissions through its dual inhibitory effect on autotrophic nitrification and nitrification coupled denitrification (Zhuet al.2019).In our study,in terms of N sources,the addition of DMPP almost completely inhibited N2O emissions induced by the urea treatment,whereas it inhibited about half of the N2O emissions in the digestate treatment (Fig.2;Appendix C).Compared to mineral fertilizers,the reduced effectiveness of nitrification inhibitors with organic fertilizers was consistent with previous work (Vallejoet al.2006;Pereiraet al.2010).Pereiraet al.(2015) reported that DMPP combined with mineral fertilizers reduced N2O emissions by 29%,while DMPP with organic fertilizers decreased N2O emissions by only 10% in a Cambisol with a pH of 6.1 under aerobic incubation conditions.This difference may be explained by: 1) a partially spatial separation of NH4+and the nitrification inhibitor,considering that the NH4+in microsites continuously released from the mineralization of organic fertilizers could not be permeated by DMPP completely (Di and Cameron 2011;Pereiraet al.2015);2) the promotion of denitrification due to the increased anaerobic micro-environments containing abundant inorganic N and organic C in the digestate treatment,in which the DMPP was ineffective for this part of the denitrification (Comfortet al.1990).However,Hatchet al.(2005) indicated that DMPP may have a greater inhibitory effect on N2O emissions when applied with organic fertilizers than with mineral fertilizers due to the inhibition of nitrification coupled denitrification by precluding a synchronized supply of NO3–(inhibited nitrification) and carbon.
Regarding soil types,DMPP worked most effectively in the fluvo-aquic soil,and then in the red soil and black soil.In the fluvo-aquic soil,these changes were obvious in the nitrification-induced N2O emissions which were almost totally inhibited by DMPP.However,considering the limited inhibitory effects of DMPP in the black and red soils,it was speculated that some non-nitrification processes dominated the N2O production.In the black soil,the high N2O yield might imply that the digestate induced significant heterotrophic denitrification (Cuiet al.2016) (Fig.5).The results in the red soil suggest that slow nitrification took place,with the products being scavenged effectively by two possible sinks: chemical decomposition of nitrite,primarily by reactions with iron(Wanget al.2016;Duanet al.2020),and heterotrophic denitrification.Furthermore,the DMPP had less of an effect on N2O emissions from the red soil compared with black soil.This difference might reflect the effects of soil textures on aggregate state,aeration status and redox potential under the same/certain soil moisture conditions(Chantignyet al.2007;Möller 2015).In addition,the effectiveness of DMPP in retarding N2O emissions was significantly higher than the nitrification rate in all three soils (Fig.6),suggesting a dual inhibitory effect of DMPP on N2O emission by reducing the nitrification rate,as well as the N2O yield of nitrification (Zhuet al.2019).

Fig.6 Comparisons between the inhibitory effects of 3,4-dimethylpyrazole phosphate (DMPP) on cumulative N2O emissions (%) and nitrification rate (%) during the 21-day incubation period in the studied soils.
4.4.Dose-dependent effects of DMPP
There were no significant differences in N2O emissions between the three concentrations of DMPP (0.5–1.5%)in any of the fertilizer sources or soils (Fig.1).The concentration of 0.5% DMPP (of applied N) almost reached the maximum inhibitory effect with both digestate and urea in the three soils (Appendix C).This finding was consistent with the results from a long-term field study (Juet al.2011),the greenhouse vegetable soils (Kouet al.2015) and paddy soils (Yinet al.2017).The application of DMPP at 0.5% of urea-N decreased the N2O emissions almost completely and delayed ammonia oxidation in the greenhouse vegetable soils (Kouet al.2015).Yinet al.(2017) also concluded that 0.5–1% DMPP was the optimal concentration to reduce the N2O emitted from paddy fields.The optimal application rate of DMPP in most soils was basically independent on the soil type,that is,0.5% DMPP achieved almost the maximum inhibition in all three of the studied soils.However,the inhibitory effects of DMPP on N2O-emissions were different for the three soils (48–89% in the fluvo-aquic soilvs.18–52%in the black soilvs.59–73% in the red soil) and for the two nitrogen sources (32–68% in the digestate treatmentvs.18–89% in the urea treatment).The same optimal application rate may be due to the consistency of the hydrothermal conditions (19% gravimetric water content,20°C) in this incubation experiment,and the differences in the inhibitory effects are because the dominant processes differed distinctly between the soils and fertilizers,as discussed above.
5.Conclusion
Our results show a double effect on the N2O emissions from nitrification by reducing the overall nitrification rate as well as the N2O yield in the alkaline soil,suggesting a selective inhibitory effect of DMPP on AOB.The nitrification in acidic soils is marginally affected by DMPP,implying the dominance of AOA in these soils.The results for the acidic soils suggest that heterotrophic denitrification and possibly abiotic reactions (chemodenitrification)can play a role as the sources of N2O in such soils,especially with the fertilization by digestate,affecting both the measured NO3–production and the emission of N2O.Therefore,bio-digestate combined with DMPP has considerable potential for the reduction of N2O emissions.Further studies on the appropriate use of nitrification inhibitors should consider the nitrogen sources,the soil properties,and the underlying dominant N2O production processes.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31861133018,41830751,and 42107320) and the Hainan University Startup Fund,China (KYQD(ZR)-20098).We sincerely thank Prof.Zhang Xiaojun in Shanghai Jiao Tong University,China for providing the digestate.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Less hairy leaf 1,an RNaseH-like protein,regulates trichome formation in rice through auxin
- Characterization of a blaCTX-M-3,blaKPC-2 and blaTEM-1B co-producing lncN plasmid in Escherichia coli of chicken origin
- Consumers’ experiences and preferences for plant-based meat food: Evidence from a choice experiment in four cities of China
- Farmers’ precision pesticide technology adoption and its influencing factors: Evidence from apple production areas in China
- Visual learning graph convolution for multi-grained orange quality grading
- lnfluence of two-stage harvesting on the properties of cold-pressed rapeseed (Brassica napus L.) oils
