Advances in studies on the physiological and molecular regulation of barley tillering
2023-01-06AsadRlAZAhmadALQUDAHFarahKANWALKlausPlLLENYELingzhenDAlFeiZHANGGuoping
Asad RlAZ ,Ahmad M.ALQUDAH ,Farah KANWAL ,Klaus PlLLEN ,YE Ling-zhen ,DAl Fei ,ZHANG Guo-ping
1 Department of Agronomy,College of Agriculture and Biotechnology,Zhejiang University,Zijingang Campus,Hangzhou 310029,P.R.China
2 Department of Agroecology,Aarhus University at Flakkebjerg,4200 Slagelse,Denmark
3 Institute of Agricultural and Nutritional Sciences,Martin-Luther-University Halle-Wittenberg,Betty-Heimann-Straße 3,06120 Halle(Saale),Germany
Abstract Tillering is a crucial trait closely associated with yield potential and environmental adaptation in cereal crops,regulated by the synergy of endogenous (genetic) and exogenous (environmental) factors.The physiological and molecular regulation of tillering has been intensively studied in rice and wheat.However,tillering research on barley is scarce.This review used the recent advances in bioinformatics to map all known and potential barley tiller development genes with their chromosomal genetic and physical positions.Many of them were mapped for the first time.We also discussed tillering regulation at genetic,physiological,and environmental levels.Moreover,we established a novel link between the genetic control of phytohormones and sugars with tillering.We provided evidence of how environmental cues and cropping systems help optimize the tiller number.This comprehensive review enhances the understanding of barley’s physiological and genetic mechanisms controlling tillering and other developmental traits.
Keywords: barley, development,genetic regulation,phytohormone,tillering
1.lntroduction
Barley (Hordeum vulgareL.) ranks as the world’s fourth largest cereal crop in terms of planting area,only after wheat,rice,and maize (http://faostat.fao.org).The Green Revolution,characterized by the introduction of semi-dwarfing genes into cereal crops,results in a dramatic increase in grain yieldviaincreasing tiller and spike number per plant and,simultaneously,the reduction of plant height and lodging risk (Yeet al.2019).Tillering,one of the major agronomic traits in cereal crops,is closely related to grain yield formation and stability (Dockter and Hansson 2015).Tillers are the lateral branches that grow from the main shoot or basal meristem of non-elongated internodes and produce their adventitious roots and spikes during their development(Beveridge and Kyozuka 2010).
The development of tillers is regulated by a complex network of multiple factors involved in genetics,physiology,and environment.Several mutants related to tillering have been identified in cereal crops,such as barley.Specifically,many noded dwarf(mnd) (Babb and Muehlbauer 2003) andhigh number of tillers1(hnt1) (Yeet al.2019) produce more tillers,whileuniculm2(cul2)(Okagakiet al.2013) anduniculme4(cul4) (Tavakolet al.2015) have no tiller.Tillering is also influenced by many environmental factors,including water (Rominaet al.2014),nutrients (Tanaka and Nakano 2019),temperature(Wanget al.2010),and light (Miralles and Richards 2000).Some tillers initiated in cereal crops at the vegetative stage,and only a few could grow into the shoots with spikes.Most tillers stopped development and died before the heading stage,becoming so-called non-productive tillers (Kebromet al.2013).These non-productive tillers compete with effective tillers for resources,such as light,nutrients,and water (Islam and Sedgley 1981).The effective regulation of tiller development is crucial to optimize the number of productive tillers (Xing and Zhang 2010).The proposed ideotype of cereal plants consists of fewer unproductive tillers (Jiaoet al.2010).In wheat,mutanttiller inhibition(tin) produced fewer tillers which could develop into larger spikes under waterlimited conditions (Mitchellet al.2013),revealing that the potential tiller number should be ideal,not too high or very low.The enhanced productivity of some cereal crops,followed by domestication,was complemented by fewer tillers,such as maize and foxtail millet (Doebleyet al.2006;Doust and Kellogg 2006).Particularly,the limitation of tiller development factors may lead to fewer tillers but higher productivity,e.g.,wealthy farmer’s panicle(wfp) in rice (Miuraet al.2010) and overexpression of a cytokinin dehydrogenase gene (AtCKX1) in the transgenic barley which resulted in more tillers but lower yield (Pospíšilováet al.2016).However,a well-balanced number of tillers is the foremost requirement,as unproductive tillers waste nutrients (Jiaoet al.2010).Thus,elucidating the molecular and physiological mechanisms associated with tillering is significant for increasing crop productivity.
Considerable progress has been made in deciphering the genetic basis of tillering development in barley.However,there is a gap in the combined knowledge of genetic improvement,including genetics,genomics,and molecular physiology.It is important to compile and overview all new discoveries and gaps for an efficient breeding program.In the current review,we put steps forward for understanding tiller development and how genetics influence the process either separately or through interaction with other factors.We then discussed the molecular physiology,including hormonal and sugar regulation of tiller development,and highlighted the potential of their manipulation to increase barley yield through regulating tillering development.As the first molecular evidence,the role of sugars in the development of lateral branches in barley was discussed because it acts as an important signal transducer and source of nutrients during tiller development.Moreover,we provided a brief account of the environmental influence of tiller development and its association with barley yield.Finally,we provided a perspective on the applications of recent advances in molecular physiology with functional genomics-based approaches for optimizing tillering and,thus,grain yield.
2.Tiller formation and development
Tillering is determined by the activity of shoot apical meristems (SAMs) and axillary meristems (AXMs).Shoot development occurs continuously throughout a plant’s life in repeated stacked units called phytomers.In barley,a phytomer consists of upper and lower half-nodes separated by a portion of the stem,an internode.Leaf primordia develop on the upper half-node,and axillary buds (AXBs) and root initials on the lower half-node(Forsteret al.2007).Tillering in barley begins during germination,and new AXBs develop adjacent to the internode,covered by the leaf sheath of the previously formed phytomer (Fig.1-①).Usually,two AXBs are already formed in mature embryos,the first being in the axil of the coleoptile (T0 AXB) and the second (T1 AXB) in the axil of the first leaf (Kirby and Appleyard 1981).
Tiller development mainly consists of two phases: i)AXMs initiation and bud formation,as shown in Fig.1-①,and ii) bud growth into tiller (Fig.1-②) (Schmitz and Theres 2005).Barley mutantcul2could not develop its axillary buds,having only one main culm with no-tillers due to losing the ability to initiate or maintain axillary buds(Babb and Muehlbauer 2003).In contrast,an increased number of tillers was observed in thehnt1mutant of barley with accelerated axillary bud initiation (Yeet al.2019).On the other hand,bud growth fate is determined by a complex network of endogenous and environmental factors.The barley mutant oflow number of tillers1(lnt1)produces fewer tillers due to weak axillary bud growth and suppresses the formation of secondary tillers (Dabbertet al.2010).The wheat mutant oftinand the rice mutant ofasp1are also good examples of suppressed bud outgrowth (Domagalska and Leyser 2011;Yoshidaet al.2012).
During the seedling developmental phase,dozens of tiller buds are initiated,while few pass from the first tiller phase (bud initiation and formation) to the second phase of tiller development and growth.The first developed tiller is usually visible when seedlings have three leaves(Okagakiet al.2018),and the initiation of reproductive growth indicates the end of tillering in grasses (Fig.1).Plants can develop primary tillers which arise from axillary buds of the main culm (Fig.1-②-T1),and secondary tillers which arise out of leaf axils of primary tillers(Fig.1-③-T2) (Kirby and Appleyard 1987).Some tillers will develop inflorescences called spikes,while other tillers fail to form spikes,becoming non-productive or vegetative tillers (Jones and Kirby 1977),as shown for T4 in the 5th part of Fig.1.The proportion of tillers that develop into spikes depends on endogenous factors and environmental conditions (Okagakiet al.2018).

Fig.1 Architecture of barley plant with its developmental stages of tillers (Shaaf et al. 2019).①,the first stage of tiller development:bud initiation,which can be seen after removal of the en-sheathing leaf.②,the second stage of tiller development: bud outgrowth into tiller: T1;from leaf axil &T0;from coleoptile.③&④,secondary tillers.⑤,productive tillers,T1 and T2 with grain bearing spikese and non-productive tillers;T0 and T4.T0,the tiller from coloeptile;T1,tiller from the first leaf of the main shoot;T2,tiller from the second leaf of the main shoot;T3,tiller from the third leaf of the main shoot;T4,the secondary tiller from the first tiller.
3.Genetic regulation of tiller development
Tillering is a highly complex trait,and its genetic determinants have been well studied in rice and maize,while the relevant knowledge in barley is relatively limited(Hussienet al.2014).The recent advancements in nextgeneration sequencing offer an excellent opportunity to identify and map causal mutations and genes that can be directly used in barley breeding programs.A number of QTLs involved in the regulation of tillering have been mapped,and some of them have been cloned using a map-based cloning strategy.To illustrate the progress in molecular physiology and genetics regulating tiller development,we listed the known genes and mutants of barley tiller development (Fig.2;Appendix A).The genetic and physical map of tiller developmental genes/mutants will support the understanding of molecular mechanisms underlying tiller development.

Fig.2 Chromosomal map of barley tillering related genes.Upside scale shows the name/number of chromosome,number of genes mapped on each chromosome and the size of chromosome in Mb.The white mark line indicates the physical location and the black bubble with black text is the name of specific gene.
Based on phenotypic observations,tillering mutants of barley could be categorized into four classes: 1)uniculm2(cul2) mutants having only one culm with no developed tillers (Babb and Muehlbauer 2003),2) mutants with fewer tillers,such aslnt1(Dabbertet al.2010),absent lower laterals1(als1) (Agharkaret al.2007),anduniculme4(cul4) (Tavakolet al.2015),3) mutants with moderate tiller numbers,such asintermedium-b(int-b) andsemibrachytic(uzu) (Babb and Muehlbauer 2003),and 4) mutants with higher tiller number,such ashnt1(Yeet al.2019),granum-a(gra-a),many noded dwarf1(mnd1),mnd6andIntermedium-c(Int-c) (Babb and Muehlbauer 2003;Drukaet al.2011),Grassy tillers(Grassy) (Drukaet al.2011),HvD14(Marzecet al.2016),Many noded dwarf1/5(Mnd1/5),Many noded dwarf3(Mnd3) (Franckowiak and Lundqvist 2002),andmany nodeddwarf 4/6 (Mascheret al.2014).Mutations are the primary source of genetic variations used to understand tiller development mechanisms.Therefore,uncovering the molecular genetics and physiology of the desirable and causative mutations have prospects for their utilization in breeding.
Studies have revealed the genetic and morphological characterization of certain tillering mutants.Cul2mutants,exhibiting only the main culm,are described as suppressing axillary buds (Babb and Muehlbauer 2003).Lnt1,cul4,andals1showed fewer tillers because axillary meristems cannot develop into primary tillers (Dabbertet al.2009;Dabbertet al.2010;Tavakolet al.2015).Interestingly,when breeders crossed low tillering barley mutants with high tillering mutants,such asalsorcul2withgra-aormnd1,all hybrid plants produced a low tillering or uniculm phenotype,suggesting epistatic behavior of low tillering to higher tillering mutants (Okagakiet al.2013;Yeet al.2019).Like the rice SL mutants,the barleyHvD14mutant showed a dwarf phenotype with high tillering(Marzecet al.2016).Theuzugene encodes an ortholog ofBRI1inArabidopsisandD61in rice,which is associated with tillering regulation (Babb and Muehlbauer 2003).CUL4encodes a BROAD COMPLEX,TRAMTRACK,BRIC-À-BRAC (BTB)-ankyrin domain-containing protein homologous toArabidopsisBLADE-ON-PETIOLE 1(BOP1) and BOP2,which regulates certain tiller processes,including outgrowth of tillers and development of secondary buds (Babb and Muehlbauer 2003).
VRS1encodes the homeodomain-leucine zipper class I(HD-ZIP1) transcription factor,which pleiotropically regulates tillering in barley by inhibiting bud outgrowth.It is found to be a homolog ofgrassy tillers 1(gt1) in maize (Lilleret al.2015).VRS5(Int-C) encodes a TEOSINTE BRANCHED 1/CYCLOIDEA/PCF1 (TCP)transcription factor,which is the homolog of TB1 in maize,enhancing tiller number at the early development stage but suppressing bud outgrowth at the late development stage (Ramsayet al.2011).VRS4is associated with trehalose-6-phosphate synthase (T6PS) and trehalose-6-phosphate phosphatase (T6PP),which are orthologues of maize RAMOSA2,encoding a LATERAL ORGAN BOUNDARIES (LOB) transcription factor promoting spikelet and floret determinacy (Koppoluet al.2013).This suggests the potential roles of sugar pathways through T6PS and T6PP in determining plant stature developmental traits,including tillering (as discussed below).JUBEL2 encodes a BEL-like homeodomain transcription factor,which is an ortholog ofArabidopsisBELLRINGER (BLR) and the low tillering mutant,low number of tillers 1(lnt1),suggesting correspondence ofLNT1to JuBel2 (Mülleret al.2001).ELI-Aencodes a conserved protein that may be a transposon.Although it has the ability to inhibit thecul2mutant phenotype,the single mutant with strongeli-aalleles produces fewer tillers,typically holding about half as many tillers as wild plants (Chatfieldet al.2000).High tillering phenotypes correspond with a mutation inINT-CandMND.INT-Cis a member ofTB1and an ortholog of the branching inhibitor of maizeTB1,and loss-of-function mutants have a moderately high tillering phenotype (Ramsayet al.2011).MNDencodes a cytochrome P450 in the CYP78A family homologous to ricePLASTOCHRON1(PLA1),andpla1mutants have a similar phenotype tomndones(Le Briset al.1999).Gra-aproduced more axillary buds (Schmitz and Theres 2005) and,similar tomnd1,showed excessive development of tillers and semi-dwarf phenotype (Drukaet al.2011).Comparatively differential expression of the genes in tillering mutants from those in non-mutants revealed that many up-regulated genes in low tillering mutants were involved in stress responses,such as the production of reactive oxygen species and calcium signaling,which are involved in organ development (Agharkaret al.2007;Arendet al.2009;Okagakiet al.2013).Spike morphology in barley also affects tiller number,with two-rowed barley having higher tillers than six-rowed barley (Tucker 1977).In addition,the mutation in the barley row-typeVRS1gene affected the tiller number pleiotropically (Lilleret al.2015).Genetic regulation of tiller development in barley revealed a significant variation in germplasm collection as well as the bi-parental population (Abeledoet al.2004;Borràset al.2009).Taken together,we confirmed that these genes/mutants have a strong potential for improving barley grain yield by optimizing the productive tiller number.The molecular mechanism of the cross-talk between the genes regulating tiller development and spike development is still unexplored.
4.Physiological regulation of tiller development
4.1.Hormonal regulation
At present,auxin,strigolactones (SLs),cytokinins (CTKs),gibberellins (GAs),abscisic acid (ABA),and jasmonic acids (JAs) are reported to play essential roles in tiller development.Usually,the final phenotype of tiller development is a function of the interaction between a number of phytohormones.
AuxinAuxin is an important growth regulator that controls tiller development by regulating AXMs formation(Agusti and Greb 2013).It is actively synthesized in the shoot apex and indirectly suppresses the axillary bud outgrowth indirectly (Agusti and Greb 2013).The auxin transportation downwards from the shoot apex is termed polar transport (PAT).It is determined by auxin efflux carriers of the adenosine triphosphate (ATP)-binding cassette B and the PIN-FORMED (PIN) protein families present in xylem parenchyma (Petrášek and Friml 2009;Zazimalováet al.2010).The mutation affecting auxin transport could result in various abnormalities,such as the inability to establish axillary meristems in inflorescences(Yoshidaet al.2012).Recently,a new mutanthnt1of barley was reported to have more tillers due to accelerated bud formation and initiation.It was suggested that HNT1 might regulate PAT-related genes (Yeet al.2019).It was reported that BA1,a homolog of LAX1,controls AXM formation by regulating auxin transport(Gallavottiet al.2004,2008).BA1 acts downstream and is a direct target of the protein kinase BIF2,a PINOID ortholog important for PAT (Skirpanet al.2008,2009).Arabidopsis max1mutants showed increased axillary branching because of higher expression of PIN1 and auxin transport (Shinoharaet al.2013).For example,mutations in the genes that specify organ boundaries,like CUP-SHAPED COTYLEDON (CUC) genes inArabidopsis,disturb PAT,resulting in reduced branching,loss of leaf serration,and abnormal inflorescences(Vroemenet al.2003;Nikovicset al.2006;Ramanet al.2008;Bilsboroughet al.2011).Vegetative and reproductive axillary meristem formation is also inhibited in three maize mutants with impaired PAT:barren stalk 1(ba1),Barren inflorescence 1(Bif1),andbif2(Mcsteenet al.2007).Several lines of evidence fromArabidopsisand other species suggest that the diverse phenotypes resulting from reduced or ectopic expression of class 1 KNOX genes are attributed to an increased or decreased PAT,respectively (Shinoharaet al.2013).Auxin could down-regulate CTK levels by inhibiting the expression of IPT (ISOPENTENYL TRANSFERASE) genes,resulting in suppressed AXM (Ferguson and Beveridge 2009).The exogenous application of auxin in barley plants inhibits tillering by suppressing bud formation (Woodward and Marshall 1988).However,the molecular mechanism for regulating barley tillering by auxin is still not completely clear.
CytokininsCytokinins are fundamental regulators of plant growth,including axillary bud activation and delay of senescence (Sakakibara 2006).InArabidopsis,supershoot(sps) enhanced shoot propagation and AXM activity with CTK accumulation at bud initiation by suppressing aSPSgene (Tantikanjanaet al.2001).It was also reported that a particularArabidopsisKNOTTEDlike homeobox (KNOX) protein SHOOTMERISTEMLESS(STM) promoted the expression of (IPT7) ISOPENTENYL TRANSFERASE7 (Jasinskiet al.2005) and downregulated gibberellin biosynthesis genes,resulting in low GA and high-CTK contents in the meristem.This may be crucial for maintaining meristematic activity (Jasinskiet al.2005).Transgenic rice plants overexpressing OsIPTs showed enhanced axillary bud activity by CTK overexpression (Sakamotoet al.2006).In barley,theHvCKX1gene regulates the CTK status due to an enhanced cytokinin dehydrogenase activity that degrades CTK.HvCKX1knock-out mutants produced more tillers and grains than wild plants (Holubováet al.2018),suggesting that the exploitation of CTK-regulating genes in barley may be profitable for optimizing the number of productive tillers.
StrigolactonesSLs are recently reported to function as growth regulators by inhibiting AXB internode elongation(Gomez-Roldanet al.2008).SLs may also induce the expression of transcription factors orthologous to maizeTB1,riceFINE CULM1(FC1),andArabidopsis BRANCHED1,which act downstream of strigolactones to inhibit internode elongation (Minakuchiet al.2010).Five barley genes,HvD14,HvD27,HvMAX1,HvCCD7,andHvCCD8,are orthologous to genes in rice andArabidopsisand involved in SLs’ function of inhibiting tiller development (Wanget al.2018).InArabidopsis,SLs are synthesized in the roots upon expression ofMAXgenes and then transported to AXB (Gomez-Roldanet al.2008).SLs interact with auxin in a dual-loop pathway to control axillary bud outgrowth,but the nature of this regulatory loop is still unresolved (Kebrom and Richards 2013).Arabidopsis max1mutants have increased axillary branching associated with overexpression ofPIN1and enhanced auxin transport (Shinoharaet al.2013).The increased branching phenotype depends on the overexpression ofPIN1,asmax1/pin1double mutants exhibit fewer lateral branches (Bennettet al.2006).InArabidopsis,max1andhigh tillering dwarf(htd) in rice showed enhanced shoot branching regulated by SLs(Zouet al.2006).Future research should focus on the interactions of SLs and auxin to better understand the role of SLs’ crosstalk with other phytohormones in affecting tiller development.
Gibberellins (GAs)GA has been reported to play a role in internode elongation in grasses.Bioactive GA is deactivated by an enzyme encoded by theGA2ox1gene.The deactivation of GA probably prevents GA from reaching nodes under the shoot apex and results in the inhibition of internode elongation (Sakamotoet al.2001).It was observed that overexpression ofGA2oxproduced more tillers,suggesting that the increase in bioactive GA and lessGA2ox1activity inhibits tillering and permits internode elongation in rice (Loet al.2008).More tillers were observed in the GA-responsive mutant of turfgrass(Agharkaret al.2007).In rice,MOC1(MONOCULM1),a tillering regulator,is guarded by belting with the DELLA protein ofSLR1(SLENDER RICE1).GA has a role in the degradation ofSLR1,resulting in stem elongation and reduced tiller number (Liaoet al.2019).In barley,theLNT1(LOW NUMBER OF TILLERS1) gene encodesJUBEL2,which is a homolog to the KNOX protein regulatingGA2ox,as reported in maize (Dabbertet al.2009).The expression ofGA2ox1in thelnt1barley mutant should be investigated to elucidate the regulation of tiller development by GAs.
Abscisic acid (ABA)Previous studies indicated that ABA inhibits bud outgrowth,as shown after exogenous ABA treatment inArabidopsis(Chatfieldet al.2000).The possibility that ABA may control tiller development has been widely explored.Functional connections exist between the biosynthetic pathways of ABA and other phytohormones,such as SLs.The effect of this connection on tillering has been proved in barley (Wanget al.2018).In barley,two transgenic lines accumulating ABA as a result of RNAi-mediated down-regulation of HvABA 8´-hydroxylase 1 and 3 were developed.LC-MS/MS analysis confirmed higher ABA levels in stem base tissues in these transgenic lines.Both lines showed enhanced tiller formation and lower expression levels of HvD27,HvMAX1,HvCCD7,and HvCCD8,indicating that ABA suppresses SL biosynthesis,leading to enhanced tiller formation (Wanget al.2018).Recently,a study has also shown that ABA-related gene expression increased in bud-containing tissues ofArabidopsisunder low red light to far-red light exposure,which led to a general reduction in branch number.This study suggests that a high red light to far-red light exposure could inhibit the ABA effect(González-Grandíoet al.2013).Endogenous ABA exerts a direct effect on regulating axillary bud outgrowth in intactArabidopsis,acting as a general inhibitor (Yao and Finlayson 2015).The biosynthetic pathways of ABA and SLs appear to be connected,but so far,the mechanism has not been well explored.
Jasmonic acid (JA)JA is one of the emerging endogenous growth regulators affecting many developmental processes in plants.It was previously known as a growth inhibitor but is now identified as a signal transducer related to stress responses.For example,JA may affect nutrient uptake and sugar transportation,leading to stress tolerance (Ruanet al.2019).The very first investigation of JA’s role in tillering in grass species was reported in sorghum,where it was shown that exogenous application of JA promoted in situ bud growth (Liu and Finlayson 2019).The ERF109 transcription factor regulates auxin transport-related genes (Xuet al.2020),and the sorghum homolog of ERF109 associated with JA GO terms was strongly induced by leaf removal (Liu and Finlayson 2019).Based on the results obtained in a study on sorghum,there is a possibility that JA application on buds induces ERF109,resulting in overexpression of the auxin transport-related genes,thus leading to bud growth acceleration (Liu and Finlayson 2019).It would be interesting to investigate the JA-auxin interaction at the molecular level to elucidate JA’s role in bud regulation related to tiller development.
4.2.Cross-talk of phytohormones in regulating tiller development
It is well-documented that phytohormones interact in a complex network to regulate tiller formation.The individual and the cumulative effect of different interacting phytohormones on tillering were shown in Fig.3.For phytohormonal cross-talk in tiller development,the major and direct roles are played by CTKs and auxin.CTKs promote tillering by boosting the auxin transport that enhances the bud outgrowth,while auxin inhibits CTKs,which lowers or stops the auxin transport,resulting in auxin accumulation and bud dormancy.The other phytohormones play indirect roles in regulating tiller development by inhibiting or promoting auxin transporters(or other phytohormones),resulting in low or high tillering phenotypes,respectively.SLs and JAs are the most recently identified phytohormones playing their roles in shoot branching.Previous studies revealed that SLs inhibited bud outgrowth by blocking the auxin transport,while JA had an inhibitory effect on SLs and promoted the auxin transport,resulting in enhanced tiller development.Likewise,ABA and GAs inhibit the SLs,which indirectly stimulates bud outgrowth.
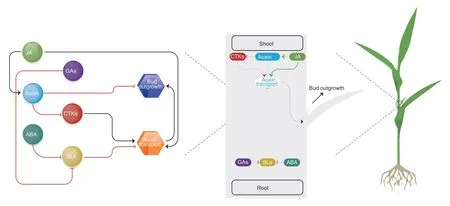
Fig.3 Summary of hormonal cross-talk and their relationship in regulation of tillering.The arrow-shaped lines indicate the promoting effect and T-shaped arrows indicate the inhibitory effect of phytohormones.SLs,strigolactones;CTKs,cytokinins;GAs,gibberellins;ABA,abscisic acid;JA,jasmonic acid.
4.3.Regulation of tiller development by sugars
In addition to their metabolic roles,sugars can also act as a mediator in many important developmental processes of plants.Sugars are an energy provider and resource of carbon for protein synthesis.Sucrose functions as a signal to control growth and differentiation with its related products,glucose and fructose (Ruan 2012;Lunnet al.2014).Very few studies discussed the role of sugars in tiller development from a developmental and physiological viewpoint or showed the genetic regulation of sugar content and composition and its role in tiller development in cereals.This section emphasizes the role of sugars in regulating tiller development in barley.
In shoot branching,sugar is crucial for enhancing bud outgrowth.During the development of lateral branches,sugars seem to play a signaling role,notable through trehalose 6-phosphate,interacting with phytohormones.In wheat,the tin mutant showed a lower tiller number with low sucrose levels in the inhibited buds due to the downregulation of sucrose inducible genes (Kebromet al.2012).Likewise,the defoliation causing bud inhibition was found to be associated with the up-regulation of sucrose starvation and down-regulation of sucroseinducing genes in dormant buds of sorghum (Kebrom and Mullet 2015),suggesting that the outgrowth may be dependent on the overall status of plant sugar.Recently,the molecular mechanism of regulation of shoot branching by sucrose has been reported inArabidopsisand rice(Zafaret al.2020;Fichtneret al.2021).In barley,some reports shed light on the role of sugar-related genes,speciallyHEXOKINASE(HXK),SUCROSE TRANSPORTER(SUC),and trehalose 6-phosphate(Tre6P) for regulating shoot branching (Barbieret al.2021;Fichtneret al.2021).Three sugar-related QTLs(HvSUT1,HvHXK9,andHvHXK6) associated withHEXOKINASEandSUCROSE TRANSPORTERgenes were found to be related to the regulation of tillering in barley (Alqudahet al.2016).Although the role of sugar in the regulation of shoot branching has been highlighted in very recent reports to our knowledge,no prior study has been conducted in barley to explore the role of sugars and the molecular mechanisms in the regulation of shoot branching (Fichtner and Lunn 2021).Therefore,we mapped around 20 sugar-related genes in barley for the first time.Among them,HEXOKINASE-and Tre6Prelated genes are strongly supported by recent studies(Barbieret al.2021),ultimately offering an opportunity to understand their molecular basis.These findings raise the importance of sugar-related genes in affecting most plastic traits,including tillering in grasses.Thus,it can be concluded that further investigations should be conducted at the molecular level on the regulation of tillers by sugars.
5.Regulation of tiller development by the environment-responding genes and agronomic factors
Tillering is also influenced by many environmental factors,including temperature,photoperiod,water,and nutrient availability (Skinner and Simmons 1993;Miralles and Richards 2000;Rominaet al.2014).With respect to global warming,changing phonological properties may be an efficient method for planting cereal crops,especially winter barley and wheat.Tillering was inhibited by high temperature,substantial vernalization,and less photoperiod sensitivity (Wanget al.2010).Tiller production in barley was shown to be significantly affected by the genes in response to the environment,such as vernalization genesVERNALIZATION-H1(Vrn-H1) andVrn-H2and the photoperiod response genePpd-H1(Von Korffet al.2006;Wanget al.2010).Photoperiod affects tiller number by altering the duration of the vegetative growth.Barley genotypes carrying the photoperiod-sensitivePpd-H1allele had high expression levels ofVrn-H3(Campoliet al.2012).Alqudahet al.(2016) reported that the barley accessions carrying the reduced photoperiod sensitivity (Ppd-H1) allele produced much more productive and non-productive tillers per plant than the accessions sensitive to photoperiod.In addition,the genes associated with barley flowering time,includingPpd-H1,Sdw1,Vrn-H1,andVrn-H3exerted pleiotropic effects on plant development,including tillering (Wiegmannet al.2019).These pleiotropic effects were found to be strongly regulated by the response to environmental factors,such as day length and temperature (Herziget al.2018;Wiegmannet al.2019).A number of quantitative trait loci (QTLs) that control tillering have been described in wheat (Naruokaet al.2011;Yanget al.2013;Xieet al.2016).In a study on the tillering traits,a number of QTLs were identified under the short day and artificially vernalized conditions,where the Ppd genes were active,and the QTLs were located on chromosomes 6B (QTLs 45,48) and 4B(QTL 29),respectively (Giuntaet al.2018).Floweringassociated genes may influence wheat tiller number.Overexpression ofTaZIM-A1caused delayed heading and increased effective tiller number by regulating TaFT and VERNALIZATION1 (VRN1) expression (Liuet al.2019).The photoperiod-sensitivity genePpd-1influences tiller number in wheat (Dycket al.2004).The later-headingvrn-A1allele was associated with more tillers per plant in a wheat cultivar Cappelle-Desprez (Katoet al.2000).
It may be suggested that the wild barley germplasm can improve plant development to boost grain yield.Several agronomic factors or agricultural management practices also influence tillering and productive tillers.For example,the cropping pattern can affect tillering,as cereal crops deplete nutrients from the soil,lowering the number of productive tillers in the next crop.There is a higher number of productive wheat tillers in a wheat–rice cropping pattern compared to a wheat–sunflower cropping pattern (Nawabet al.2011).Cereal crops are exhaustive compared to legumes that facilitate the soil with nitrogen (N) and phosphorus (P) (Stagnariet al.2017).In addition,a higher barley yield was observed when grown in a crop rotation,including legumes,rather than in a continuous barley crop sequence (Jones and Singh 2000).Water availability also regulates tillering,as water-limited conditions result in fewer tiller formations(Chaturvediet al.1981).Improvements in soil moisture through mulch application reduced the number of nonproductive tillers and increased rice yield (Jabranet al.2015).Excessive evaporation resulting in drought stress reduces the number of productive tillers in wheat and maize (Balwinder-Singhet al.2016;Zhanget al.2017).Nutrient management is also an important factor affecting tillering (Bakhtet al.2010).Although N and P are essential nutrients with regard to tillering in grasses,potassium (K) also plays a role in tiller regulation,such as increasing the tiller number in rice (Bahmaniar and Ranjbar 2007).Planting density or seeding rate also influences the morphology and number of tillers,with an increased number of non-productive tillers at higher planting densities in barley (Kirby and Faris 1972).Similarly,a low tiller number was reported with dense seeding of spring wheat (Ottesonet al.2008).In short,the influence of environmental and agronomic factors on tillering and productive tillers have been intensively studied,but the precise roles of all these factors are still not fully described.It is suggested that some integrated experiments be conducted to understand the mechanisms of these factors toward the regulation of tiller initiation and development.
6.Conclusion and perspective
Tillering is a major yield determinant in cereal crops and is controlled by different endogenous and environmental factors.The misregulation of developmental genes may affect various physiological processes,resulting in higher or lower tiller numbers.Tiller development involves bud initiation and outgrowth.The bud initiation is severely inhibited in theuniculm2mutant with zero tillers.The mutants related to bud outgrowth are mainly determined bylnt1,als1,andcul4;the candidate gene ofals1has not yet been identified.LNT1 encodes a JuBel2 homeodomain transcription factor,andcul4encodes BOP-like BTB-ankyrin protein,which plays crucial roles in a weak bud outgrowth of tillers.The mutantsgra-a,grassy,mnd1,mnd3,mnd5,aps1,andint-menhance tiller production,whereascst1,int-b,andcul2inhibit tillering in barley.
However,the candidate genes for these mutants are yet to be identified.Some candidate genes of mutants that increase tiller production have been identified,e.g.,HNT1,INT-C,HvD14,MND6,andUZU1,which encode proteins to regulate tillering in barley.SDW1 regulates the biosynthesis of GA phytohormone and enhances tiller production.Few genetic studies have targeted and functionally characterized tillering genes in barley.Further investigations and functional validation of tillering genes are necessary for exploring more-nat ural tilleringenhancing alleles for crop breeding.Here,we provide an essential foundation for uncovering the biological functions of sugar-related genes,suggesting that sugarregulated genes potentially regulate tillering in barley and,most likely,other temperate cereals such as wheat.Targeting such genes by genome editing approaches such as an efficientCRISPR/Cas9system to produce heritable and desirable alleles is expected to accelerate crop breeding significantly.Abiotic stresses,such as extreme temperature,drought,and phosphorus depletion,inhibit the growth of lateral branches in barley,while nitrogen availability and photoperiod insensitivity promote barley tillering.Breeders prefer to select the lines with low non-productive tillering and high-productive tillers(carrying spikes) to increase grain yield.It is also critical to explore the tillering-enhancing alleles to maximize grain yield.A deep understanding of tillering as affected by genetic and agronomic factors will broaden our knowledge of biological processes and may allow breeders to better control and optimize tillering.Future studies should focus on the regulatory roles of phytohormones and their molecular interaction to define tillering and yield formation in barley and other cereal crops.
Acknowledgements
This study is funded by the National Key R&D Program of China (2018YFD1000706),the Key Research Project of Science and Technology Department of Zhejiang Province,China (2021C02064-3),the Jiangsu Collaborative Innovation Center for Modern Crop Production,China.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Less hairy leaf 1,an RNaseH-like protein,regulates trichome formation in rice through auxin
- Characterization of a blaCTX-M-3,blaKPC-2 and blaTEM-1B co-producing lncN plasmid in Escherichia coli of chicken origin
- Consumers’ experiences and preferences for plant-based meat food: Evidence from a choice experiment in four cities of China
- Farmers’ precision pesticide technology adoption and its influencing factors: Evidence from apple production areas in China
- Visual learning graph convolution for multi-grained orange quality grading
- lnfluence of two-stage harvesting on the properties of cold-pressed rapeseed (Brassica napus L.) oils
