Role of microRNA-regulated cancer stem cells in recurrent hepatocellular carcinoma
2023-01-03LeiLiChenXunChunHongYu
Lei Li, Chen Xun, Chun-Hong Yu
Abstract Among the most common cancers, hepatocellular carcinoma (HCC) has a high rate of tumor recurrence, tumor dormancy, and drug resistance after initial successful chemotherapy or radiotherapy.A small subset of cancer cells, cancer stem cells (CSCs), exhibit stem cell characteristics and are present in various cancers, including HCC.The dysregulation of microRNAs (miRNAs) often accompanies the occurrence and development of HCC.miRNAs can influence tumorigenesis, progression, recurrence, and drug resistance by regulating CSCs properties, which supports their clinical utility in managing and treating HCC.This review summarizes the regulatory effects of miRNAs on CSCs in HCC with a special focus on their impact on HCC recurrence.
Key Words: Hepatocellular carcinoma; Cancer stem cells; MicroRNAs; Recurrence.
lNTRODUCTlON
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers in the liver, accounting for about 75% of all liver cancers, with a poor clinical prognosis, resulting in 500000-600000 deaths each year[1-4].In recent years, there has been substantial progress in the diagnosis and treatment of HCC, but the high recurrence and metastasis rates of HCC still pose a headache for doctors and patients.The proposal of cancer stem cell (CSC) theory provides us with a direction.CSCs are considered one of the very small cell types in tumor cells with unlimited proliferative potential, which can drive tumorigenesis, and development.They can confer unique drug resistance, recurrence, and metastasis capabilities to tumors[5-8].Conventional cancer treatments only kill common cancer cells, but CSCs remain.When in the right microenvironment, CSCs begin to proliferate and differentiate, leading to cancer recurrence.In recent years, many studies have focused on liver cancer stem cells (LCSCs) and achieved satisfactory results.Therefore, targeting CSCs is considered a more promising approach to improving the outcomes of conventional treatments (Figure 1).
An example of a microRNA (miRNA) is a small, non-coding RNA that is produced by endogenous cells and can be used to regulate gene expression by binding to the 3' untranslated region (UTR) of genes to inhibit their translation[9,10].It has been shown that miRNAs can regulate tumorigenesis, progression, invasion, and even tumor recurrence in HCC by acting as tumor promoters or suppressors[11,12].Another important finding is that miRNAs can modulate the stemness profile of LCSCs to combat conventional therapy further.Pollutriet al[13] reported that miR-494 induces sorafenib resistance in HCC and is associated with stem cell phenotypes.Further research has demonstrated that miR-181 family members play a critical role in maintaining the stem cell characteristics of HCC cells in a study by Aiet al[14].Therefore, we believe miRNAs play a key role in LCSCs, and understanding this information will help our further research and development of HCC therapies.This review summarizes recent years' research findings and reports, outlines the role of miRNAs in LCSCs, and discusses potential therapeutic strategies for HCC recurrence, intending to provide clinical practitioners with information about how to treat HCC patients effectively.
SURFACE MARKERS OF LCSCS AND THElR ROLE lN HCC
A number of characteristics of LCSCs are similar to those of normal stem cells, including their ability to self-renew and differentiate.LCSCs are more prevalentin vivothan other tumor cell types.They can promote the growth of primary cancer cells and facilitate the metastasis of transplanted secondary tumors, and they are crucial in the recurrence of HCC.In order to identify and isolate CSCs effectively, it is mostly necessary to take advantage of surface markers.Common LCSCs are CD133, CD90, CD44, CD13, CD47,etc.During the past few decades, a growing body of evidence has been generated concerning the properties of specific surface markers on LCSCs, which has provided opportunities for investigating potential biological functions, signaling pathways, and therapeutic approaches for HCC (Figure 2).Table 1 summarizes the major surface molecular markers of LCSCs and their potential roles in HCC.
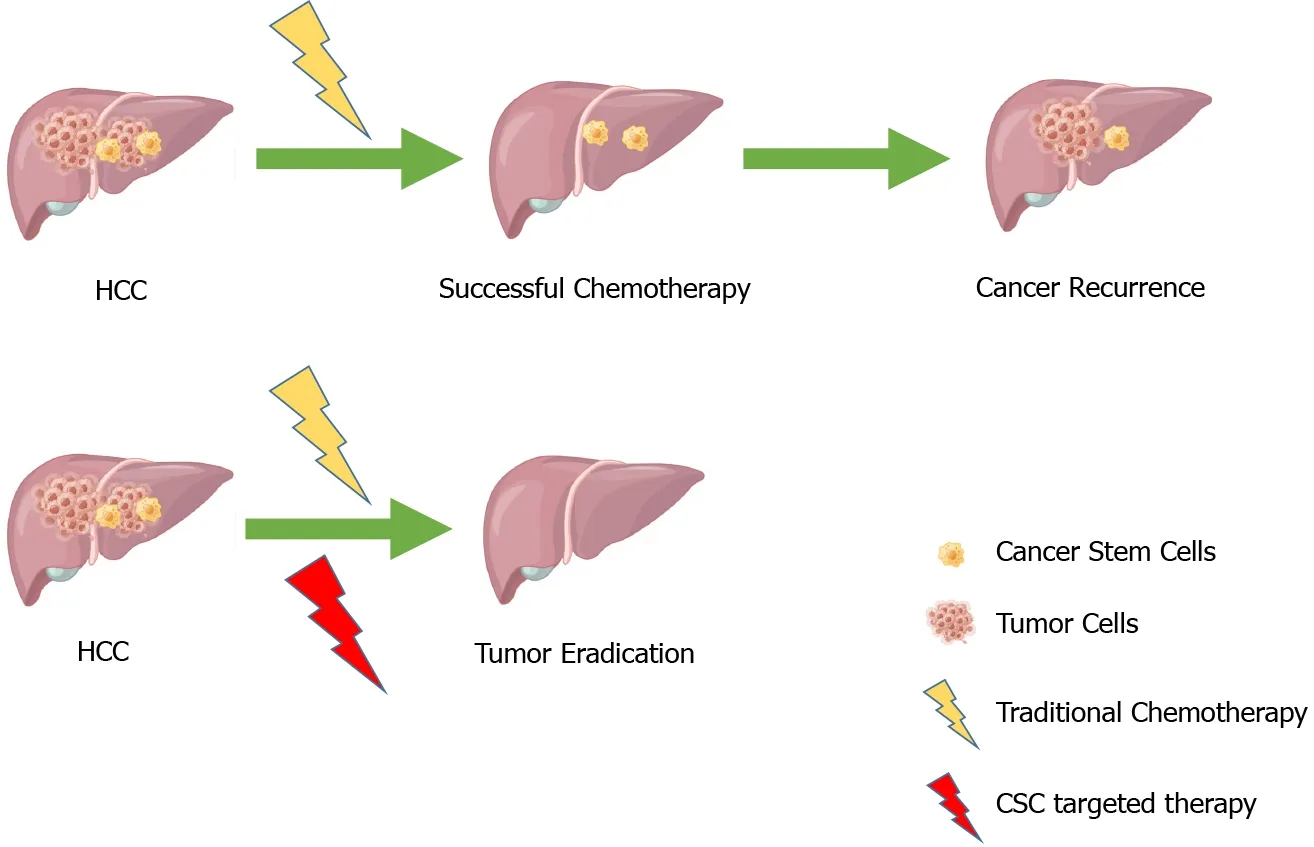
Figure 1 Combination therapy for hepatocellular carcinoma.Top: Conventional treatment may lead to tumour recurrence due to cancer stem cell reactivation.Bottom: Combination therapy leads to increased efficacy of tumour eradication.Adapted from Dzobo et al[8].HCC: Hepatocellular carcinoma; CSC: Cancer stem cell.

Figure 2 Liver cancer stem cells markers and their potential related functional pathways in hepatocellular carcinoma.HCC: Hepatocellular carcinoma; LCSCs: Liver cancer stem cells.

Table 1 Hepatic cancer stem cell markers and their roles in hepatocellular carcinoma recurrence
CD133
In 1997, CD133 was discovered as the first protein on the surface of neuroepithelial stem cells[15].A transmembrane glycoprotein consisting of five transmembrane domains, two extracellular glycosylation chains, and three transmembrane domains is an important surface glycoprotein that serves as a cell surface marker.CD133 is expressed in embryonic epithelial stem cells, colon cancer, prostate cancer, pancreatic cancer, brain tumor, HCC, hematopoietic stem cells, and the like.CD133 was identified as a liver CSC marker in 2007[16-18].According to studies conducted by our laboratory, the expression of CD133 in HCC cells is negatively related to the overall survival rate of patients with HCC and the rate of recurrence[19].HCC patients with higher CD133 expression levels in the primary lesion tend to live shorter and have a higher recurrence rate postoperatively than those with lower CD133 expression levels[20].HCC patients with higher CD133 expression levels also responded poorly to the conventional chemotherapy drug sorafenib.Several molecular mechanisms have been involved in the action of CD133 on tumors, including angiogenesis, self-renewal, growth, invasion, and chemoresistance.CD133+cells in HCC contribute to chemoresistance by preferentially activating the Akt/PKB and Bcl-2 cell survival receptors during the chemoresistance response[21].As a result of the interaction between neurotensin and interleukin-8 and CXCL1 signals in the liver, CD133 controls tumorigenesis, growth, and self-renewal of liver tumor-initiating cells[22].The expression of iNOS in CD24+CD133+LCSCs, but not CD24-CD133-LCSCs, enhanced Notch1 signaling, and accelerated HCC initiation in the mouse xenograft tumor model[23].
CD90
CD90+cells from HCC Cell Lines were reported to have higher tumorigenic and metastatic potential than CD90-cells in 2008, suggesting that CD90+cells can be used as a marker of metastatic HCC[24,25].Consistent with these findings, CD90 expression is positively correlated with HCC progression and poor prognosis[26-28].CD90 is involved in varies molecular mechanisms, including inflammation, circulation, drug resistance, and lipid metabolism.In HCC 97H cells, the cyclin D1-mediated activation of Smad2/3 and Smad4 is an important regulatory mechanism in enhancing single sphere formation, enhancing the CD90+population, increasing stemness gene expression, and increasing chemoresistance[29].Therefore, CD90 may also be a surface marker for poor prognosis of HCC and a potential therapeutic target.
CD44
A transmembrane glycoprotein named CD44 has been found to be expressed on numerous cells, including hepatocytes, endothelial cells, lymphocytes, and mesenchymal stem cells.It plays a role in extensive proliferation, self-renewal, invasion, and tumorigenicity[30].It is possible to isolate cancer cells with stem cell markers by using CD44 alone or in combination with other markers.CD44v6, a variant of CD44, participates in the proliferation of HCC cells by stimulating the Ras/MAPK signaling cascade through interaction with c-Met[31].Several studies have indicated that CD44s are associated with poor prognoses in hepatocellular carcinoma patients and regulate the TGF-β-mediated mesenchymal phenotype[32].TGF-β1 and CD44 are synergistic in that they contribute to epithelial mesenchymal transition (EMT) induction and the development of CSC properties in tumor cells by interactingviathe AKT/GSK-3β/β-catenin pathway in HCC cells[33].In addition, CD44 is known to enhance HCC migration and local metastases by triggering the AKT/ERK pathwayviathe CXCR4 receptor[34].Therefore, CD44 may be a potential treatment target for HCC and a marker of poor prognosis for HCC[35,36].
CD24
It is known that CD24 is a glycoprotein that is expressed on the surface of stem cells, mature granulocytes, and B cells, as well as in malignant tumors, such as HCC, breast cancer, colon cancer, and small cell lung carcinoma[37,38].As well as driving CSC development, CD24 is involved in the differentiation of progenitor and stem cells in the liver and in metastatic development, self-renewal, and chemotherapy resistance of HCC cells[39].CD24+liver tumor-initiating cells are driven to self-renew and initiate tumorsviaSTAT3-mediated NANOG signaling[40].An IL-6/STAT3 axis regulates CD24 and epithelial cell adhesion molecule (EpCAM) expression in liver cancer stem cells through long noncoding RNA DILC[41].
CD13
A membranous glycoprotein called CD13 is associated with the progression of cancer and drug resistance.Cell cycle, self-renewal, and tumorigenicity are all regulated by CD13, which is involved in tumorigenesis, cell proliferation, and chemoresistance[42].The combination of CD13 with other surface markers could lead to prostate cancer tumorigenesis.The CD13 gene is expressed in LCSCs that are slow-growing or semi-quiescent, which contributes to the formation of HCC tumors[43].Quiescent CD13+CSCs accumulate after chemotherapy in HCCs, serving as a source of recurrence[44].
CD47
CD47 is a transmembrane member of immunoglobulin associated with immune evasion, tumor apoptosis, metastasis, tumor-initiating ability, chemoresistance, and proliferation in various cancers.In addition to tumor initiation and self-renewal, CD47 also plays an important role in metastasis in HCC.HCC growth can be inhibited by suppression of CD47, which inhibits CTSS/PAR2 signalingin vivoand causes chemosensitization[45].There is a positive correlation between CD47 and NF-κB expression in HCC samples from clinical trials[46].Patients with HCC with upregulated CD47 expression had poor overall survival and recurrence-free survival, and IL-6 derived from macrophages infiltrating the tumor was shown to activate STAT3 and upregulate CD47 expression on hepatoma cells[47].
OV6
OV6, a monoclonal antibody raised against hepatic progenitor cells isolated from rat livers treated with carcinogens, was shown to be a marker for such cells.An HCC cell line expressing OV6+tumorinitiating cells has a greater potential for invasiveness and metastatic spread, bothin vitroandin vivo, which promotes the metastasis and progression of HCC[48].There was an association between higher levels of OV6+tumor cells, aggressive clinicopathologic features, and a poor prognosis.Inhibition of βcatenin signaling leads to a decrease in the proportion of OV6+cells in HCC cell lines and primary HCC tissues, which indicates the role of Wnt/β-catenin signaling in OV6+HCC cells[49].
EpCAM
As another transmembrane glycoprotein found in most epithelial tissues, the EpCAM plays a role in signal transduction, cell adhesion, migration, proliferation, and differentiation[50-54].EpCAM was discovered as a biomarker early in the diagnosis of HCC.A strong correlation was found between EpCAM expression in LCSCs and differentiation, chemoresistance, high invasion, and tumorigenesis in HCC.EpCAM is a target gene for Wnt-beta-catenin signaling that may help improve HCC prognosis.
MlRNAS lN HEPATOCELLULAR CARClNOMA
Dysregulated miRNAs contribute to many critical processes in HCC, ranging from growth, proliferation, apoptosis, and differentiation to migration, invasion, and progress.Moreover, miRNAs are important in tumor recurrence and metastasis.Understanding miRNAs' biological roles and specific targets will help further research and development of HCC therapies.Table 2 summarizes the major miRNAs in HCC and their potential roles in HCC.

Table 2 The regulatory roles of miRNAs in hepatocellular carcinoma
The upregulated miRNAs in HCC
Cells from HCC cell lines and patients express high levels of miR-21.There is a positive correlation between miR-21 expression and HCC migration and invasion.As a result of silencing miR-21, the protein levels of PTEN, RECK, PDCD4, and KLF5, as well as the protein and mRNA levels of KLF5, increase, leading to a reduction in HCC cell migration and invasion[55,56].Hepatocellular carcinoma growth is promoted by exosomal miR-21 regulation of the TETs/PTENp1/PTEN pathway, and three novels predicted miR-21 targets (CAMSAP1, DDX1, and MARCKSL1) correlate with HCC patient survival[57,58].There is an association between miR-130b-3p up-regulation in HCC and a poor prognosis[59].Patients who undergo HCC resection are at an increased risk of recurrence if their miR-135a expression is high[60].A direct target of TP53INP1 is MiR-155, which regulates the migration and invasion of liver cancer cells, EMT, and CSC acquisition (which is positively correlated with CD90 and CD133)[61,62].Patients with HCC who express MiR-182-5p in tumor tissues are more likely to experience poor prognosis and recurrence of the disease at an earlier stage.miR-182-5p activates AKT/FOXO3a pathway and Wnt/β-catenin signaling by targeting FOXO3a, enhancing HCC proliferation, motility, and invasion bothin vitroandin vivo[63].As miR-221 targets PTEN and TIMP3 tumor suppressors through activation of the AKT pathway, liver cancer cells express high levels of miR-221[64].Upon Fas-induced fulminant liver failure, miR-221 is upregulated, which regulates liver expression of the p53 upregulated modulator of apoptosis[65].
The downregulated miRNAs in HCC
Several miRNAs like miR-9-3p, miR-26, miR-30a, miR-122, miR-125b, miR-142, miR-142-3p, miR-199b-5p, miR-200a, miR-203, miR-449a, and miR-541 showed lower levels in HCC than in healthy donors.HBGF-5 expression is significantly downregulated by miR-9-3p overexpression, HCC viability and proliferation are reduced, and ERK1/2 is strongly downregulated[66].Apoptosis is promoted by MiR-26 by targeting ULK1, EphA2, TAK1, and TAB3, which enhance chemosensitivity and radiosensitivity in HCC cells[67-69].MiR-30a inhibits HCC cell proliferation by targeting FOXA1viathe Ras/ Raf/MEK/ERK signaling pathway, suppressing autophagy-mediated resistance and metastasis[70-72].It facilitates tumor cell invasion, migration, and EMT when miR-30a is downregulated[73].By downregulating miR-122, HCC cells proliferate, colonize, migrate, invade, metastasize, and activate IGF-1R and RAS/RAF/ERK pathways[74-77].When miR-122 expression levels are elevated in HCC cells, it inhibits the EMT process by upregulating the expression of E-cadherin and downregulating ZEB1/2, Snail1/2, N-cadherin, and Vimentin[78].miR-125b is correlated with cell proliferation, differentiation, metastasis, apoptosis migration, and EMT[79-81].miR-125b overexpression attenuates EMT-associated chemores-istance, migration, and stemness and negatively correlated with CSC marker, EpCAM and CD13 expressions in HCC specimens by targeting SMAD2 and SMAD4[82].Increasing the amount of miR-142 in the cells results in a decrease in vitality, proliferation, and EMT outcomes, as well as an increase in THBS4 which is overexpressed by cancer cells, resulting in more rapid migration and vascular invasion[83,84].As a result of miR-142-3p inhibiting self-renewal, initiating tumor growth, invasion, migration, inducing angiogenesis and resisting chemotherapy in HCC cells, miR-142-3p is directly targeting CD133 to control the ability to confer cancer and stem cell-like characteristics[85].It was found that overexpression of miR-19b-5p increases cell aggregation, suppresses migration and invasion in HCC cells, and inhibits the metastasis of xenograft tumors in nude mice.Akt phosphorylation is inhibited by miR-199b-5p overexpression, and N-cadherin and DDR1 are directly targeted and inhibited by miR-199b-5p overexpression[86,87].In HCC, microRNA-200a directly targets GAB1 and FOXA2 to suppress cell invasion, migration, and metastasis[88,89].MiR-203 expression is significantly associated with tumor recurrence and poor survival in HCC patients with early-stage tumors.In contrast, miR-203 overexpression suppresses Ki67 and CAPNS1 expression to inhibit proliferation, invasion, and metastasis of hepatic residual HCC[90,91].Activating EMTviathe Notch pathway promotes invasivenessin vitroby
downregulating Calpain 6 and POU2F1; mir-449a induces apoptosis in liver cancer cells by downregulating Calpain 6 and POU2F1, it inhibits Met signaling and Snail accumulation in cells by targeting its 3'-UTR, and miR-449a contributes to short-term HCC recurrence[92-94].HCC cellsin vitroandin vivoare inhibited by miR-541 by inhibiting growth, metastasis, and autophagy, and the target genes are ATG2A and RAB1B[95].
Therapeutic potential of miRNAs targeting CSC
It has been demonstrated that miRNAs could be therapeutic targets for HCC, but miRNA-based therapies have not been well developed for clinical applications.CSC therapies targeting miRNA are considered to be one of the most promising cancer treatments.In this way, miRNAs can regulate multiple genes at once, contributing to the regulation of CSC-related pathways.For example, miR-365 can regulate LCSCs through the RAC1 pathway[96]; miR-520f-3p is involved in altering the sensitivity of HCC cells to sorafenib treatment under hypoxic conditions by increasing stem cell phenotype[97]; miR-4320 inhibited epithelial-mesenchymal transition and reduced stemness characteristics in HCC cells by targeting FOXQ1 expression[98]; miR-206 inhibited LCSCs expansion by regulating EGFR expression[99]; Liet al[100] found that miR-613 inhibits LCSC proliferation and differentiation through regulation of SOX9; therapeutic delivery of miR-125b in a mouse model reduces the expression of CSC markers and inhibits HCC metastasis[82].The findings of these studies suggest that miRNA therapy combined with targeting CSCs can treat HCC.However, the development of miRNA therapy remains challenging.The development of miRNA delivery systemsin vivohas always been an area of interest for clinical treatment research.A specific, stable, low toxicity and durable delivery system is our hope, but currently, in the clinical treatment of HCC, there is still no very suitablein vivodelivery system.Furthermore, CSCs have great heterogeneity between patients, and how to accurately target CSCs is also a problem that needs to be addressed further.
CONCLUSlON
In recent years, although research focusing on CSC has entered a trend of rapid growth, there are still many problems to be solved in clinical translation and practical application, especially in HCC patients.Targeting CSCs is considered as a potential therapeutic approach that can overcome the shortcomings of traditional treatments and significantly inhibit tumor recurrence.miRNAs play key roles in the posttranscriptional regulation of genes, and miRNAs are involved in various biological processes, including tumorigenesis.miRNA therapy has been used in some tumors and has entered the clinical stage, such as miR-34a has been used in a phase 1 study in patients with advanced solid tumors[101].In clinical treatment, miRNAs can enhance the sensitivity of LCSCs to treatment, and targeting the deregulated key miRNAs in LCSCs can effectively reduce the role of LCSCs in metastasis and recurrence[102-104].El-Mahdyet al[105] summarized the key signaling pathways associated with miRNAs (such as TP53, PI3k/AKT/mTOR, JAK/STAT, Wnt/β-catenin, and MAPK pathways), through which miRNAs can further affect the cellular processes and responses of HCC to clinical treatment.Therefore, investigating the role of miRNAs in LCSCs can help improve the prognosis of HCC patients and inform the development of new therapies.
ACKNOWLEDGEMENTS
Thanks to the China Scholarship Council for the scholarship to Dr.Li L.Thanks to FIGDRAW for providing the picture material.
FOOTNOTES
Author contributions:Li L, and Xun C completed the drawing of the picture and the writing of part of the content; Yu CH conceived and supervised the writing of this article.
Conflict-of-interest statement:All the authors report having no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:New Zealand
ORClD number:Lei Li 0000-0001-5062-3538; Chun-Hong Yu 0000-0002-6751-7851.
S-Editor:Liu GL
L-Editor:A
P-Editor:Liu GL
杂志排行
World Journal of Hepatology的其它文章
- lmmunological classification of hepatitis B virus-positive hepatocellular carcinoma by transcriptome analysis
- Liver chemistries in severe or non-severe cases of COVlD-19: A systematic review and meta-analysis
- CLlF-SOFA and CLlF-C scores for the prognostication of acute-onchronic liver failure and acute decompensation of cirrhosis: A systematic review
