Chlorine Dioxide Accelerates the Wound Healing Process of Potato Tubers by Eliciting Phenylpropanoid Metabolism
2022-12-30CHAIXiuweiKONGRuiZHENGXiaoyuanZHUYatongLIANGWeiZHAOShijiaLIBaojunBIYangDovPRUSKY
CHAI Xiuwei, KONG Rui, ZHENG Xiaoyuan, ZHU Yatong, LIANG Wei, ZHAO Shijia,LI Baojun, BI Yang,*, Dov PRUSKY
(1. College of Food Science and Engineering, Gansu Agricultural University, Lanzhou 730070, China;2. Department of Postharvest Science of Fresh Produce, Agricultural Research Organization, Rishon LeZion 7505101, Israel)
Abstract: Chlorine dioxide (ClO2) is a safe and effective disinfectant that is widely used for preserving fruits and vegetables as well as controlling microorganisms. However, the mechanism by which ClO2 treatment affects wound healing in potato tubers remains unclear. The half cut potato tubers ‘cv. Atlantic’ were soaked with 25 mg/L ClO2 for 10 min, which were stored for 0, 3, 5, 7, 14, and 21 d at room temperature in dark for wound healing. The mass loss rate and the disease index of wounded tubers inoculated with Fusarium sulphureum were determined, the deposition of suberin poly phenolic (SPP) and lignin at wound sites were observed, and the activities of the key enzymes of phenylpropanoid metabolism and peroxidase(POD) as well as the content of phenylpropanoid metabolism products and H2O2 at wound sites were measured. This result showed that ClO2 at 25 mg/L accelerated SPP and lignin deposition and increased cell layer thickness at wounds.Furthermore, the mass loss rate and disease index (after inoculated with F. sulphureum) of potato tubers significantly was reduced by 20.8% and 45.3%, respectively, on day 14 after ClO2 treatment. Additionally, ClO2 enhanced the contents of five phenolic acids (cinnamic, p-coumaric, caffeic, ferulic, and sinapic acids) and three lignin monomers (p-coumaryl, sinapyl,and coniferyl alcohols) by increasing phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), and cinnamyl alcohol dehydrogenase (CAD) activities. Moreover, ClO2 treatment significantly promoted the synthesis of total phenolics,flavonoids and lignin and elevated H2O2 content and peroxidase activity. In conclusion, ClO2 treatment accelerated the deposition of SPP and lignin at wound sites, decreased the mass loss rate and disease index of damaged potato tubers during healing, triggered the phenylpropanoid metabolism pathway, and increased H2O2 content and POD activity. These findings provide evidence that ClO2 treatment can accelerate wound healing in potato tubers.
Keywords: chlorine dioxide; potato tubers; phenylpropanoid metabolism; wound healing; suberin poly phenolic; lignin
Potato (Solanum tuberosumL.) tubers have wound healing capacity and can effectively inhibit mass loss and prevent pathogen infection by forming a closed layer and wound-periderm on wounds[1]. However, potato tubers need a long time to heal naturally; therefore, some effective measures should be taken to accelerate the wound healing process. Therefore, accelerating the healing process to effectively reduce the infestation rate of wound pathogens and reducing mass loss rate are urgent issues to be addressed in postharvest potato tubers.
Recent studies have found that certain chemicals also have the ability to promote wound healing in fruits and vegetables. For example, benzo-(1,2,3)-thiadiazole-7-carbothioic acidS-methyl ester (BTH) could accelerate the healing of potato tubers after harvesting[2]; and preharvest sprays with amino oligosaccharide could promote healing of harvested muskmelons[3]. Chlorine dioxide (ClO2)is an internationally recognized A1 disinfectant; it is safe, highly efficacious, eco-friendly and non-toxic, and noncarcinogenic[4]. ClO2is highly germicidal to bacteria,viruses, and fungi[5-6]. Because of its strong oxidation ability,ClO2rapidly oxidizes and destroys amino acids (especially tyrosine) and enzymes in microbial proteins as well as inhibits the synthesis of new microbial proteins, eventually leading to microbial metabolic dysfunction and death[7]. In addition, ClO2induces resistance to gray mold in strawberries caused byBotrytis cinereaand blue mold in apples caused byPenicillium expansum[8-9]as well as to bacterial soft rot caused byErwinia carotovoraand dry rot caused byFusarium anantum, inpotato tubers[10]. The mechanism of resistance induced by ClO2is closely associated with the activation of phenylpropanoid metabolism[11], accumulation of phenolics and flavonoids[12], and enhancement of antioxidant enzyme activities[13]. Relative research indicated that ClO2accelerated suberin poly phenolic (SPP) and lignin deposition by activating phenylpropanoid metabolism in wounds and effectively boosted the wound healing of muskmelon[12],indicating that ClO2has the ability to promote wound healing of fruit and vegetables. Although there are studies indicating that ClO2induces resistance in fruits and promotes wound healing in muskmelons, no report is available on the chemical effects of ClO2on wound healing of potatoes.
This study evaluated the effects of ClO2on potato tuber healing by 1) determining the phenylpropanoid metabolismrelated enzyme activities and product contents, as well as H2O2level and peroxidase (POD) activity; 2) observing the deposition of SPP and lignin; 3) measuring the mass loss rate and disease index of the treated potato tubers. In order to provide a method for rapid healing of potato tubers.
1 Materials and methods
1.1 Materials and reagents
Mini potato tubers (Solanum tuberosumcv. Atlantic)were harvested from the potato cultivation base in Dingxi,Gansu Province of China.
ClO2was purchased from Tianjin Zhangda Technology Co., Ltd., with an effective concentration of 10% (m/V), and stored at 4 ℃.
The pathogenF. sulphureumcausing dry rot of potatoes was supplied by the Institute of Plant Protection, Gansu Academy of Agricultural Sciences.
Hexane, sodium nitrite, acetic acid, aluminum nitrate,acetone, boric acid, hydroxylamine chloride, acetyl bromide Sinopharm Chemical Reagent Co., Ltd.; guaiacol, neutral red Tianjin Guangfu Fine Chemical Research Institute;toluidine blue Shanghai Zhongqin Chemical Reagent Co., Ltd.;β-mercaptoethanol, berberine hydrochloride,polyvinylpyrrolidone Beijing Solarbio Technology Co., Ltd.;folinol, Triton X-100 Beijing Coolaber Technology Co., Ltd.
1.2 Instrument and equipment
LRH-105F constant temperature incubator Shanghai-Heng Science Instrument Co., Ltd.; DW-86L416G cryogenic preservation box Qingdao Haier Biological Medical Co., Ltd.; TGL-20M low temperature high speed centrifuge Changsha Banli Instrument Co., Ltd.; U-LH100-3 fluorescence microscope Shanghai Yongke Optical Instrument Co., Ltd.; UV-2450 UV-visible spectrophotometer Japan Shimadzu Company; 1510-04087 microplate reader Thermo Fisher (Shanghai) Instruments Co., Ltd.; DFT-50 research prototype machine Shanghai Guning Instrument Co., Ltd.; high performance liquid chromatograph (HPLC)USA Waters Corporation.
1.3 Methods
1.3.1 Tubers artificial wounding
The selected tubers were equal in size and shape and had no infection or mechanical damage; they were transported to the laboratory and kept at (20 ± 5) ℃ for experiments.Wounds of tubers were done according to Dastmalchi et al[14].The tubers were cleaned and disinfected by immersion in sodium hypochlorite (1% (V/V), 3 min) before washing with sterile water and then air-dried. Subsequently, tubers were cut in half (diameter of wounded tubers: 20–30 mm) with a stainless steel knife perpendicu lar to its equator. Finally,all ClO2-treated group was immersed in 25 mg/L ClO2for 10 min (sterile water as control) and air-dried.
1.3.2 Determination of mass loss rate and disease index
Evaluation of mass loss rate during healing by the gravimetric method at 0, 3, 5, 7, 14, and 21 d, and the result was expressed in percentage. Triplicate determinations for 18 tubers per treatment were recorded.
The method reported by Jiang Hong et al.[15]for disease index was employed. In brief, 20 μLF. sulphureumspore suspension (1 × 106spores/mL) was inoculated on the wounded site of each treated or control tuber on different healing days(days 0, 3, 5, 7, 14, and 21), spread evenly, and air-dried. The disease index was analyzed after 7 d of incubation. The absence of infection on the wound surface was given a score of 0; the appearance of infection on 1/4 of the surface was scored as 1; 2/4 was scored as 2; 3/4 was scored as 3; and complete infection was scored as 4. The formula for calculating the disease index is:
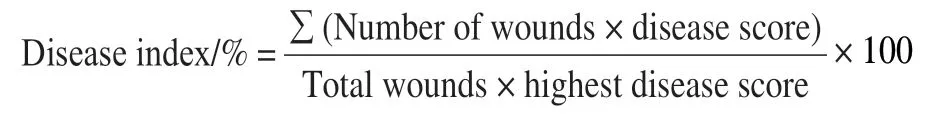
Three replicates (18 tubers per treatment) were evaluated.
1.3.3 Determination of SPP and lignin deposition
SPP deposition was visualized by berberine staining[15].Tissue samples 0.2–0.4 mm thickness were cut perpendicular to the wound surface with a stainless-steel blade. Slices were washed 6–8 times with distilled water and placed onto glass slides with tweezers. The SPP was visualized by fluorescence microscope.
Lignin deposition was observed using the abovementioned microscope according to the method of Jiang Hong et al.[15].
SPP and lignin thicknesses were measured using ISCapture software.
1.3.4 Preparation of biochemical samples
Wounded tissues of tubers were collected according to Yang Ruirui et al.[16], 3 mm thick healing tissues were sampled on days 0, 3, 5, 7, 14, and 21 after wounding and rapidly frozen in liquid nitrogen. The obtained tissues were homogenized into powder, loaded into centrifugal tubes(50 mL), and stored at –80 ℃ prior to further determination of biochemical indexes.
1.3.5 Determination of enzyme activities
Phenylalanine ammonia-lyase (PAL) activity was analyzed using Koukol et al.[17]method. Cinnamate-4-hydroxylase (C4H) activity was measured based on the method of Lamb et al.[18]. Cinnamyl alcohol dehydrogenase (CAD)activity assay was done as detailed before Goffner et al.[19].PAL, C4H, and CAD activities were indicated as U/g, where U = 0.01 OD290nm/h, U = 0.01 OD340nm/min, and U = 0.01 OD400nm/min, respectively.
POD activity was determined based on an amended method described by Venisse et al.[20]. POD activity was indicated as ΔOD470nm/(min·g).
1.3.6 Determination of total phenolics, flavonoids and lignin contents
The total phenols and flavonoids contents were measured using the methods of Scalbert et al.[21]. Total phenol and flavonoid contents were indicated as mg gallic acid equivalents per 100 g and mg catechin equivalents per 100 g,respectively.
The lignin content by using the approach of Hammerschmidt[22]was analyzed. The result was indicated as OD280nm/g.
1.3.7 Determination of phenolic acids and lignin monomers contents
The phenolic acids and monolignin content were measured by HPLC[23]. 5 mL of 70% (V/V) precooling acetone was added to 3.0 g of samples, undergoes ultrasonication for 30 min at 40 kHz before being centrifuged. The supernatant was concentrated in a concentrator up to one milliliter with the volume fixed. The concentrated solution was filtered into two milliliters autosampler bottles by an organic membrane(0.22 μm). The spectral information of SPP and lignin monomers were recorded at 325 nm. Phenolic acids and lignin monomer content were indicated as μg/g.
1.3.8 Determination of H2O2content
H2O2was analyzed using the approach of Prochazkova et al.[24].1.0 g frozen power was homogenized in 3 mL acetone and centrifuged (12 000 ×g, 4 ℃, 10 min). H2O2reactions contained supernatant (1 mL), TiCl4(100 μL, 20%(V/V)), and concentrated ammonia liquor (100 μL). After centrifugation, pellets were dissolved in concentrated sulfuric acid (2.0 mL, 1 mmol/L) and OD values were recorded at 410 nm. The H2O2content was indicated as μmol/g.
1.4 Statistical analysis
All above-mentioned determinations were done at least thrice. The data were shown as mean ± standard error via Excel 2016 software. Statistical significance was determined by an independent-samplet-test using SPSS 19.0 software,and thettest was used to compare the means when the overallPvalue of the experiment was below the value of significance(P< 0.05).
2 Results and analysis
2.1 ClO2 accelerated SPP and lignin deposition
The two primary components, SPP and lignin, are in the closing layer of the potato healing process. In this study,their deposition in wounds gradually increased over time.The difference in SPP and lignin deposition (red arrows) was obvious on day 3, and the ClO2treatment induced distinctly more deposition in wounded tubers than in control group(Fig. 1A, B). During healing, the difference in the SPP and lignin cell layer thicknesses between ClO2-treated and control tubers was noted on day 5 and day 3, respectively,which were 16.7% and 23.1% higher than in control group,respectively (Fig. 1C). ClO2treatment increased SPP and lignin deposition at wounds.
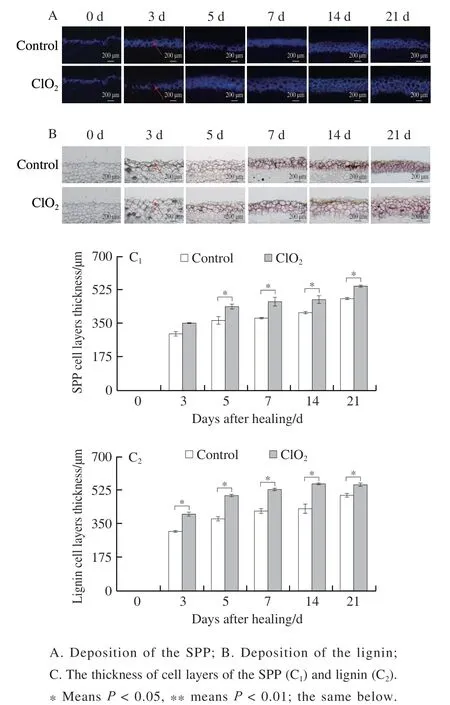
Fig. 1 SPP and lignin deposition and cell layer thickness at wounds of potato tubers during healing
2.2 ClO2 treatment reduced mass loss rate and disease index
Two vital indexes, mass loss rate and disease index were used to evaluate the level of tuber healing. Wounded tubers in both treatment and control groups steadily lost weight over 21 days. While treated ones showed lower less than the control group (20.8% less on day 14 of healing) (Fig. 2A).Disease index gradually reduced in both groups. Compared with the control, disease index of treated tubers was lower,and was 45.3% lower on day 14 (Fig. 2B), indicating that ClO2treatment promoted the potato tubers healing.
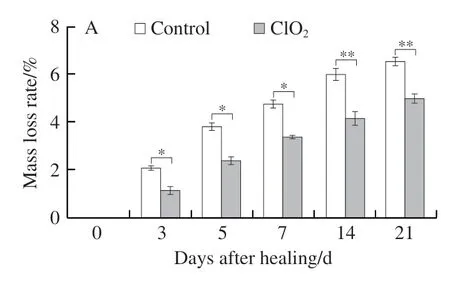
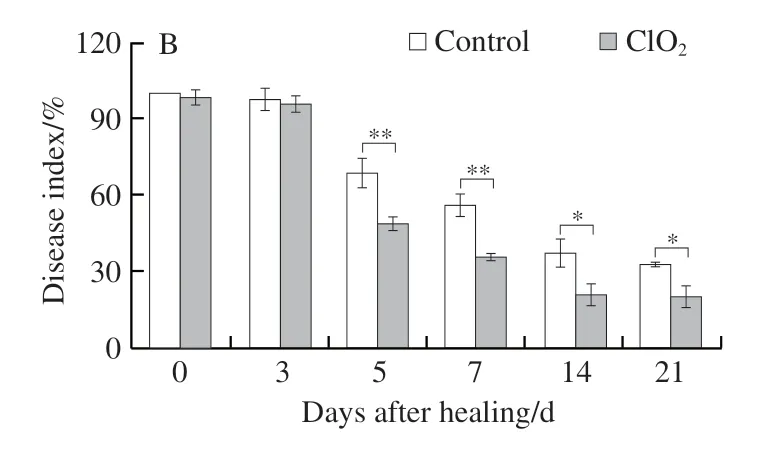
Fig. 2 Mass loss rate (A) of wounded tubers and disease index (B) of inoculated tubers of potato during healing
2.3 Effects of ClO2 treatment on phenylpropanoid metabolism
2.3.1 ClO2promoted PAL, C4H, CAD activities and flavonoids content
The key enzymes activities of phenylpropane metabolism at wounds is a reflection of the ability of phenolic substances to form during healing. During healing, PAL and C4H activities of the ClO2-treated and control groups showed single peaks that appeared on days 5 and 7, respectively.However, the activities of PAL and C4H were higher in ClO2-treated group than in the control group (56.6% and 40.0%higher on day 14, respectively, Fig. 3A, B). CAD activity also increased in all wounded tubers with the healing time,but it was evidently higher in the ClO2-treated group than in the control group (27.7% higher at day 14, Fig. 3C). The flavonoid content increased in the control tubers during healing. However, the flavonoid content first increased and then reduced in the ClO2-treated group. The maximal difference was observed on day 14 (49.5% increases relative to the control, Fig. 3D). Thus, ClO2treatment activated phenylpropanoid pathway and boosted the synthesis of flavonoids at wounds.


Fig. 3 Activities of PAL (A), C4H (B) and CAD (C) and flavonoids content (D) at wounds of potato tubers during healing
2.3.2 ClO2enhanced 5 phenolic acids and total phenol contents
Phenolic acids are precursors of SPP. Content of five phenolic acids and total phenol is shown in Fig. 4. During healing, cinnamic, ferulic, and sinapic acids in wounded tubers in both ClO2-treated and control groups showed single peaks, and the content of phenolic acids were substantially higher in the ClO2-treated group than in the control group at day 7 (Fig. 4A, D, E). The content ofp-coumaric acid after ClO2treatment showed a bimodal pattern over time,with peaks appearing on the 5thand 14thday, showing higher values in the ClO2-treated group than in control group(Fig. 4B). ClO2treatment enhanced the synthesis of caffeic acid and total phenolic content (Fig. 4C, F). These findings proved that ClO2treatment promoted the synthesis of phenolic acids and total phenolics at wounds.
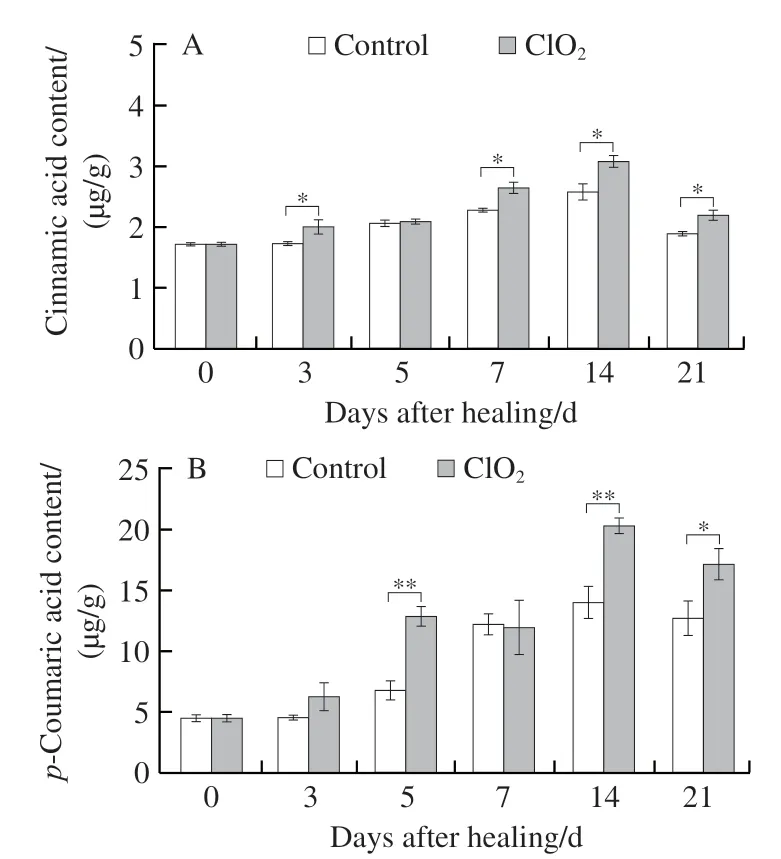
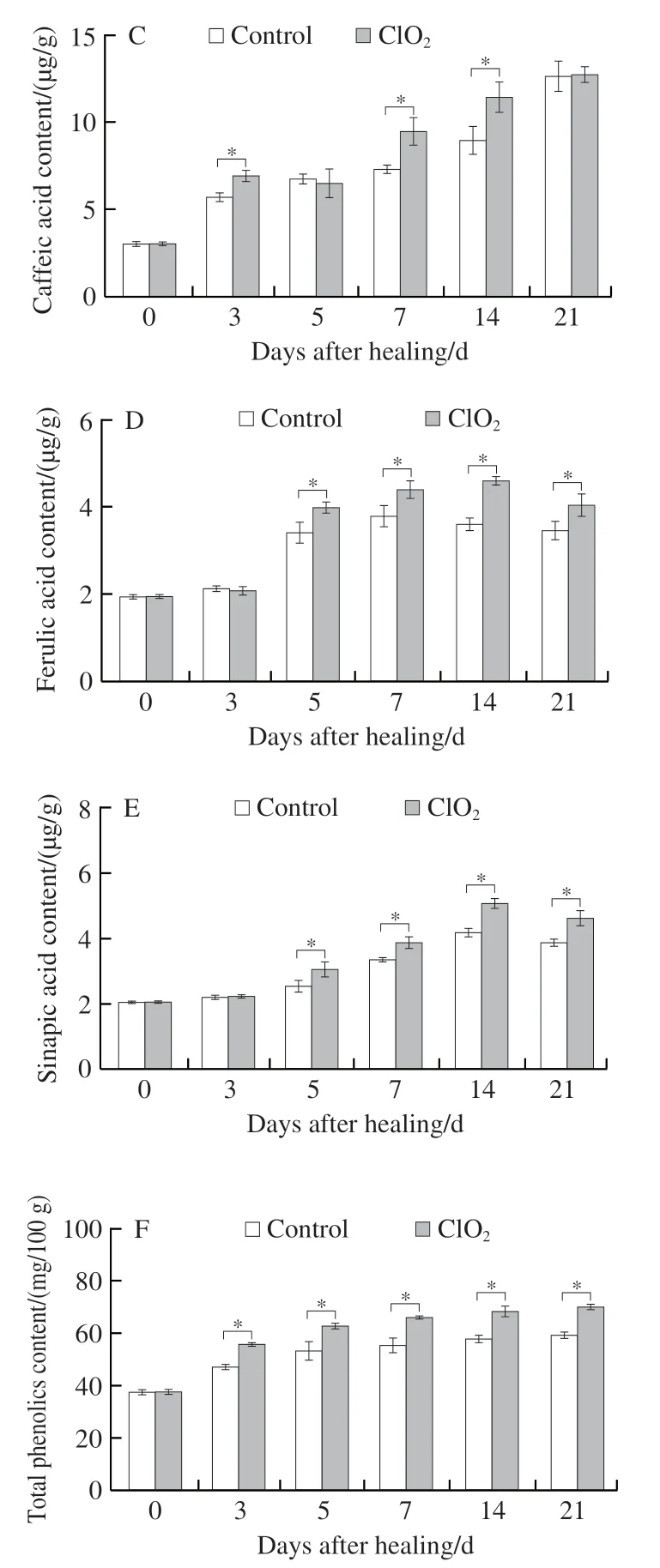
Fig. 4 Contents of cinnamic acid (A), p-coumaric acid (B), caffeic acid (C), ferulic acid (D), sinapic acid (E) and total phenolics (F) at wounds of potato tubers during healing
2.3.3 ClO2treatment enhanced 3 lignin monomers and lignin contents
Coumaryl, sinapyl, and coniferyl alcohols are three substrates for lignin synthesis. During healing,p-coumaryl,sinapyl, and coniferyl alcohols all showed a single peak.Peaks from ClO2-treated group were substantially higher than those from control group after day 7 (Fig. 5A-C). The contents of lignin in ClO2-treated and control groups also increased with healing time. Lignin content was higher in the ClO2-treated group than in control group, except on day 3 (38.7% higher at day 14, Fig. 5D). The above results suggested that ClO2treatment promoted lignin monomer productionand lignin synthesis at wounds.
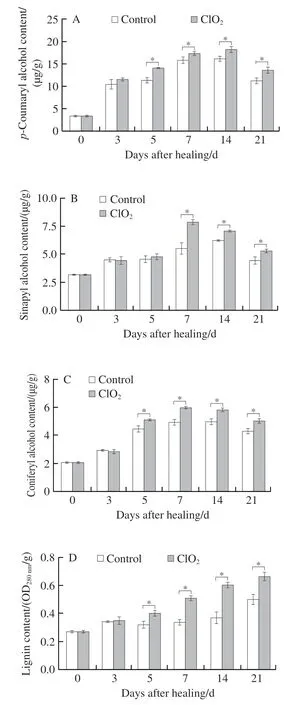
Fig. 5 Contents of p-coumaryl alcohol (A), sinapyl alcohol (B), coniferyl alcohol (C) and lignin (D) at wounds of potato tubers during healing
2.3.4 ClO2treatment increased H2O2content and POD activity
POD and H2O2participate in promoting the oxidative cross-linking of SPP and lignin precursors. The content of H2O2in all wounded tubers increased with healing time, and the ClO2-treated group showed significantly higher than the control (23.0% higher on day 14, Fig. 6A). POD activity in wounded tubers showed an upward tendency during healing, and ClO2-treated group showed higher POD activity than the control group (33.6% higher on day 14 of healing,Fig. 6B). The results indicated that ClO2treatment increased H2O2production and POD activity in potato tubers.
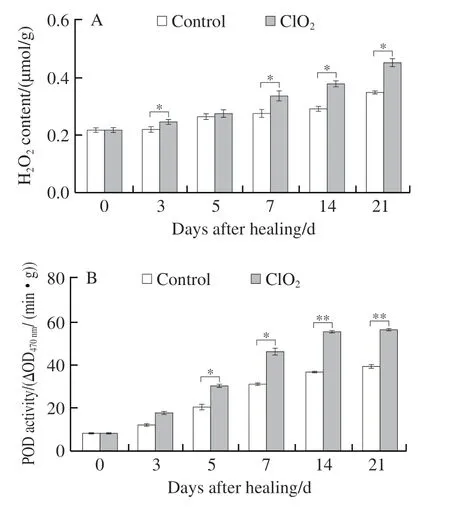
Fig. 6 Content of H2O2 (A) and activity of POD (B) at wounds of potato tubers during healing
3 Discussion
Both SPP and lignin act as major ingredients of the closing layers of tubers during healing. SPP is mainly composed of cinnamic acid, ferulic acid, and other phenolic acids. Through the function of feruloyl transferase, they are connected via esters and ether bonds. By the function of H2O2and POD, lignin is chiefly formed by ether-bondedp-coumaryl, sinapyl, and coniferyl alcohols[25]. ClO2treatment accelerated SPP and lignin deposition at wounds in the current study (Fig. 1A–C), which was consistent with the research results obtained by Zheng Xiaoyuan et al.[12]in muskmelon.
This study showed that ClO2treatment reduced mass loss rate in wounded tubers (Fig. 2A) and agrees with findings that ClO2treatment inhibited mass loss rate in muskmelon[12].SPP and lignin deposition on wounds is reported to decrease mass loss rate in muskmelon during healing[12]. ClO2treatment reduced mass loss rate in tomato fruit by inhibiting respiration intensity to reduce the substrate consumption[26]. Furthermore,ClO2treatment effectively reduced the disease index (Fig. 2B),which was consistent with the results of the effects of ClO2on muskmelon[12]. The physical barrier formed by SPP and lignin deposition in wounded tubers resulted in reduced disease index of muskmelon during healing[12]. Moreover, ClO2treatment promoted total phenols and flavonoids contents by activating phenylpropanoid pathway, thereby significantly inhibiting the infection caused by pathogens[11].
Phenylpropanoid pathway is vital for tuber healing. It provides substrates for SPP and lignin formation, as well as promotes the synthesis of antimicrobial substances, such as phenolics and flavonoids[27]. PAL is a key enzyme initiating this metabolism by the deamination ofL-phenylalanine totrans-cinnamic acid[28-29]. Next, C4H catalyzes the formation ofp-coumaric acid fromtrans-cinnamic acid. Subsequently,caffeic acid is catalyzed by thep-coumaric acid reaction with caffeic acid-3-hydroxylase, and ferulic acid reaction with 3-O-methyltransferases. Ferulic acid is initially converted to 5-hydroxyferulic acid and then to sinapic acid by 5-hydroxyferulic acid-O-methyltransferase[30]. These phenolic acids are first reduced to coumaric aldehyde, sinapis aldehyde, and coniferal dehyde under the catalyzation of 4-coumarate-CoA ligase and cinnamyl-CoA reductase, which are further converted to three lignin monomers by CAD[31].According to the experiment data, we found that ClO2-treated group dramatically improved PAL, C4H, and CAD activities(Fig. 3A–C), thus increasing flavonoid content (Fig. 3D).In addition, ClO2treatment promoted the synthesis of five phenolic acids, total phenols, and three lignin substrates(Fig. 4, Fig. 5A–C). The results were consistent with the reports in apples and muskmelons, which indicated that postharvest ClO2treatment increased PAL activity as well as total phenol and flavonoid content[11-12].
Both H2O2and POD participate in the oxidative crosslinking of SPP and lignin substrates. In this study,we found that ClO2significantly improved H2O2content at wounds of tubers (Fig. 6A), which is similar to the observations in muskmelons[12]. NADPH oxidase (NOX) is the main source of H2O2for healing in potatoes. The damage induces extracellular Ca2+influx, leading to increased intracellular Ca2+concentrations. Calcium-dependent protein kinase (CDPK) senses the increase and becomes activated by binding to Ca2+in the cytoplasm. CDPK then activates the downstream NOX through phosphorylation, resulting in· production[27]. Because of the short survival life ofit is quickly disproportionated to H2O2under the effect of superoxide dismutase (SOD)[27]. ClO2upregulatedNOX(RbohD) andSODexpression, enhanced the SOD activity,and further promoted H2O2production in longan fruit[32].Therefore, we infer that ClO2may promote the synthesis of H2O2by increasing NOX and SOD activities at the transcriptional level. ClO2treatment significantly improved POD activity (Fig. 6B), which was consistent with the result that ClO2treatment increased POD activity in longan fruit[33].It has been reported that ClO2induced rapid production of H2O2in longan fruit during early storage[32]. H2O2, as a signaling molecule, can activate phenylpropanoid pathway[34].Therefore, we infer that ClO2first induces the synthesis of signal molecules and then promotes the contents of phenolic acids and lignin substrates by activating phenylpropanoid metabolism. However, further studies are warranted to reveal potential mechanisms of ClO2related to regulated phenylpropanoid pathway during healing.
4 Conclusions
ClO2treatment elicits phenylpropanoid pathway by enhancing the key enzymes activities and promoting the synthesis of five phenolic acids and three lignin monomers at wounds of tubers. ClO2also improved H2O2level and POD activity. The phenolic acids and lignin monomers were crosslinked by H2O2and POD to form SPP and lignin, which were deposited at wounds. SPP and lignin acted as a physical barrier resulting in less mass loss rate and disease index of ClO2-treated group. Considering that ClO2is a cheap and safe agent, it might serve as an accelerator of wound healing in fruits and vegetables.
