In vitro Antibacterial Activity of Palmitoleic Acid Isolated from Filamentous Microalga Tribonema minus Against Fish Pathogen Streptococcus agalactiae
2022-12-27WANGFeifeiGUOYuhaoCAOYanandZHANGChengwu
WANG Feifei , GUO Yuhao CAO Yan and ZHANG Chengwu,
1)School of Modern Industry for Selenium Science and Engineering, Wuhan Polytechnic University, Wuhan 430023, China
2) School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Nanjing 210023, China
3)Engineering Research Center of Tropical and Subtropical Aquatic Ecological Engineering, Ministry of Education,Guangzhou 510632, China
4)Department of Ecology and Research Center for Hydrobiology, Jinan University, Guangzhou 510632, China
Abstract Filamentous microalgae from genus Tribonema are promising sustainable sources of omega-7 palmitoleic acid, but their ability to accumulate this compound varies among species and depends on the initial nitrogen concentration (INC)supply. In this study,the palmitoleic acid accumulation capacities of five Tribonema species were examined under three different INCs to select the alga species with the highest production. Results showed that a high INC was associated with increased palmitoleic acid accumulation but led to decreased biomass concentration in all tested species. In particular, T. minus grown at 18 mmol L-1 INC had the highest palmitoleic acid content (20.72% of dry weight)and productivity (90.88 mg L-1 d-1). The combination of alkali metal freezing precipitation(AMFP)and urea complexation successfully isolated and enriched palmitoleic acid from T. minus and obtained a purity of 80.11%and a yield of 7.39 g (100 g)-1 of algal powder. The compound was identified as (9Z)-hexadecenoic acid (C16:1 ω-7). Antibacterial activity evaluation for the highly concentrated palmitoleic acid (10 mg mL-1)against Streptococcus agalactiae revealed the formation of a 12.10 mm-diameter inhibition zone and the minimum inhibitory concentration of 31.25 μg mL-1, indicating that palmitoleic acid is an effective antibacterial agent. This study is the first to report that palmitoleic acid derived from T. minus can antagonize S. agalactiae,which further broadens the potential application of Tribonema biomass in green aquaculture.
Key words Tribonema; palmitoleic acid; enrichment; Streptococcus agalactiae; antibacterial activity
1 Introduction
Streptococcus agalactiae, or group B streptococcus, is a Gram-positive pathogenic bacterium and a major etiologic agent of septicemia and meningoencephalitis in fishes that causes huge annual economic losses to the aquaculture industry (Chideroliet al., 2017; Denget al., 2019). Vaccines and antibiotics are the most promising measures of controlling infections caused byS. agalactiae(Liuet al.,2016; Denget al., 2019). However, no approved vaccine is suitable for preventingS. agalactiaeinfections in fish farms,and the repeated use of antibiotics leads to theserious antibiotic residues and drug resistance (Betriuet al., 2003;Denget al., 2019). Therefore, efforts have been devoted to discovering and developing new antibacterial compounds from different natural resources, including plants, animals,microorganisms, and microalgae (Genilloud, 2019; Abdel-Razeket al., 2020), which can replace antibiotics with biosafety and environmental protection.
Microalgae are a large group of organisms that are extremely diverse and heterogeneous from evolutionary and ecological viewpoints (Sharma and Rai, 2011). These species contain various bioactive compounds, such as unsaturated fatty acids (UFAs), carotenoids, polysaccharides,and phycobiliproteins, which are widely applied in the medical, food, cosmetic, and aquaculture industries (Chu and Phang, 2019; Pereiraet al., 2020). Palmitoleic acid, which is an omega-7 monounsaturated fatty acid (MUFA), is enriched in some microalgae, such asPhaeodactylum tricornutum(Zhanget al., 2018a),Eustigmatoscf.polyphem
(Wanget al., 2018), andTribonema minus(Zhouet al., 2017)and has emerged as a functional fatty acid beneficial to human health (Huet al., 2019). These molecules also possess antibacterial activity against a wide range of Gram-positive bacteria, includingStaphylococcus epidermidis,Corynebacteriumsp.,Bacillus cereus, andBacillusweihenstephanensis, and can significantly inhibit the growth of multidrug resistantS. aureus(Wille and Kydonieus, 2002; Desboiset al., 2008). Palmitoleic acid in microalgae as a natural antibacterial agent has attracted attention because it prevents pathogenic bacterial infection and provides available nutrients; however, few studies have investigated its antimicrobial activity against fish pathogenS. agalactarius.Therefore, research must first focus on the selection of algal species rich in palmitoleic acid, the optimization of culture conditions, the isolation of palmitoleic acid, and the evaluation of its antimicrobial activity.
Yellow-green filamentous microalgae from genusTribonema(class Xanthophyceae)are potential candidates for biodiesel production due to their high lipid contents and excellent cultivation advantages, such as easy harvesting and anti-pollution attributes (Zhouet al., 2017; Wanget al.,2020). Some species in this genus can also accumulate large amounts of palmitoleic acid, indicating their potential application in the large-scale production of this compound.However, the ability to accumulate palmitoleic acid varies among species and depends on the initial nitrogen concentration (INC)supplied in the growth medium. In this study,the effects of different INCs on palmitoleic acid accumulation capacity of fiveTribonemaspecies were investigated to select the strain with the highest production. The palmitoleic acid produced by the most productive microalga was then isolated and enriched to evaluate its activity against fish pathogenS. agalactiae.Tribonemaspp. could be used as a feed ingredient to increase growth performance, flesh quality, antioxidant capacity, and immunity in fishes (Chenet al., 2019; Zhaoet al., 2020). Therefore, this research on the antibacterial activity of palmitoleic acid from aTribonemaspecies will broaden the potential applications of these microalgae in aquaculture and help reduce antibiotic use in aquaculture.
2 Materials and Methods
2.1 Algal Species and Fish Pathogen
Filamentous microalgaeTribonemasp. 2172,T. minus,T.ulotrichoides,T. viride,andT. vulgarewerepurchased from the Culture Collection of Algae at the University of Göttingen (SAG)(Göttingen, Germany), maintained in modified BG-11 (mBG-11)medium (Gaoet al., 2016), and deposited in the laboratory. Fish pathogenS. agalactiaewas kindly supplied by the Molecular Biology Laboratory, Institute of Hydrobiology, Jinan University, Guangdong Province, China and was grown on brain heart infusion (BHI)agar plates at 28℃.
2.2 Microalgae Culture Conditions
EachTribonemaspecies was cultivated in mBG-11 containing different INCs (3, 9, and 18 mmol L-1)to investigate their biomass and palmitoleic acid accumulation ability.
The starter culture of each species was prepared in a Ø6× 60 cm (inner diameter × length)glass column photobioreactor containing 1.2 L of normal mBG-11 medium and grown under low-light irradiation (70 – 80 μmol m-2s-1)with 1% CO2compressed air (v/v)agitation. After approximately 7 days of cultivation, the sediments were collected separately through filtration and washed with sterile water prior to inoculation. Batch cultures of each species were supplied with different INCs and cultivated in Ø6 × 60 cm glass column photobioreactors at an inoculum density of 0.30 – 0.35 g L-1under continuous high-light irradiation((300 ± 10)μmol m-2s-1)and bubbling of 1% CO2(v/v)compressed air from the bottom of the column. On day 15, palmitoleic acid productivity (mg L-1d-1)was calculated as biomass concentration × palmitoleic acid content / culture time.
2.3 Isolation, Enrichment, and Identification of Palmitoleic Acid
Palmitoleic acid was isolated and enriched fromT. minusbiomass by combining total lipid extraction, saponification,alkali metal freezing precipitation (AMFP), and urea complexation. Fatty acid profile was analyzed by gas chromatography (GC, see Section 2.5.2)at each stage of this serial process. In brief, 20 g ofT. minusalgal powder was first pretreated with dilute acid to facilitate the efficient extraction of total lipids by the mixed solvents ofn-hexane/ethanol (1:1,v/v)(Wanget al., 2020). The total lipids were then saponified with 10% KOH-ethanol solution at 70℃for 1.5 h and subjected to low-temperature treatment (-20℃,36 h)for the removal of saturated fatty acid (SFA)potassium salts (insoluble). The resulting liquid phase, which was rich in UFA potassium salts, was hydrolyzed with strong acid (HCl, 2 mol L-1)to produce free fatty acids. These free fatty acids were treated with urea and 95% ethanol at a mass ratio of 1:5:20 (w/w/w)and then crystallized overnight at 4℃, followed by dissolving the recovered urea crystals in 40℃ water and partitioning them withn-hexane. The upper phase was then evaporated to produce concentrated palmitoleic acid, which was characterized by GC-mass spectrometry (GC-MS)as described in Section 2.5.3.
2.4 Evaluation of the in vitro Inhibitory Activity of Palmitoleic Acid Against S. agalactiae
2.4.1 Disk diffusion assay
In brief, 0.2 mL of fresh bacterial suspension aliquot (1× 106colony-forming unit (CFU)mL-1)prepared with BHI broth medium was uniformly coated on the surface of a BHI agar layer in a Petri dish (9 cm diameter), and three sterile filter paper disks (6 mm diameter)were then symmetrically placed on top to form a triangle. One of the disks was dripped with 6 μL of the concentrated palmitoleic acid(10 mg mL-1)dissolved in 5% dimethyl sulfoxide (DMSO),and the other two were separately dripped with palmitoleic acid standard (Sigma-Aldrich; > 98.5% (GC))and 5%DMSO as positive and negative controls, respectively. The plates were allowed to stand for approximately 30 min for pre-diffusion and then incubated at 28℃ for 24 h. Anti-S.agalactiaeactivity was evidenced by the formation of an inhibition zone with a diameter > 6 mm.
2.4.2 Microbroth dilution assay
Various concentrations of the concentrated palmitoleic acid (20 μL, dissolved in DMSO)in the range of 39.06 –5000 μg mL-1were first prepared in the wells of a 96-well culture plate by using the twofold dilution method to determine its minimal inhibitory concentration (MIC)againstS. agalactiae. DMSO and sterile water were used as controls. Then, 180 μL of fresh bacterial suspension (1 × 106CFU mL-1)was inoculated into each well to obtain a total volume of 200 μL and a final concentration range of 3.91– 500 μg mL-1. The plates were incubated at 28℃ for 24 h with gentle shaking (40 r min-1), and MIC was defined as the lowest concentration of the concentrated palmitoleic acid that inhibited the visible growth ofS. agalactiaeas indicated by turbidity changes in the solution.
2.5 General Analytical Methods
2.5.1 Determination of biomass concentration
The biomass dry weight of each culture was measured at day 15 following the method of Wanget al. (2020). In brief, 10 mL of each culture was filtered through a 0.45 μm pre-weighted GF/B filter paper (M0)and dried at 105℃overnight to a constant weight (M1). Biomass dry weight(DW, g L-1)was then calculated as [(M1– M0)× 100].
2.5.2 Fatty acid profile analysis
Fatty acid profiles were analyzed using the gas chromatography-flame ionization detector method described by Zhanget al. (2018a). The fatty acid composition was measured by comparing the retention times with fatty acid methyl ester standards (Sigma, St. Louis, MO, USA). The content of each fatty acid was calculated by comparing its peak area with that of the internal standard (heptadecanoic acid,C17:0).
2.5.3 GC-MS analysis
The concentrated palmitoleic acid was subjected to GCMS analysis with an Agilent Technologies 7890B GC system equipped with an autosampler, a split-splitless injector,and a mass spectrometer under the chromatographic conditions described by Mehariet al. (2019). The palmitoleic acid was identified by comparing the retention time and mass spectra with the National Institute of Standards and Technology Mass Spectral Library Ver. 2.0.
2.6 Statistical Analysis
All experiments were conducted in triplicate, and the experimental data are presented as means ± standard deviations. Differences between treatments were compared using one-way ANOVA, followed by Student-Newman-Keuls test in the statistical analysis system software package SPSS 19.0. APvalue < 0.05 was considered statistically significant.
3 Results and Discussion
3.1 Biomass Concentration and Palmitoleic Acid Production of Five Tribonema Species in Response to Different INCs
Nitrogen is one of the most important nutrients that affect the growth and biochemical composition of microalgae (Akgul, 2020). Despite numerous studies showing thatTribonemaspp. exhibit unique biochemical responses to nitrogen (Guoet al., 2014; Wanget al., 2020), only a few works have comprehensively examined interspecific differences in palmitoleic acid production under different levels of nitrogen supply. In the present research, fiveTribonemaspecies were cultured in mBG-11 medium supplied with three different INCs (3, 9, and 18 mmol L-1)to investigate the influence of nitrogen on their biomass concentrations and palmitoleic acid production levels.
The biomass concentration of all fivespecies significantly decreased with the increase in INC (Fig.1a). Except forT. vulgare, all tested species exhibited a maximum biomass concentration at 3 mmol L-1INC, withT. virideobtaining the highest biomass concentration (5.45 g L-1± 0.20 g L-1),followed byT. ulotrichoidesandTribonemasp. 2172. Their biomass concentration at 18 mmol L-1INC ranged from 3.95 to 4.83 g L-1. By contrast, their palmitoleic acid contents showed the opposite response to a change in nitrogen concentration. A high INC led to a high palmitoleic acid content ranging from 14.68% to 20.72% of DW in all tested species grown in mBG-11 medium containing 18 mmol L-1INC (Fig.1b). This finding is consistent with previous studies (Xuet al., 2018; Wanget al., 2020). At 18 mmol L-1INC,T. minusobtained the highest palmitoleic acid content which was 20.72% ± 1.02% of DW but had minimal biomass accumulation.
Given these contrasting responses of biomass concentration and palmitoleic acid content to INC change, the productivity of each species was used as an index to further evaluate its ability to accumulate palmitoleic acid.T. minusandT. virideshowed an advantage over the other species in respect to their remarkable palmitoleic acid productivity of (90.88 ± 3.21)and (88.43 ± 2.16)mg L-1d-1(Fig.1c), respectively, when grown with 18 mmol L-1INC. These results showed that all fiveTribonemaspecies exhibited similar responses to the change of nitrogen concentration in terms of biomass and palmitoleic acid accumulation but had different abilities for palmitoleic acid accumulation.T.minusshowed the highest palmitoleic acid productivity due to its high palmitoleic acid content. In subsequent analyses,this species was used as the biomass feedstock for the isolation and enrichment of palmitoleic acid.
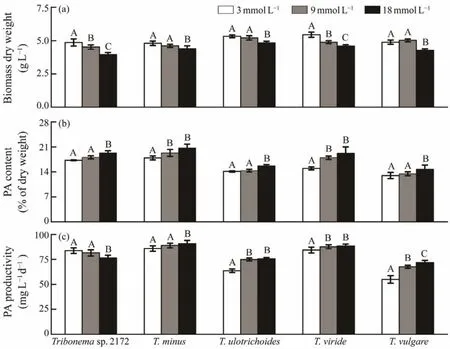
Fig.1 Biomass concentration (a), palmitoleic acid content (b), and palmitoleic acid productivity (c)of five Tribonema species under different initial nitrogen concentrations. PA, palmitoleic acid; Values are expressed as mean ± standard deviation of three replicates; Different capital letters on the columns within a species indicate significant differences (P < 0.05).
3.2 Isolation, Enrichment, and Identification of Palmitoleic Acid
Palmitoleic acid is a functional fatty acid that has attracted increasing commercial attention (Huet al., 2019). Obtaining high-purity palmitoleic acid from microalgae at a reasonable cost is the key to its downstream applications.Here, alkali metal freezing precipitation (AMFP)method and urea complexation were combined to isolate and enrich palmitoleic acid fromT. minus.
The fatty acid profile at each isolation step is presented in Table 1 and Fig.2. The total lipids extracted fromT. minusmainly consisted of myristic acid (C14:0), palmitic acid(C16:0), palmitoleic acid (C16:1), oleic acid (C18:1), and eicosatetraenoic acid (C20:4)(Table 1 and Fig.2b), which altogether accounted for 98.31% of total fatty acids (TFAs).For the isolation and enrichment of palmitoleic acid from the total lipids, AMFP was first used to fractionate UFAs and SFAs depending on the solubility differences of their fatty acid potassium salts at a low temperature. As a result,palmitoleic acid comprised the major peak (Fig.2c), and its content increased significantly from 50.95% to 73.00%of TFAs (Table 1)with a yield of 14.47 g (100 g)-1of algal powder. In addition, the content of SFAs decreased from 33.55% to 5.72% of TFAs, with the C16:0 amount decreasing rapidly from 26.92% to 2.08% of TFAs. Thus, AMFP is an effective approach for SFA removal (Zhanget al., 2018a).
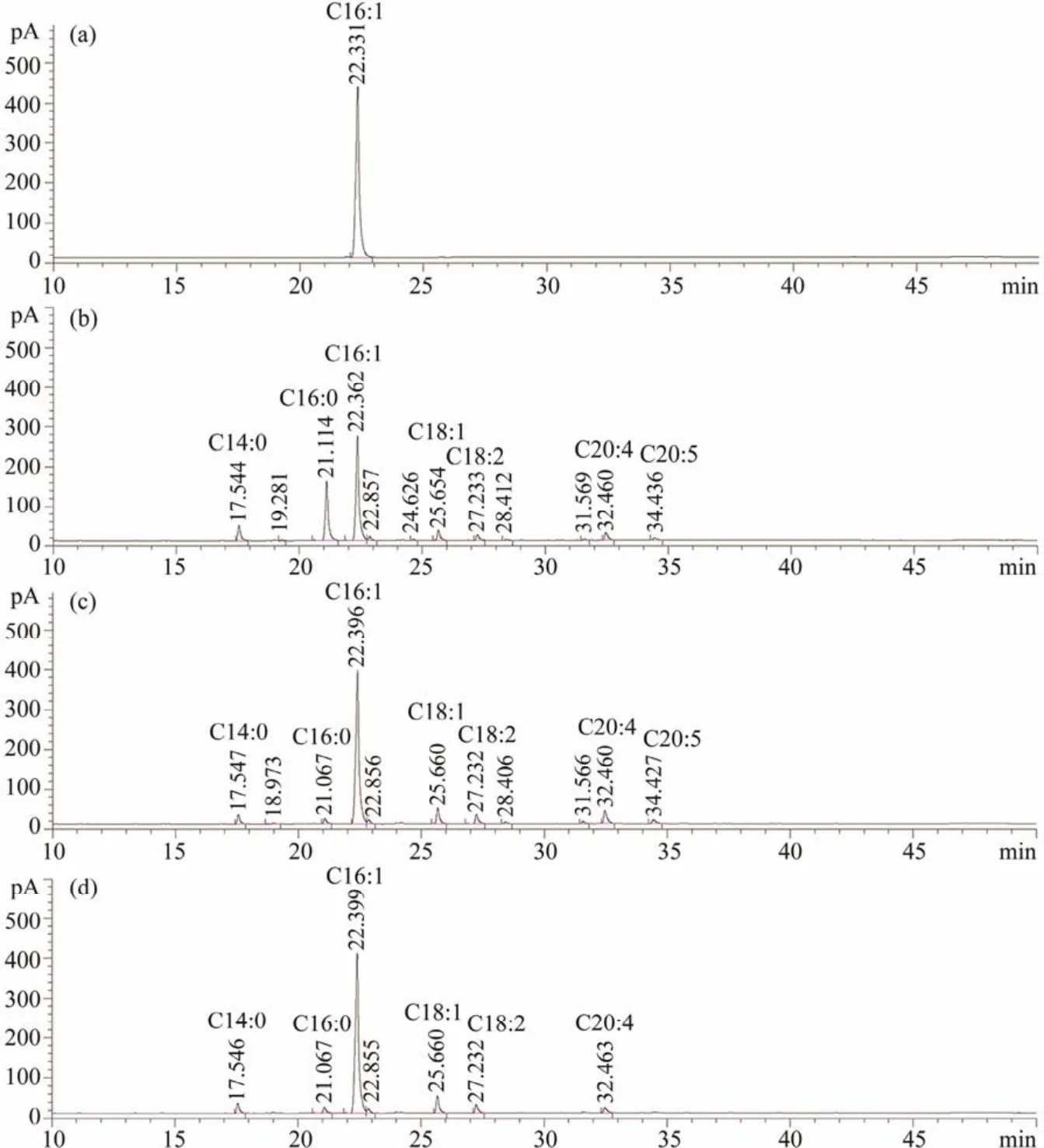
Fig.2 Gas chromatograms of palmitoleic acid during the isolation and enrichment processes. (a), palmitoleic acid standard;(b), total lipids; (c), after alkali metal freezing precipitation; (d), after urea complexation. The fatty acids are annotated by comparing them with the standard of fatty acid methyl ester; those with content less than 2% of total fatty acids are not annotated.
Urea complexation, a separation method depending on unsaturation degree, is one of the most promising methods for polyunsaturated fatty acid (PUFA)enrichment at an industrial scale (Vazquezet al., 2017). In this study, urea complexation was used to enrich palmitoleic acid because straight chains and MUFAs more easily form urea inclusion compounds than PUFAs (Ratnayakeet al., 1988). With this treatment, the content of palmitoleic acid was further increased to 80.11% of TFAs (a yield of 7.39 g (100 g)-1of algal powder), and the content of PUFAs was simultaneously decreased from 13.20% to 6.52% of TFAs (Table 1). No peaks corresponding to C18:3 and C20:5 were observed (Fig.2d). Thus, urea complexation successfully removed most of the PUFAs, but some fatty acids (C18:2,C20:4)remained in the urea complexing fractions due to the solvent retention effect. This procedure also increased C18:1 content from 4.74% ± 0.03% to 7.09% ± 0.02% of TFAs due to the similar physical properties of urea to palmitoleic acid.
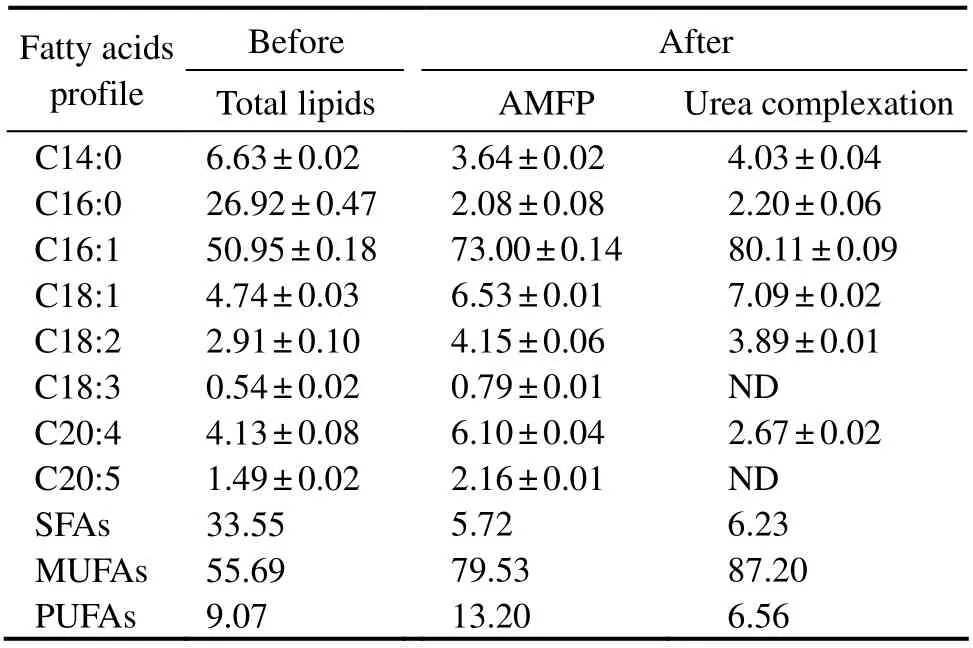
Table 1 Fatty acid profile (% of total fatty acids)of the lipid extract from Tribonema minus before and after enrichment
The chemical composition of the concentrated palmitoleic acid was investigated using GC-MS. According to the spectroscopic data, a major peak corresponded to palmitoleic acid (Fig.3)and was identified as (9Z)-hexadecenoic acid (C16:1 ω-7). Its fragmentation showed a characteristic base peak atm/z55, a prominent ion peak atm/z236([M - 31]+)due to the loss of a methoxy group, and an ion atm/z194 ([M - 74]+)due to the loss of a McLafferty ion(Fig.4).
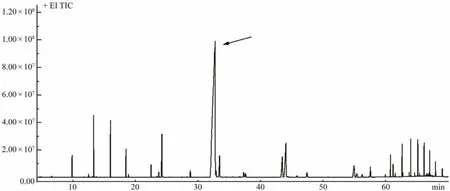
Fig.3 Total ion chromatogram of the concentrated palmitoleic acid showing the composition of fatty acid methyl esters.
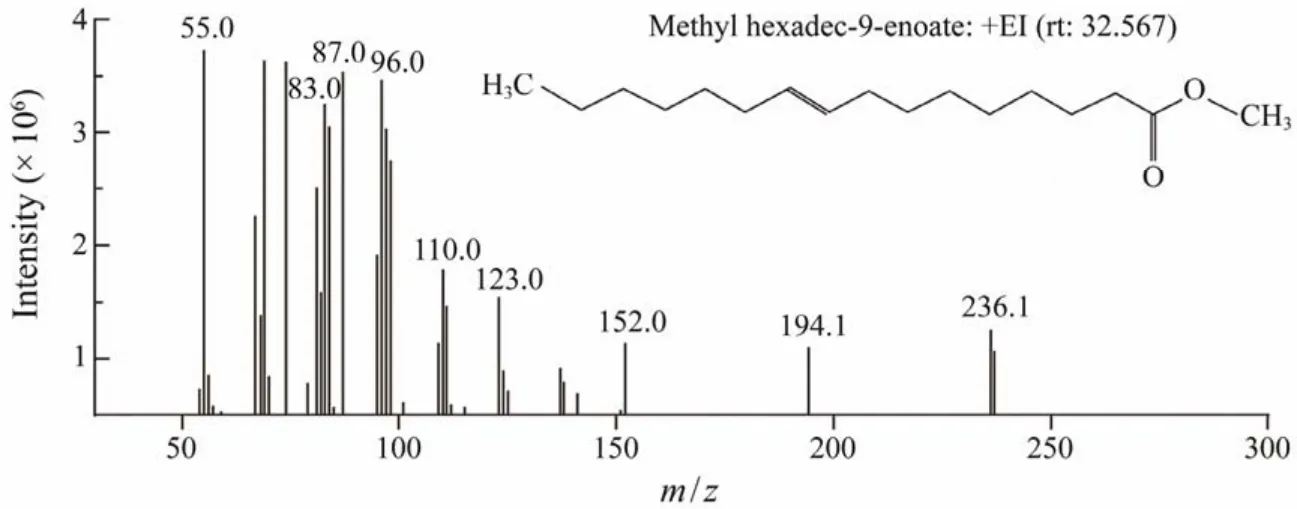
Fig.4 Mass spectrum analysis of methyl palmitoleic acid (C16:1, ω7)isolated from Tribonema minus.
This study is the first to successfully obtain such highly concentrated palmitoleic acid by combining AMFP with urea complexation as the isolation method. This approach is superior over molecular distillation or carbon dioxide supercritical extraction due to its relatively low capital and operating costs. However, the high-purity palmitoleic acid could not be obtained through urea complexation alone.Optimizing the extraction conditions and applying other innovative separation techniques will be the focus of future research to further improve the purity of palmitoleic acid.
3.3 In vitro Antibacterial Activity of the Concentrated Palmitoleic Acid Against S. agalactiae
Palmitoleic acid is an effective antibacterial agent against a range of Gram-positive pathogenic bacteria, includingS.aureus,Corynebacteriumsp.,Bacillus cereus, andB.weihenstephanensis; however, only a few studies have investigated its inhibitory effects on fish pathogens such asS. agalactiae. In the present work, the antibacterial effect againstS.agalactiaeof highly concentrated palmitoleic acid obtained fromT. minuswas further investigated to expand the application of palmitoleic acid isolated from microalgae for the control ofS. agalactiaeinfections in aquaculture.
Given that the concentrated palmitoleic acid contains other fatty acids, its antibacterial activity againstS. agalactiaewas evaluated in reference to palmitoleic acid standard (> 98.5%). The concentrated palmitoleic acid significantly inhibited the growth ofS. agalactiaeat 10 mg mL-1and formed an inhibition zone diameter of 12.10 mm ± 0.39 mm, which was comparable with that of the standard (13.12 mm ± 0.16 mm)(Figs.5a and b). Thus, palmitoleic acid was the dominant contributor of the anti-S. agalactiaeeffect. In addition, microbroth dilution assay showed that the MIC of the concentrated palmitoleic acid againstS. agalactiaewas only 31.25 μg mL-1(Fig.5c), which is lower than those for essential oils and terpinen-4-ol (Claudiaet al., 2018; Zhanget al., 2018b). This study is the first to provide evidence that palmitoleic acid antagonizes fish pathogenS. agalactiae, and the palmitoleic acid derived fromT. minusis a good candidate for the development of a novel agent to preventS. agalactiaeinfections in aquaculture.
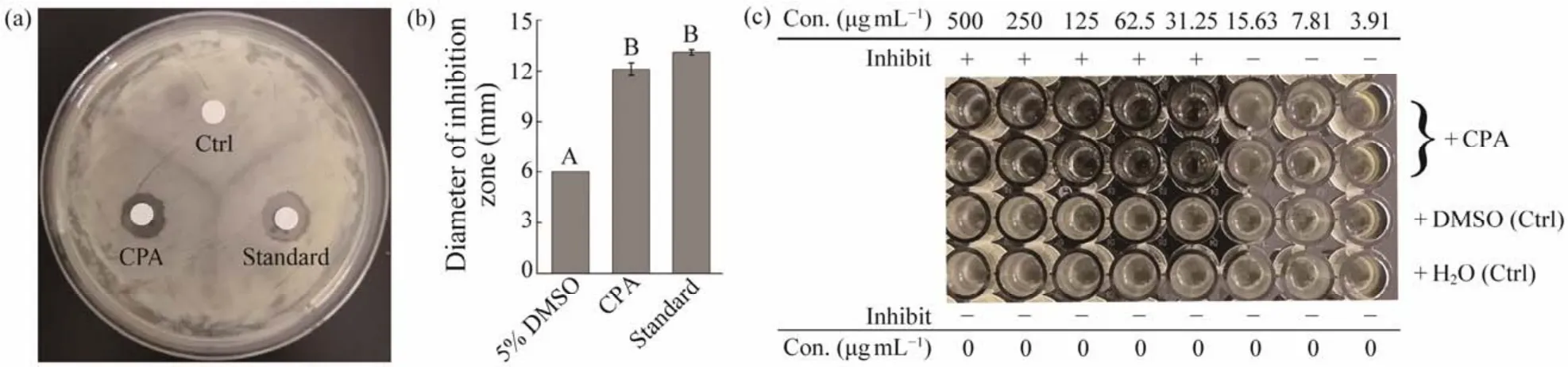
Fig.5 Inhibitory effects of the concentrated palmitoleic acid on the growth of Streptococcus agalactiae. (a), disk diffusion assay, Ctrl, 5% dimethyl sulfoxide; (b), diameters of the inhibition zones; (c), microbroth dilution assay. CPA, concentrated palmitoleic acid; Standard, high-purity palmitoleic acid (> 98.5%); Con, concentration; DMSO, dimethyl sulfoxide. In (c),‘+’ indicates that the concentration of sample can inhibit S. agalactiae growth, and ‘-’ indicates vice-versa. Values are expressed as means ± standard deviations of three replicates. Different capital letters above the bars indicate significant differences at P < 0.05.
4 Conclusions
Among the five testedTribonemaspecies cultured with different INC,T. minusshowed the highest palmitoleic acid productivity when grown at 18 mmol L-1INC. Hence, this species was consequently used for the isolation of palmitoleic acid. Isolation and enrichment through combined AMFP and urea complexation significantly increased the palmitoleic acid content to obtain a purity of > 80%. Moreover, the concentrated palmitoleic acid showed strong antibacterial activity againstS. agalactiaewith a relatively low MIC value. This study presents the first evidence that palmitoleic acid can inhibit fish pathogenS. agalactiae, and the palmitoleic acid derived fromT. minusshows potential as a novel antibacterial agent to preventS. agalactiaeinfections in aquaculture.
Acknowledgements
This work was supported by the Jiangsu Provincial Natural Science Foundation (No. BK20200734), the Hubei Provincial Natural Science Foundation (No. 2021CFB224),the Engineering Research Center for Tropical and Subtropical Aquatic Ecological Engineering, Ministry of Education, Jinan University, P. R. China (No. 2021A0401), the Research and Innovation Initiatives of Wuhan Polytechnic University (WHPU)(No. 2021Y06), the Hubei Key Laboratory for Processing and Transformation of Agricultural Products (WHPU, China)(No. 2020HBSQGDKFB17), and the Sinopec Joint Program of China Petroleum and Chemical Corporation (No. ST18005-2).
杂志排行
Journal of Ocean University of China的其它文章
- A Theoretical Model for the Microwave Emissivity of Rough Sea Surfaces
- Mechanism of Regional Subseasonal Precipitation in the Strongest and Weakest East Asian Summer Monsoon Subseasonal Variation Years
- Analytical and Experimental Studies on Wave Scattering by a Horizontal Perforated Plate at the Still Water Level
- Theoretical Prediction of the Bending Stiffness of Reinforced Thermoplastic Pipes Using a Homogenization Method
- Penetration Resistance of Composite Bucket Foundation with Eccentric Load for Offshore Wind Turbines
- Application of Converted Displacement for Modal Parameter Identification of Offshore Wind Turbines with High-Pile Foundation
