Feasibility of Surfgrass Phyllospadix iwatensis Transplantation Along the Rocky Coasts of Shandong Peninsula, China
2022-12-27ZHANJiaxinCHENGRanHOUXinWANGHuanZHANGPeidongKANGBinandLIWentao
ZHAN Jiaxin, CHENG Ran, HOU Xin, WANG Huan, ZHANG Peidong, KANG Bin,and LI Wentao
The Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao 266003, China
Abstract Surfgrass Phyllospadix iwatensis is a dominant seagrass species along the east coast of Shandong Peninsula, China. Like other seagrasses in the world, surfgrass has been declining in the past decades. To assess the possibility of transplanting P. iwatensis in the coastal area, a new surfgrass transplant system was designed and applied to conduct a surfgrass transplantation experiment in a tide pool and a subtidal area, and its survival, morphological and physiological parameters were examined from May 2019 to June 2020. The results showed that after a year since transplantation, more than 65% of the transplants survived and the survival rate was higher in the subtidal site than in the tide pool. The morphological measurements including shoot height, leaf width, number of leaves per shoot, rhizome diameter and elongation rate, and number of roots per shoot were all higher in the subtidal area than in the tide pool, showing that the P. iwatensis transplants grew better in the subtidal area than in the tide pool in the study area. These results indicated that surfgrass can be transplanted in the two areas, and it is probably more suitable in subtidal area than in tide pool in the study area.
Key words surfgrass Phyllospadix iwatensis; rocky substrate; seagrass transplantation; transplant survival; growth
1 Introduction
Seagrasses are higher plants growing in shallow areas of temperate, tropical and subtropical coastal waters, and generally have flowers, seeds, rhizomes and roots (Orthet al., 2000). At present, there are 72 seagrass species identified in the world, among which 22 species were found in China and all belong to 4 families and 10 genera (Shortet al.,2011). Seagrass meadows are ecologically important for many reasons, such as serving as a habitat and a food source for a wide variety of marine animals, and improving the stability of sediments (Kochet al., 2006; Sheppardet al.,2007). However, due to human activities and global climate changes, the seagrass beds have been declining worldwide in the past decades (Shortet al., 2011).
In genusPhyllospadix, there are five species in the world,i.e.,Phyllospadix torreyi,P. serrulatus,P. scouleri,P. iwatensisandP. japonicus. The first three species are found only along the North American Pacific coast (Hartoget al.,2007), and the latter two species are endemic to the rocky coasts of northeastern Asia, including the coasts of Japan and Korean Peninsula (Shortet al., 2007). According to the IUCN, the three American surfgrass species are stable,while the two Asian species are listed as vulnerable or endangered ones (https://www.iucnredlist.org/). In China, the two surfgrass species are reported in the coastal area of Liaoning, Hebei, and Shandong Provinces, China (Zhenget al., 2013). In Shandong Province, surfgrass had been a widespread and dominant species, which had been conventionally used for thatching houses along the coastal area.However, due to various causes (e.g., human activities),most of the surfgrass beds have disappeared, especially those in the tide pools in the intertidal areas and in the shallow subtidal areas. Though the tide pools are generally small water bodies, their ecosystems usually have a relatively high biodiversity, and the surfgrass meadows may have been playing an important role (Shelton, 2010).
Until now, most of the researches about seagrass restoration are for the species growing in soft bottoms, such as eelgrassZostera marina(Williams, 1995). Unlike the seagrass species inhabiting in soft-bottom habitats (e.g.,Z. marina)with mild waves and currents, surfgrass grows in rocky areas where waves and currents are usually very strong (Turner, 1985; Williams, 1995; Park and Lee, 2009). Very few researches have been conducted in the surfgrass restoration(Holbrooket al., 2002; Bullet al., 2004; Park and Lee,2010), which might be due to their limited distribution and the harsh environments.
To assess the possibility of restoring the surfgrass beds,aP. iwatensistransplantation experiment was performed in two typical habitats: tide pool and subtidal area at the east end of Shandong Peninsula, China. Moreover, a recently developed transplanting technique for surfgrass transplantation was applied in the experiment (Houet al., 2021).The survival of the transplants in the two habitats was evaluated, and the morphology, growth, and physiological characteristics of the transplants were examined to analyze the growth responses ofP. iwatensisto the habitats. The experiments might provide some clues for the future restoration of degraded surfgrass beds.
2 Materials and Methods
2.1 Study Site
Mashanli (37˚19.3′N, 122˚35.3′E)is located on the east end of Shandong Peninsula, China (Fig.1). The location was adjacent to the Yellow Sea and regularly exposed to strong waves. The coast is mostly rocky, and lots of rocks and boulders are found in the coastal water. There are many tide pools with various sizes and depths in the area, and a thin layer of sand is deposited on some areas of the rocky sea bottom. There are mainlyP. iwatensis,Z. marina,andZ. caespitosain Mashanli, among whichP. iwatensisis mostly found in subtidal areas.
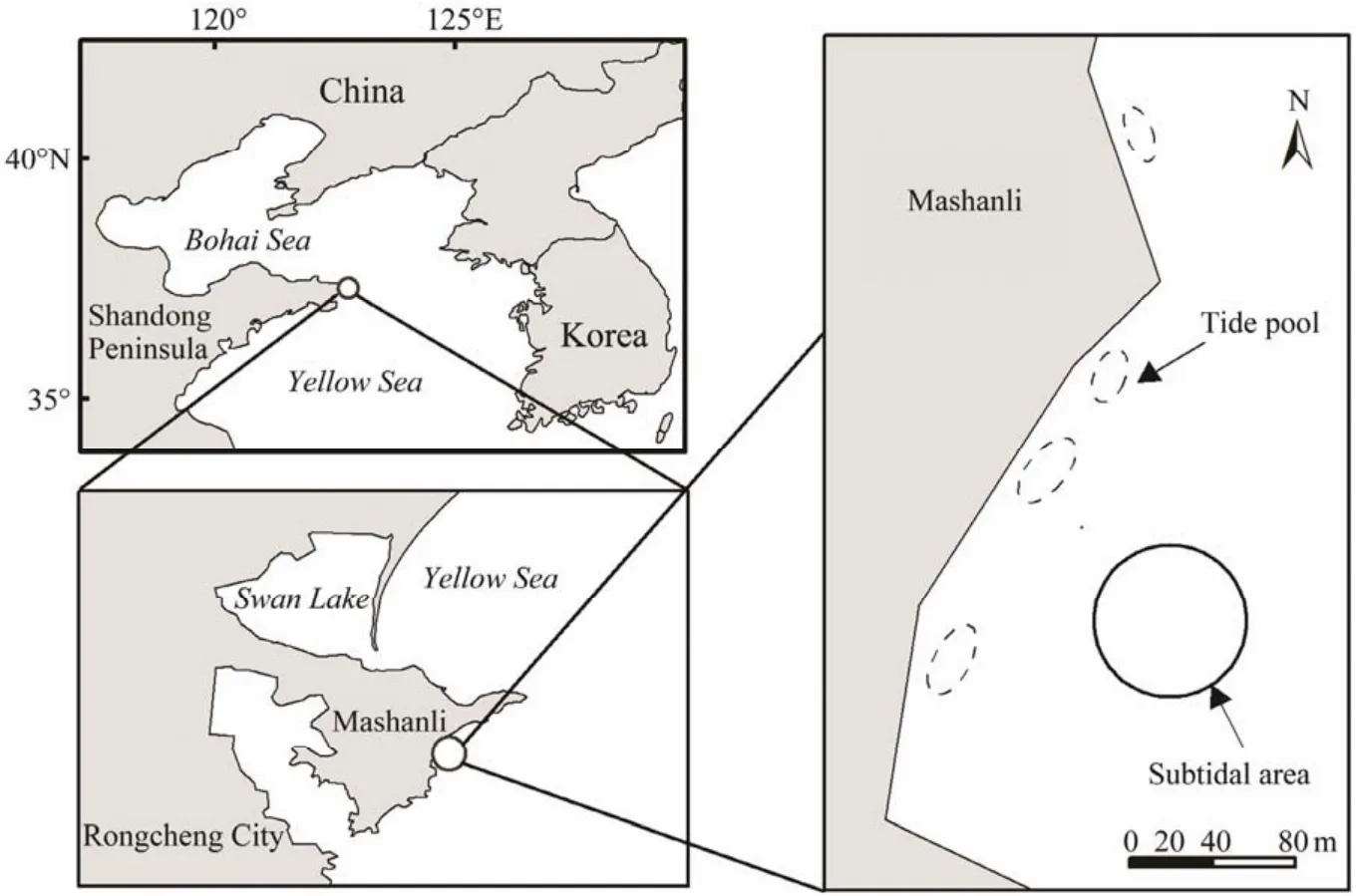
Fig.1 Study sites in Mashanli of Shandong Peninsula, China.
In this study, a subtidal site and a tide pool in the intertidal area were chosen as experiment sites, representing the two typicalP. iwatensishabitats in the study area. The sea bottom of the subtidal area for transplantation experiment was flat, with someP. iwatensispatches distributed, whereas the tide pool existed as a separate body of water only at low tide with someP. iwatensispatches. The water depth at the subtidal site for transplantation was about 0.3 m, and that in the tide pool was about 0.1 m below the mean lower low water (MLLW).
2.2 Environmental Parameters
Underwater temperature ( ℃)and light intensity (lx)atP. iwatensiscanopy level were monitored every 15 min from May 2019 to June 2020 using a HOBO data logger(Onset Computer Corp.). Light intensity recorded by the data logger was converted to photon flux density (PFD)by concurrent photon flux measurements using an LI-1400 data logger and an LI-193SA spherical quantum sensor (LICOR, Inc.). The daily average water temperature and the daily PFD (mol photons m-2d-1)were then calculated.
2.3 Transplantation of P. iwatensis
Transplantation was conducted in a tide pool in intertidal area and a subtidal area in May of 2019.P. iwatensistransplants were collected from a subtidal area (donor bed),which was several meters away from the transplant site in the subtidal area. Only the adult shoots with similar shoot height were selected as transplants. To monitor their growth,all the transplant roots were removed, and the rhizomes were also cut off with only a 1-cm rhizome segment left.Two hundredP. iwatensisshoots were fixed with cotton threads on a soft plastic plate with 144 holes (diameter 2 cm; 2 shoots for each hole in the central area of the plates),and the plastic plate attached with transplants were then tied to a cement plate (25 cm × 25 cm; with a 1-cm diameter hole at each corner of the cement plate for mounting the plastic plate)as a transplant unit (Fig.2a). A total of 12 transplant units were used in this experiment, in which half of them were deployed in the subtidal area (named as A1 –A6)and another half were deployed in the tide pool (named as B1 – B6). At each habitat, three of the six transplant units were used for monitoring transplant survival (i.e., A1, A2,A3 in the subtidal area and B1, B2, B3 in the tide pool)and the other three were for their morphological, physiological and growth measurements (i.e., A4, A5, A6 in the subtidal area and B4, B5, B6 in the tide pool).
On May 18, 2019, the transplant units B1 – B6 (Fig.2a)were directly deployed on a flatbed area of a tide pool that can provide shelter for the plants. In the subtidal area, the waves and currents are stronger than in the tide pool. So the transplant units A1 – A6 were each tied to a large cement frame for anchoring the transplant units (Fig.2b)to resist the waves and currents, and then deployed in the subtidal area.
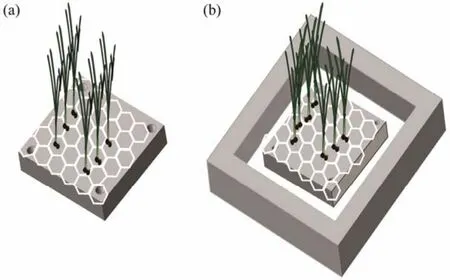
Fig.2 Diagram of planting units deployed at the tide pool(a)and the subtidal area (b).
2.4 Post-Transplant Monitoring
After the transplantation, sampling was conducted monthly. At each sampling event, the transplant units in both subtidal area and tide pool were temporally taken out of water. For transplant unit A1 – A3 and B1 – B3, all the remaining transplants at each unit were counted. The number of the remaining plants on each unit was divided by 200, and the value was defined as the survival rate on the corresponding unit.
For monitoring the transplant growth, a marking method was used (Kerr and Strother, 1985; Parket al., 2010). At each sampling time, 30 transplants were randomly collected (15 from A4, A5 and A6, and another 15 from B4, B5 and B6)and punched with a needle at their lower part of the sheath. The marked shoots were then retrieved at next sampling time for the assessment of transplant growth in the two habitats. At the same time, another 30 transplant shoots were also randomly collected (15 from A4, A5 and A6, and another 15 from B4, B5 and B6)for the morphological and physiological measurements. To assess the transplant establishment, 15 surfgrass shoots were also randomly collected in the donor bed for the morphological and physiological measurements.
Shoot height, number of leaves per shoot, leaf width, rhizome length, rhizome diameter, number of roots per shoot were measured or counted in the collected samples. The photosynthetic pigment was extracted withN,N-dimethylformamide, and then compared and determined with a spectrophotometer (Dunton and Tomasko, 1994). An anthrone method (Lewiset al., 2007)was used to determine the soluble sugar and starch contents of the above tissues.
For the measurements of transplant growth, the new leaves and old leaves were separated based on the pinhole mark on the leaves. The rhizome elongation part (including the roots)after the last sampling event (the original rhizome length was 1 cm)was regarded as the new belowground tissue. The rhizome elongation rate was calculated by dividing the length of newly grown rhizome part by the time period (in days)since the pinhole mark was made at the last sampling event. All these tissues were then dried at 60℃ to constant weight. The aboveground / belowground productivity was calculated by dividing the dry weight of newly grown leaves and rhizome/root tissues by the time period (in days)since the pinhole was made.
Due to the COVID-19 pandemic, the routine monitoring during the period from December 2019 to May 2020 was cancelled and no data were presented during the period.In addition, the aboveground productivity was also missed.
2.5 Statistical Analysis
The data are shown as mean ± standard error (± SE).A studentt-test was used to compare the differences in the transplant survival rate and growth in the two habitats. A one-way ANOVA was used to compare the differences in the morphological and physiological measurements among the reference plants and the transplants in different sampling times. A two-way ANOVA was used to compare the differences in the all measurements among the reference plants and the transplants in the two habitats and at different sampling times. All statistical analysis was analyzed by using SPSS 25.0, and the map and figures were created in Origin 2018 and ArcGIS 10.2.
3 Results
3.1 Temperature and Light
The water temperature and photon flux density (PFD)at the study site are shown in Fig.3. The water temperature varied distinctly with months. During the experiment, the mean water temperature was the highest in August (28.58℃ )and the lowest in December (-1.04℃ )during the study period. PFD reached the highest in March 2020 (28.13 mol photons m-2d-1)and the lowest in September (1.02 mol photons m-2d-1).
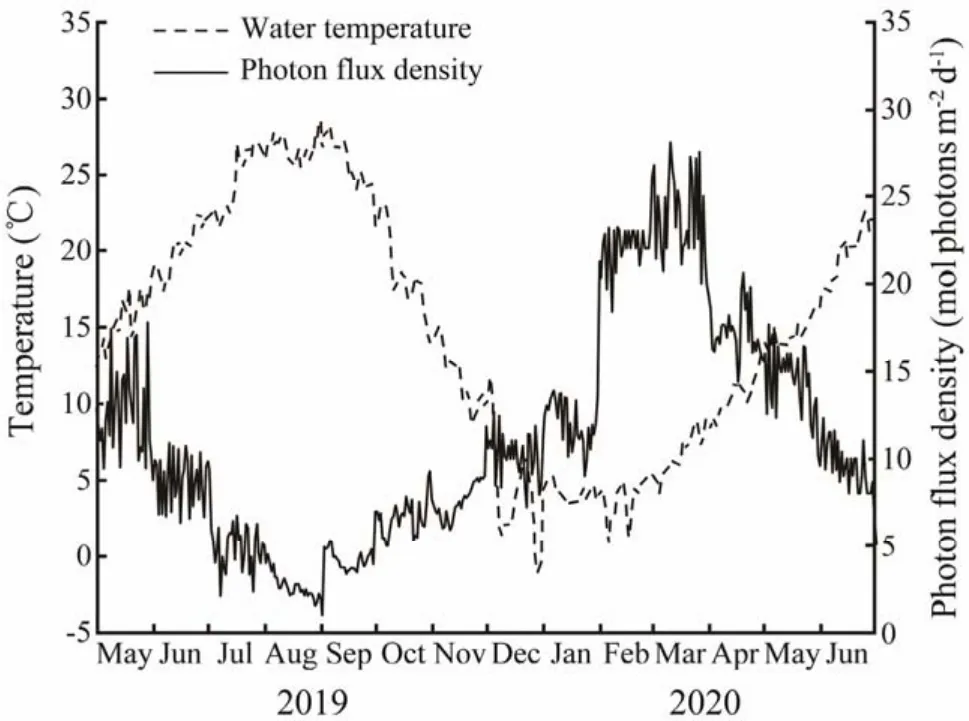
Fig.3 Temporal variations in water temperature and photon flux density (PFD)at Mashanli, Shandong Peninsula,China.
3.2 Transplant Survival
After transplantation, the survival rate of transplants decreased gradually (Fig.4). The survival rate in the subtidal area was significantly lower than that in the tide pool at the initial stage (in June and July, bothP< 0.05), and after August, the survival in the subtidal area became significantly higher than that in the tide pool (allP< 0.05). However, the transplant survival rate in the two habitats was higher than 65% during the whole experimental period.
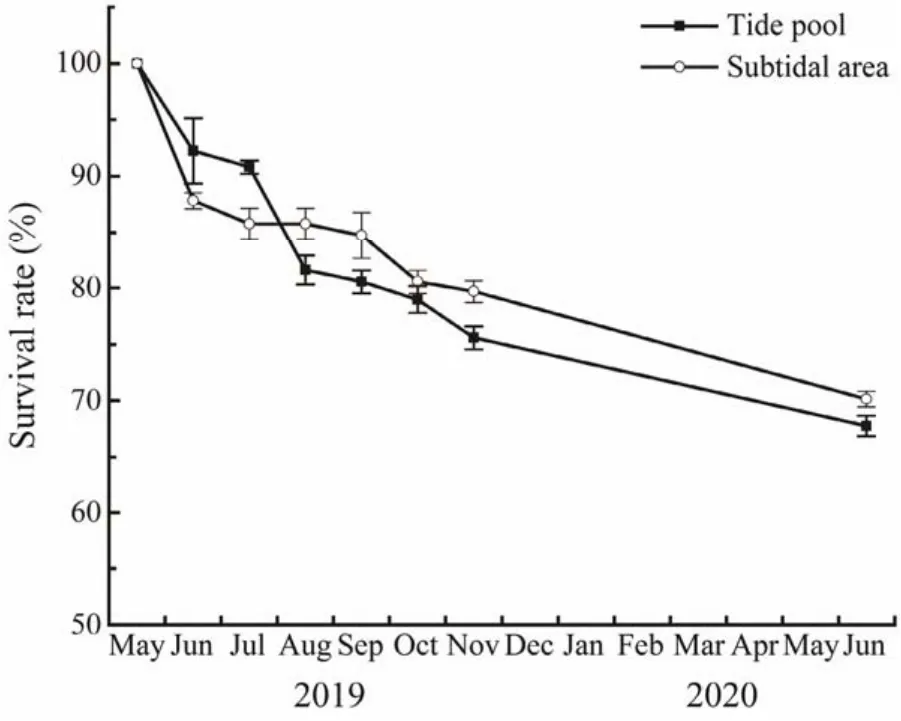
Fig.4 Transplant survival of Phyllospadix iwatensis in the tide pool and subtidal area.
3.3 Morphological Measurements
The shoot height of the transplants was significantly lower than that of the natural shoots in the sampling time (Fig.5a;P< 0.05). The maximum shoot height of the naturally growing shoots in August (75.5 cm ± 1.5 cm)was significantly higher than transplants in two habitats. Significant differences were found between the two habitats and between sampling times (two-way ANOVA; Fig.5a and Table 1). The shoot height of transplants in the subtidal area was significantly higher than that in the tide pool and the highest values was recorded in August (60.6 cm ± 1.7 cm).On the other hand, the shoot height in the subtidal area in August was significantly higher than that in other months except for June 2020 (one-way ANOVA, Duncan, allP<0.05). While in the tide pool, the highest values were recorded in June 2020 and the lowest in June 2019 (37.53 cm± 1.33 cm), and significant variations in the shoot height were observed between sampling times (one-way ANOVA,F= 13.497,P< 0.01).
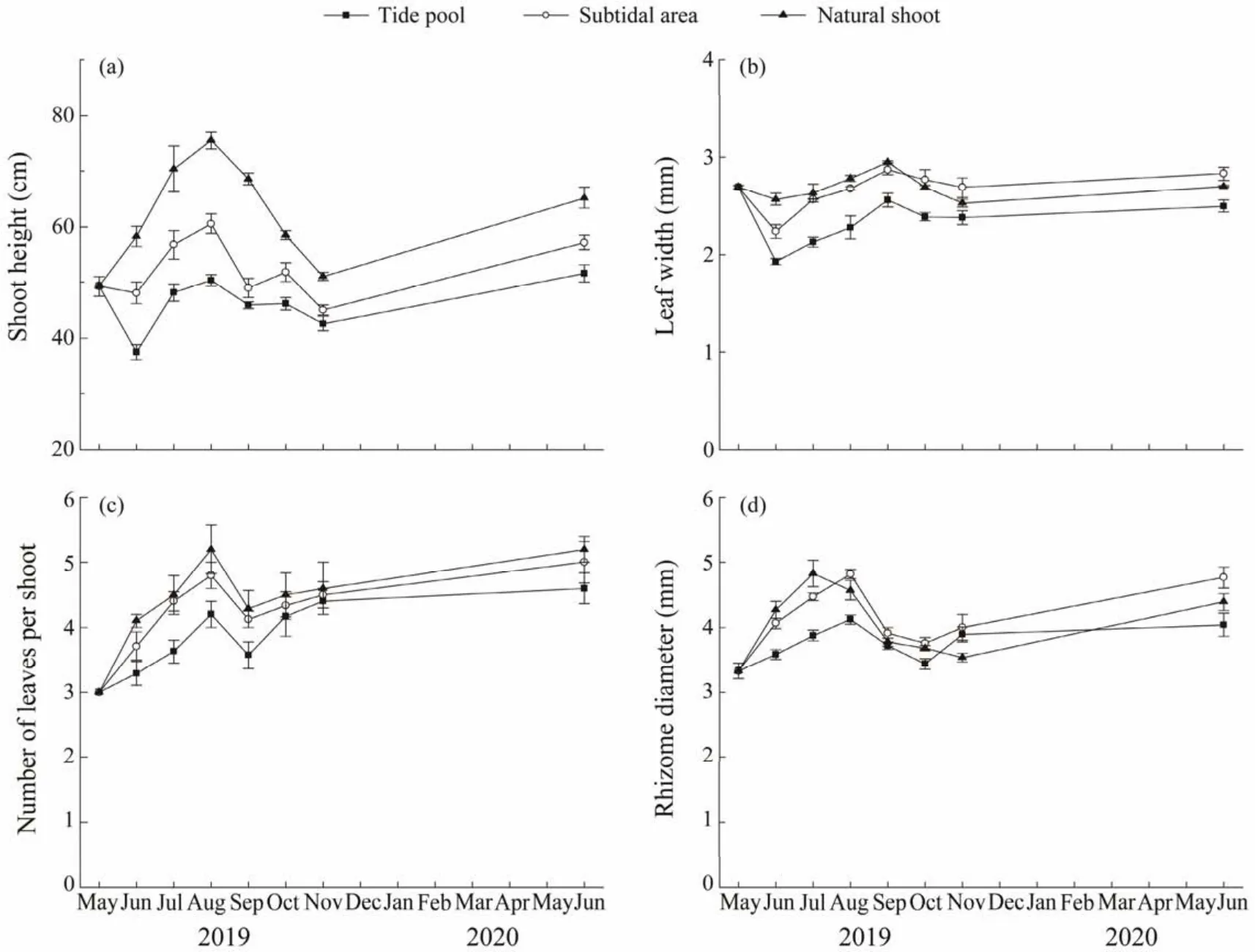
Fig.5 Morphological measurements in Phyllospadix iwatensis transplants in the tide pool and subtidal area as well as the natural plants in the subtidal area.
From May to September, the leaf width of the natural shoots was higher than that of the transplants in the tide pool except for June (P> 0.05). During the half year after the transplantation, significant differences were found between sampling times (two-way ANOVA; Fig.5b and Table 1). In the subtidal area, leaf width of transplants reached the highest values in September (2.87 mm ± 0.05 mm),and lower than the natural shoots, and significant variations in the leaf width were observed between sampling times (one-way ANOVA,F= 10.034,P< 0.01). In addition,the lowest values in the tide pool were observed in June 2019 (1.93 mm ± 0.03 mm).
The number of leaves of transplants were lower than that of the natural shoots in the sampling time, and significant difference was observed in natural shoots and transplants in the tide pool (P< 0.05). The highest value was observed in August (5.2 ± 0.4). The number of leaves in the subtidal area was significantly higher than that in the tide pool from July to September (allP< 0.05). The value in the subtidal area increased after transplantation, reaching the highest in June 2020 (5.0 ± 0.3), and significant variations in the number of leaves were observed between sampling times (oneway ANOVA,F= 11.608,P< 0.01; Fig.5c).
Rhizome diameter ofP. iwatensisin natural seagrass bed increased first and then decreased with time, which was higher than transplants in two habitats in June and July (P> 0.05). Rhizome diameter ofP. iwatensistransplants in the subtidal area was generally higher than that in the tide pool,and significant differences (allP< 0.05)were observed between the two habitats except for those in September and November (P= 0.072 andP= 0.69, respectively). In the two habitats, the diameter of rhizome had a similar variation trend, both with the highest values in August (4.82 mm ±0.07 mm for the subtidal area and 4.12 mm ± 0.07 mm for the tide pool; Fig.5d). The highest values in the subtidal area were significantly higher than those in other months except for in June 2020 (one-way ANOVA, Duncan, allP< 0.05), and a significant interaction was found between habitats and months (two-way ANOVA,P< 0.01; Table 1).
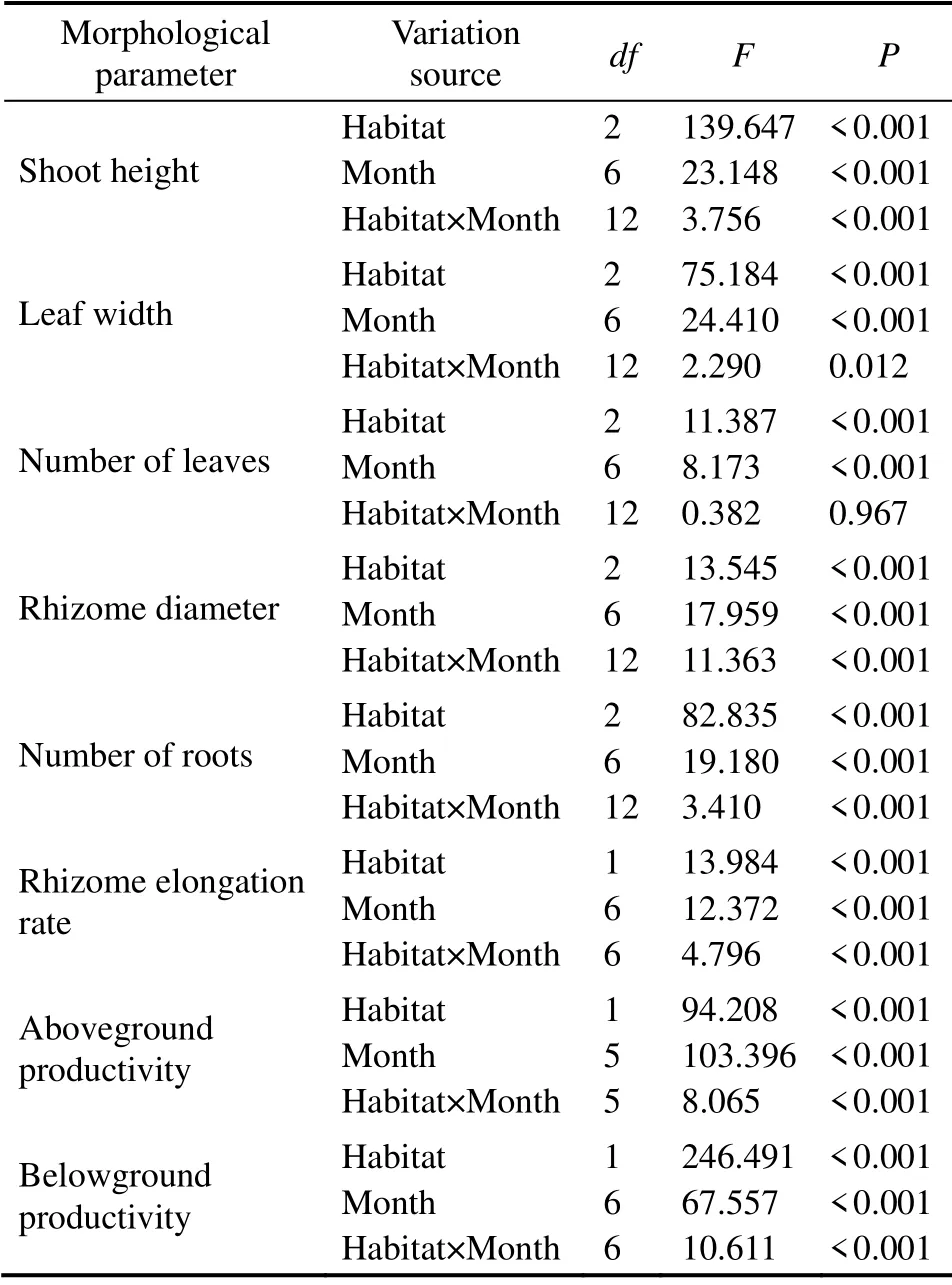
Table 1 Two-way ANOVA of spatial and temporal variations of the morphological and growth parameters of Phyllospadix iwatensis in two habitats (tide pool and subtidal area)
3.4 Transplant Growth
New roots emerged from the newly grown rhizomes of the transplants in the two habitats at the first sampling events after transplantation (Fig.6a). The number of roots in the two habitats increased gradually after transplantation, except for a temporal decrease observed in October. The number of roots in the subtidal area was significantly higher than that in the tide pool (studentt-test, allP< 0.05)The highest values (5.2 ± 0.2)was observed in June 2020, which was significantly higher than those in other months (oneway ANOVA, Duncan, allP< 0.01).
Rhizome elongation rates of the transplants in both habitats decreased first and then increased (Fig.6b). Significant differences were found between habitats and between sampling times (two-way ANOVA, allP< 0.01; Table 1).The rhizome elongation rates in the subtidal area was significantly higher than those in the tide pool in June, July,September, and November (studentt-test, allP< 0.05). The maximum value of the rhizome elongation rate in the subtidal area was observed in June 2019 ((0.30 ± 0.01)mm d-1),and the minimum value was found in September ((0.02 ±0.01)mm d-1), while the significant variations in the rhizome elongation rate were observed between sampling times(one-way ANOVA,F= 12.925,P< 0.01).
The shoot aboveground productivity in the subtidal area was significantly higher than that in the tide pool, and it also varied significantly with time (two-way ANOVA, allP< 0.01; Figs.6c and 6d). Significant differences were also observed in the aboveground productivity between the two habitats in June, July, August and November (one-way ANOVA, Duncan, allP< 0.05). The highest values were found in August in the subtidal area ((18.81 ± 0.49)mg DW shoot-1d-1)and the lowest in November in the tide pool((5.26 ± 0.20)mg DW shoot-1d-1).
For the productivity of the below-ground tissues, it can be seen that there were significant differences between the two habitats during the study period after transplantation(two-way ANOVA, allP< 0.01; Fig.6d). The belowground productivity in the subtidal area was significantly higher than that in the tide pool (studentt-test, allP< 0.01). The highest values occurred in the subtidal area in August ((8.55± 0.49)mg DW shoot-1d-1, and the lowest values occurred in the tide pool in June 2019 ((1.90 ± 0.08)mg DW shoot-1d-1).
3.5 Physiological Parameters
3.5.1 Photosynthetic pigment content
In both habitats, the photosynthetic pigment content of transplants decreased in June (one month after transplantation), and then gradually increased (Figs.7a, 7b, 7c, 7d).The contents of chlorophyllaof the natural shoots were higher than transplants during the study period, and significantly higher than that of the transplants in the tide pools(P< 0.01). The content of chlorophyllaof the transplants in the subtidal area was significantly higher than that in the tide pool (allP< 0.05, Fig.7a)except for November. The highest values in the subtidal area ((24.26 ± 0.74)mg cm-2)and the tide pool ((21.41 ± 0.65)mg cm-2)were both observed in September and the lowest values were observed in November ((4.35 ± 0.06)mg cm-2and (6.59 ± 0.17)mg cm-2for the subtidal area and tide pool, respectively), both of which were significantly different from those in other months (one-way ANOVA, allP< 0.05).
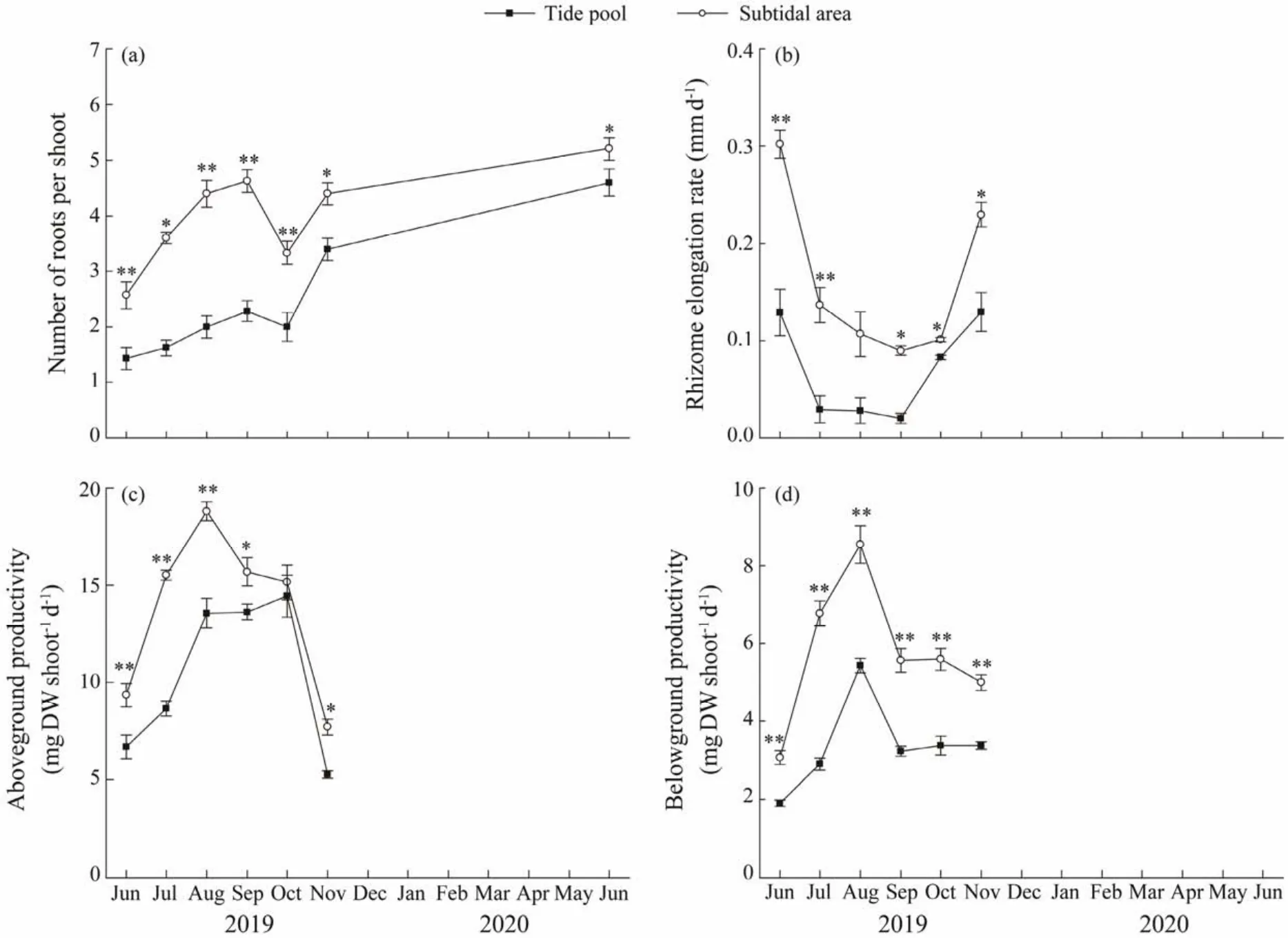
Fig.6 Growth of Phyllospadix iwatensis in the tide pool and subtidal area.
The content of chlorophyllbof the natural shoots was significantly higher than that of transplants (P< 0.05). The content of chlorophyllbin the subtidal area was higher than that in the tide pool, and significant differences were found in July, August, September, and October (allP< 0.05;Fig.7b).
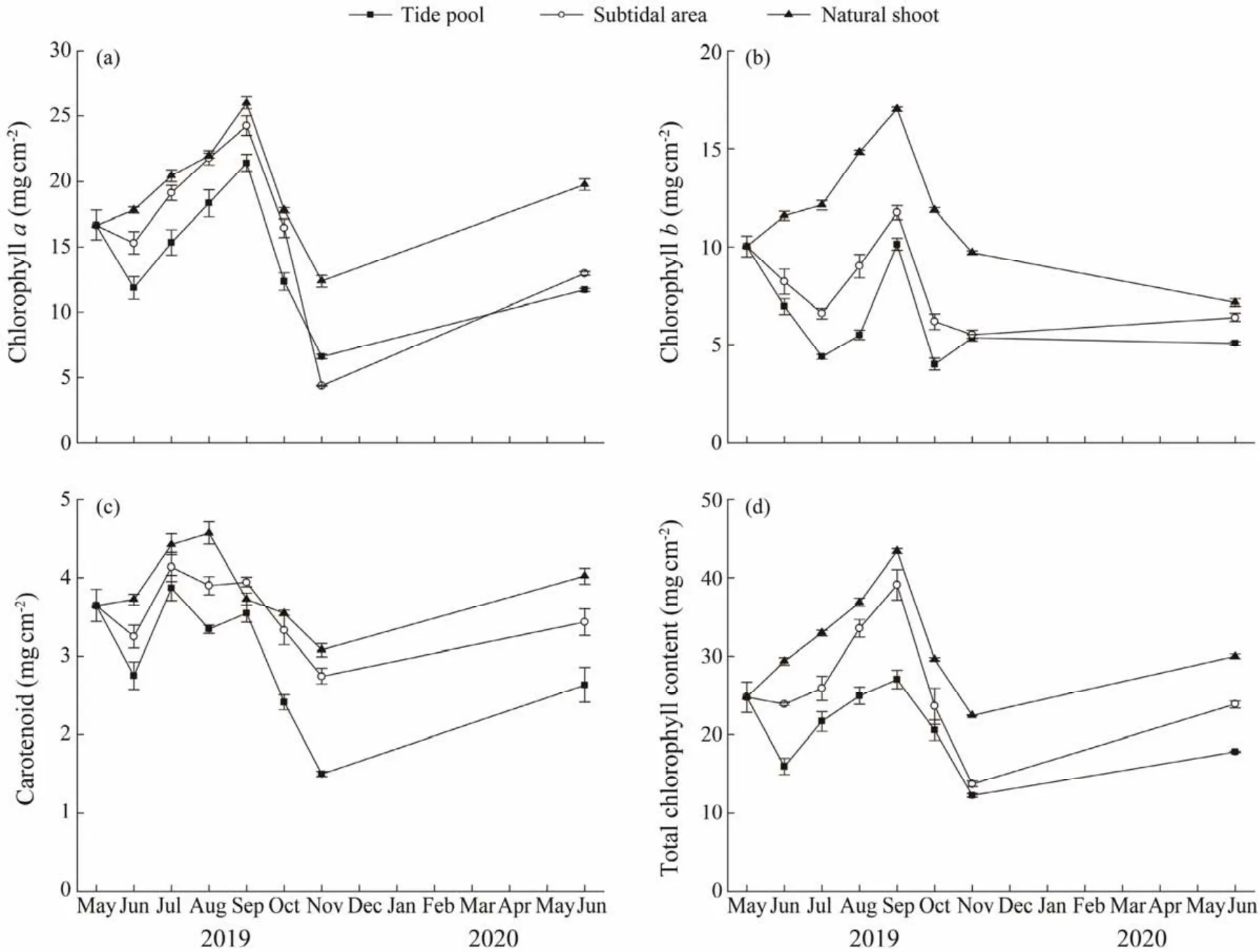
Fig.7 Chlorophyll a content (a), chlorophyll b content (b), carotenoid content (c)and total chlorophyll content (d)of Phyllospadix iwatensis transplants in the tide pool and subtidal area, and of the natural plants in the subtidal area.
The carotenoid content of the natural shoots was significantly higher than that of transplants in June, August and November (P< 0.05). The highest value was observed in natural shoots in August ((4.57 ± 0.14)mg cm-2), significantly higher than in transplants (P< 0.05). After transplantation, the carotenoid content in the subtidal area was higher than that in the tide pool, and significant differences were observed in August, September, October and November(allP< 0.05, Fig.7c). The highest values were observed in July 2019 ((4.14 ± 0.19)mg cm-2and (3.87 ± 0.09)mg cm-2for the subtidal area and tide pool, respectively), and the lowest values were observed in November ((2.75 ± 0.09)mg cm-2and (1.50 ± 0.03)mg cm-2for the subtidal area and tide pool, respectively; Fig.7c).
The total chlorophyll content of the natural shoots was significantly higher than that of the transplants in two habitats in June, November and June 2020 (P< 0.05). The content in the subtidal area was higher than that in the tide pool, and significant differences were observed in June, August, and September (allP< 0.05, Fig.7d). In the two habitats, the maximum values of total chlorophyll content both were observed in September ((39.13 ± 1.94)mg cm-2and(27.03 ± 1.24)mg cm-2for the subtidal area and tide pool,respectively)and the minimum values were observed in November ((13.73 ± 0.37)mg cm-2and (12.25 ± 0.23)mg cm-2for the subtidal area and tide pool, respectively).
3.5.2 Soluble sugar and starch content
The soluble sugar content of the natural shoots was higher than that of the transplants, with the highest values of aboveground and belowground tissues observed in August((293.50 ± 8.79)mg g-1and (360.77 ± 11.31)mg g-1, respectively). Significant differences were observed in the soluble sugar content of aboveground tissues between the two habitats and between sampling times (two-way ANOVA;Fig.8a, Table 2). The highest value was observed in subtidal area in August ((256.63 ± 13.09)mg g-1), and the lowest value was observed in tide pool in November ((40.45 ±1.29)mg g-1). And between the two habitats, significant differences were observed in June, July, September, and November (allP< 0.05). In the belowground tissues, the content of the soluble sugar in the subtidal area was significantly higher than that in the tide pool during the study period except for August and June 2020 (allP< 0.05; Fig.8b).The highest value was observed in the subtidal area in August ((338.87 ± 12.96)mg g-1), and the minimum value in the tide pool in November ((96.99 ± 2.75)mg g-1).
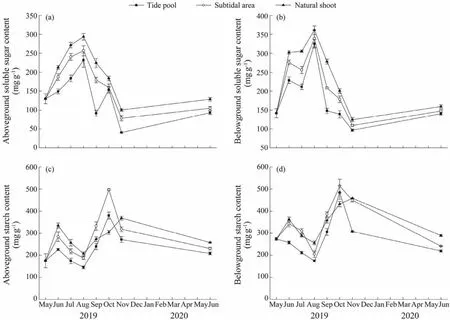
Fig.8 Soluble sugar and starch contents in aboveground (a and c)and belowground (b and d)tissues of Phyllospadix iwatensis transplants in the tide pool and subtidal area and in the natural plants in the subtidal area.
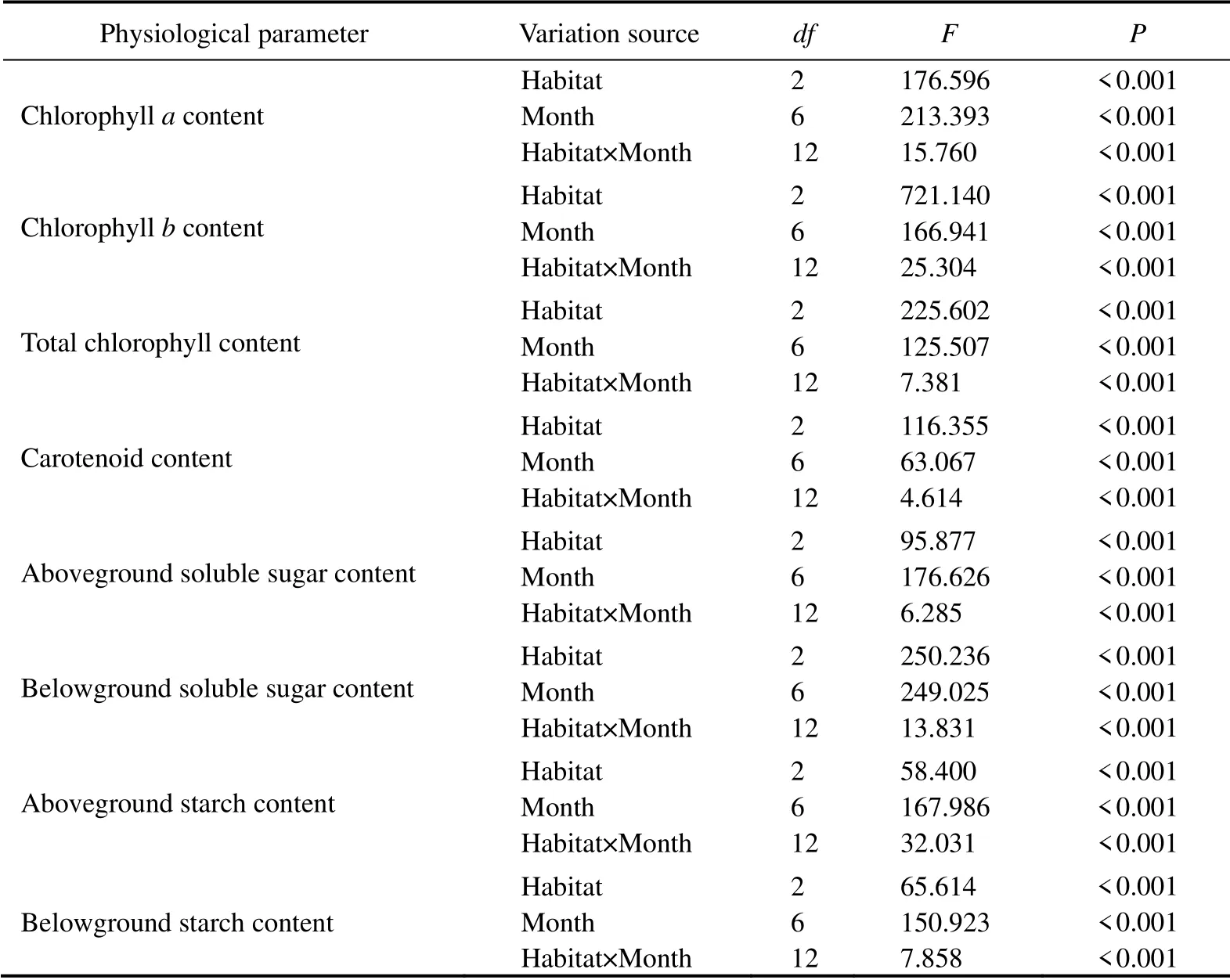
Table 2 Two-way ANOVA of spatial and temporal variation in the physiological parameters of Phyllospadix iwatensis in two habitats (tide pool and subtidal area)
The starch content of the natural shoots was higher than that of the transplants. The starch content of the aboveground and belowground tissues varied significantly with time in the two habitats (one-way ANOVA, bothP< 0.01).The highest values of aboveground tissues was observed in the subtidal area in October ((497.28 ± 3.96)mg g-1), which was significantly higher than in other months (one-way ANOVA, Duncan,P< 0.01), and the lowest values was found in the tide pool in August ((145.06 ± 6.20)mg g-1). In June, July, September and November, the starch content of belowground tissues in the subtidal area were significantly higher than those in the tide pool (allP< 0.05). The highest value was observed in subtidal area in October((513.73 ± 30.91)mg g-1), and the lowest value was found in tide pool in August ((174.23 ± 5.13)mg g-1).
4 Discussion
4.1 Transplant Survival
Failure ofZ. marinatransplantation is mainly due to the poor anchoring of the transplants at the sea bottom (Orthet al., 1999; Park and Lee, 2007). UnlikeZ. marinathat grows on the soft bottoms, especially in sheltered areas,surfgrass occupies rocky substrates where there are strong waves and currents. Therefore, anchoring the surfgrass transplant shoots to the seabed is a challenging task. Holbrook(2002)conducted transplantation experiments on surfgrassP. torreyiseedlings, in which the survival of surfgrass seedling transplants fixed on natural hosts (algae)and artificial hosts (nylon ropes and nylon nets)were assessed. The results showed that the survival rate of the seedlings on the natural hosts was only 30%, and the seedlings hooked into braided nylon netting glued to the substrate also had a comparable survival rate. Both of them were lower than the surfgrass transplant survival rate achieved in this study.Bull (2004)transplanted surfgrassP. torreyiin intertidal area and subtidal area, and higher survivorship was achieved in the latter one (71%versus48%), which was similar to the results in this study. While in this study, the transplant survival rates in the two habitats were both higher than 65%, which were both higher than those in previous studies. The differences in the transplant survival might be due to the differences in the transplanting methods, as well as to the different environmental conditions. In addition,the survival measurement in this study was also different from that in Bull’s study (2004). In this study, we counted the number of the retaining plants, including the original transplants and newly branched shoots, while in Bull’s study,the authors counted the number of leaves of the retaining transplants and then estimated the number of retaining shoots based on the mean leaf number per shoot.
4.2 Growth of P. iwatensis Transplants
Comparing the morphological and physiological characteristics of transplants and natural seagrass shoots can lead to the development of criteria for assessing transplant establishment (Balestriet al., 1998; Lee and Park, 2008). In the process of transplantation, the shoots will suffer from the impact of the graft or initial short-term pressure, which is caused by injury, drying and functional damage. Therefore, the morphology of seagrass in the early stage of transplantation is quite different from that in the natural seagrass bed. So, in this study, the transplants in two habitats were lower than the natural shoots.
According to Maxwell (2014), the morphology, non-structural carbohydrate content, leaf stable isotope ratio, and chlorophyll content of seagrasses may change with varying environmental fluctuations. Bull (2004)also compared the leaf number and biomass ofP. torreyithat were transplanted to the intertidal and subtidal habitats, and two times more leaves and a greater increase in the aerial coverage of rhizome (86%versus42%)were found in the subtidal area than those transplanted to the intertidal area. In this study, the number of leaves per shoot and rhizome elongation rate in the subtidal area was also higher than those in the tide pool, which is similar to the results inP. torreyistudy by Bull (2004).
The seagrass growth is affected by water temperature and light intensity (Zimmermanet al., 1989; Rasheed and Unsworth, 2011). In this study, the morphological measurements (shoot height, rhizome diameter, number of leaves)and productivity (aboveground and belowground)of transplants reached the highest values in August when the water temperature reached the highest, which is consistent with the previous research ofP. torreyi (Drysdale and Barbour, 1975), but opposite to the results of Park and Lee(2009)in which the aboveground productivity ofP. japonicuswas the highest in winter and early spring.
4.3 Physiology of P. iwatensis Transplants
Generally, the higher the content of chlorophyll, the stronger the ability of photosynthesis. The chlorophyll synthesis in plants is generally affected by environmental factors including temperature and light intensity (Eriander, 2017;Bertelli and Unsworth, 2018), which usually varied with months. In this study, the contents of chlorophyllaand total chlorophyll in both habitats reached the maximum values in September. Research on eelgrass showed that one of the adaptation strategies to reduce the light intensity was to improve the light utilization, and its characteristic was the increase of chlorophyll content (Dennison and Alberte,1982; Goodmanet al., 1995).
Previous studies have shown that the stress of transplantation can affect the photosynthesis of eelgrass transplants in a short period of time, and then the plants exhibit photosynthetic physiological responses by increasing the content of photosynthetic pigment (Ochienget al., 2010). In this study, the variations in photosynthetic pigment content of surfgrass transplants (a decrease in June and a gradual increase thereafter in the two habitats)may reflect temporal transplantation stress at the beginning period after transplantation.
It is found that there was a positive correlation between the sucrose content and the water temperature in the species ofZ. marinaandZ. noltii, which was due to the decrease of sucrose decomposition rate under high temperature (Touchette and Burkholder, 2000). Moreover, it has been observed that the activity of sucrose-P-synthetase, an enzyme involved in sucrose synthesis, was higher at high temperatures. In this study, the water temperature was the highest in August, and the content of soluble sugar in the aboveground and belowground tissues in the two habitats reached the maximum value in August, which might be due to the higher activity of sucrose-P-synthetase at that time. Studies have also shown that a moderate increase in temperature (from 28℃ to 34℃)caused a significant increase in the soluble sugar and starch content in the leaf tissues ofHalodule wrightiiandT. testudinum, which might help the plants to meet the increasing demand for the soluble carbohydrate (Kochet al., 2007). Peralta (2002)evaluated the relationship between light irradiance and the nonstructural carbohydrate content in the belowground tissues ofZ. noltii, and the results showed that a slight decrease was observed when the plants were exposed to higher light intensity. In this study, the soluble sugar content of the aboveground and belowground tissues was the highest in August while the light intensity was the lowest, showing a negative correlation of light intensity and soluble sugar content.
5 Conclusions
The newly developedPhyllospadixTransplant System was applied in the surfgrass transplantation experiment in subtidal area and a tide pool in intertidal area. The results showed that a higher survival and better growth were achieved in the subtidal area than in the tide pool. The survival rates were both higher than 65% in the two habitats,suggesting that the tide pool in the intertidal and the subtidal area in the study site are both suitable for transplantingP. iwatensis.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2019YFD09021 01), the National Natural Science Foundation of China (No.42076100), and the Fundamental Research Funds for the Central Universities (No. 201964002).
杂志排行
Journal of Ocean University of China的其它文章
- A Theoretical Model for the Microwave Emissivity of Rough Sea Surfaces
- Mechanism of Regional Subseasonal Precipitation in the Strongest and Weakest East Asian Summer Monsoon Subseasonal Variation Years
- Analytical and Experimental Studies on Wave Scattering by a Horizontal Perforated Plate at the Still Water Level
- Theoretical Prediction of the Bending Stiffness of Reinforced Thermoplastic Pipes Using a Homogenization Method
- Penetration Resistance of Composite Bucket Foundation with Eccentric Load for Offshore Wind Turbines
- Application of Converted Displacement for Modal Parameter Identification of Offshore Wind Turbines with High-Pile Foundation
