Nutrient uptake,physiological responses and growth of tobacco(Nicotiana tabacum L.)in soil under composite salt stress
2022-12-14JianCUIDongruiYAOJingMAXiefengYEYingPENGJiaqianSONGJinfengLIYajunCHANGJohnYANGZhenZHANGXueliLIXiaojingLIUandKhalilKARIMAN
Jian CUIDongrui YAOJing MAXiefeng YE∗Ying PENGJiaqian SONGJinfeng LIYajun CHANGJohn YANGZhen ZHANGXueli LIXiaojing LIU and Khalil KARIMAN
1Institute of Botany,Jiangsu Province and Chinese Academyof Sciences,Nanjing 210014(China)
2National Tobacco Cultivation and Physiologyand BiochemistryResearch Center,KeyLaboratoryfor Tobacco Cultivation of Tobacco Industry,College of
Tobacco Science,Henan Agricultural University,Zhengzhou 450002(China)
3Shangluo Tobacco Company,Luonan Branch,Shangluo 726000(China)
4School of Environmental Science,Nanjing Xiaozhuang University,Nanjing 211171(China)
5Department of Agriculture and Environmental Science,Lincoln Universityof Missouri,Jefferson CityMO 65201(USA)
6StaffDevelopment Institute of China National Tobacco Corporation,Zhengzhou 450000(China)
7School of Agriculture and Environment,The Universityof Western Australia,Perth WA 6009(Australia)
ABSTRACT High soil salinity imposes osmotic stress and ion toxicity in plants,leading to substantial crop yield loss worldwide.Understanding of the quantitative and dynamic physiological responses to composite soil salt stress is limited and needs to be expanded.In this study,physiological,nutritional,and biomass yield parameters of tobacco(Nicotiana tabacum L.)grown in soil with five levels of composite soil salinity(CSS),basal CSS level(control,CK)and 3(T1),6(T2),9(T3),and 12(T4)times the basal CSS level,under greenhouse were determined at days 30,60,and 90 after transplanting.Leaf dry biomass significantly(P<0.05)increased at the low salinity levels applied(T1 and T2)at all three time points,whereas it progressively declined as the CSS level further increased.The leaf physiological and photosynthetic responses were more adversely affected by CSS at the early growth stage(day 30).A path coefficient analysis demonstrated that leaf proline content had the largest direct effect(−0.66),and leaf Cu content had the most significant indirect effect(0.49)on leaf dry biomass of plants.The results suggest that lower CSS levels(T1 and T2)could stimulate tobacco growth(leaf biomass yield,in particular),and higher leaf proline and Cu levels at the early growth stage may potentially increase the ability of tobacco plants to withstand the adverse effects of salinity,which could be considered for future research and development of salinity management strategies.
KeyWords:anti-adversity metabolite,composite soil salinity,hormone,malondialdehyde,proline,translocation factor
INTRODUCTION
Soil salinity has become an overwhelming environmental issue worldwide and threatens approximately 7%of global arable land(Daliakopouloset al.,2016;Zhaoet al.,2017;Cuevaset al.,2019;Mehdizadehet al.,2020).High soil salinity lowers plant net photosynthesis and induces physiological stresses,thus reducing agricultural revenues and sustainability(Singh,2015;Hayeset al.,2019;Kamranet al.,2020;Linget al.,2020).Many studies have shown that salinity is a significant factor for crop yield reduction(Shrivastava and Kumar,2015;Butcheret al.,2016;Daliakopouloset al.,2016;Hayeset al.,2019),and it can even increase metal mobility,threatening crop safety production(Zhaoet al.,2013;Zhouet al.,2018;Nomanet al.,2021).Approximately 12–27 billion US dollars are lost annually due to soil salinization globally(Qadiret al.,2014).It has been estimated that the amount of saline soil is increasing at a rate of 10%annually,and by 2050,over 50%of global arable lands could be under threat from soil salinity to various degrees(Jamilet al.,2011;Shrivastava and Kumar,2015).Therefore,it is imperative to investigate the impact of soil salinity on crop physiology and fitness in order to identify the best management strategies to tackle soil salinity while promoting sustainability in salinity-prone agroecosystems.
Most plants,including crops,are glycophytes that tolerate only low concentration of salt before they are adversely affected,as evidenced by the decrease in growth and/or death(Bui,2013).However,even mild soil salinity affects the physiological and biochemical pathways in plants,and the underlying mechanism is very complex(Daliakopouloset al.,2016;Jinet al.,2018;Hayeset al.,2019;Linget al.,2020).Under salinity stress,plant may change the activity of Na+/H+transporters,Ca2+/K+channels,protein kinases,lipid signaling,and the expression of salt stress-responsive genes(Yaoet al.,2016;Jiet al.,2017;Sánchez-Barrenaet al.,2020).The adverse effects of salt stress on plants may be due to early osmotic and salt(such as Na+)stress(Deinleinet al.,2014;Linget al.,2020).However,most recent studies on soil salinity have focused on Na+and Cl−ions(Jinet al.,2018;Shahverdiet al.,2018;Linget al.,2020;Maet al.,2020).The Ca2+,,HCO−3,K+,and Mg2+are important mineral ions in the soil water extract that influence plant stress.For instance,a reduction in K+and Ca2+uptake inhibits proper cell function(Quinteroet al.,2007).There is a dearth of information on the physiological responses of crops to composite soil salt stress.Moreover,there is a lack of studies on composite soil salinity(CSS)threshold,which impedes crop production and field management(Maet al.,2020).Thus,it is necessary to understand how CSS affects the physiological responses of plants for assured crop production.
Tobacco(Nicotiana tabacumL.)is one of the most important economic crops worldwide,with China being a major tobacco-producing country.There are widespread saline fields in China,with a total area of 66.7 billion ha,which poses a severe threat to national agricultural development(Liet al.,2014;Yeet al.,2017;Wang Y Get al.,2018;Zhanget al.,2020).Luoyang City,an important tobacco-growing area in central China,is susceptible to soil salinization(Ye,2011;Maet al.,2019),which diminishes tobacco yield both quantitatively and qualitatively(Gaoet al.,2015;Donget al.,2017;Jinet al.,2018).Saline soils might be rehabilitated by planting well-targeted pastoral halophytes based on screening studies(Stavridouet al.,2017;Tliliet al.,2018).However,to address the soil salinity while maintaining economic productivity,we need to improve the understanding of the quantitative and dynamic responses of crops such as tobacco in composite salt soils.
We hypothesized that tobacco growth could be affected by different levels of CSS,tobacco leaves might have corresponding physiological responses at different growth stages,some crucial factors in the soil environment could control composite salt stress,and there was another key growing stage that could be used to judge whether tobacco growth was restrained.Leaf production is a fundamental economic indicator of tobacco farming.Thus,tobacco plants were grown on soil exposed to five CSS levels with the addition of various salinity-related cations/anions over a 90-d period.Tobacco growth attributes,nutrition,physiology,photosynthesis,and hormone status were characterized every 30 d to:i)quantify the impact of CSS on plant fitness,ii)identify the optimal CSS threshold,and iii)identify the key factors of soil environments and physiological parameters for tobacco growth.
MATERIALS AND METHODS
Greenhouse experiment
A greenhouse experiment was conducted in an experimental field at the Henan Agricultural University,Zhengzhou,China using a yellow soil(derived from moorstone granite)collected from the topsoil(0–20 cm)of a tobacco field in Neibu Village(112◦32′22′′E,34◦18′20′′N),Luoyang City,Henan Province,China.Detailed physicochemical properties of the soil were as follows:pH,7.52;organic matter,16.38 g kg−1;available N,81.41 mg kg−1;and available P,8.55 mg kg−1.The basal level of CSS of the soil comprised of 103.96 mg kg−1Ca2+,15.60 mg kg−1Mg2+,59.91 mg kg−1K+,18.55 mg kg−1Na+,11.65 mg kg−1HCO−3,375.64 mg kg−1SO2−4,and 5.55 mg kg−1Cl−(Maet al.,2019).Based on the routine application of K2SO4,sulphate was also considered in the experiment design.
The experiment was carried out using plastic pots(25 cm in diameter and 30 cm in height,with 25 kg soil pot−1)in a completely randomized design,with five CSS levels and three replicates:the basal CSS level(control,CK)and the CSS levels 3(T1),6(T2),9(T3),and 12(T4)times the basal CSS level,using CaCl2,Ca(HCO3)2,MgSO4,K2SO4,and Na2SO4.The sampled soil was air-dried,passed through a 2-mm sieve,and supplemented with the corresponding salt solutions and a fertilizer(N:P2O5:K2O,4.0,8.0,and 12.0 g pot−1using the fertilizers of NH4NO3,KH2PO4,and KNO3,respectively).Based on the classification and grade standards of soil salinization in China(Table SI,see Supplementary Material for Table SI),soils for CK and T1were none-and moderate saline,respectively,whereas soils for the other three treatments(T2,T3,and T4)were alkaline.Deionized water was used to water the pots to a field capacity of 18.9%.Tobacco seedlings(cultivar Zhongyan 100,sensitive to salt stress(Yeet al.,2017))were obtained from the Tobacco Research Institute of the Chinese Academy of Agricultural Sciences(Qingdao,China),and five seedlings were transplanted into each pot.
Plant sampling and chemical analysis
Plant samples were collected every 30 d(days 30,60,and 90)after transplanting.Growth and agronomic characteristics of the tobacco plants were recorded following a standard protocol(Investigating and Measuring Methods of Agronomical Character of Tobacco,YC/T 142-2010).At each time point,the root,stem,and leaf tissues(one whole plant per pot)were collected,washed with tap water,rinsed with deionized water,and oven-dried at 105◦C for 30 min and then 60◦C until constant weight.Mineral element contents in the plant samples were determined using the dry-ashing method described by Bao(2000).
One seedling from each pot was selected for the measurements of physiological parameters,hormones,and photosynthesis.Malondialdehyde(MDA)and proline(Pro)contents,the indicators of stress damage and tolerance,of fresh leaves were determined using thiobarbituric acid colorimetry(Shahet al.,2001;Yanget al.,2018)and sulfosalicylic acid colorimetry as described by Bateset al.(1973).Hormone contents of the fresh leaves,including abscisic acid(ABA),indole acetic acid(IAA),gibberellic acid(GA3),and zeatin nucleoside(ZN),were determined using an enzyme-linked immunosorbent assay(He,1993).During 9:30–11:30 a.m.,the soil and plant analyzer development(SPAD)values for the chlorophyll content of the upper leaves were determined(three leaves per plant and five measurements per leaf)using the SPAD chlorophyll meter(SPAD-502,Minolta Camem Co.,Osaka,Japan).Four other indicators of photosynthesis,net photosynthetic rate(Pn),intercellular CO2concentration(Ci),stomatal conductance(Gs),and transpiration rate(Tr),were also measured on the 8th leaf(from the bottom)of each seedling using a portable photosynthetic system(Li-COR 6400,LI-COR Corporate,Lincoln,USA).
Data processing and statistical analysis
The translocation factor(TF)of mineral elements,one of the key factors used to assess plant nutritional status,was calculated using the following equation:

where TFa-bis TF of an element from tissues a to b,andMaandMbare the contents of a mineral element in tissues a and b,respectively(mg kg−1).
Direct or indirect contribution of the selected soil or plant factors to crop performance was determined using the path coefficient analysis,as described by Dewey and Lu(1959)and Du and Chen(2010)using SPSS 22 software.Data were analyzed using one-way analysis of variance(ANOVA),and the means were compared using the Duncan’s multiple range test at a significance level ofP<0.05 using SAS 9.4 software.A correlation analysis was also performed using the Pearson test(two-tailed)at significance levels ofP<0.05 andP<0.01.
RESULTS
Nutrient contents in plant leaves
At high CSS levels(T2–T4),the leaf K,Ca,Fe,Mn,and Cu contents decreased,whereas leaf Na,Mg,and P contents increased,compared to CK(Table I).However,leaf Zn content exhibited an inverted“U”shape,being the highest at T2.At the early growth stage(day 30),plants subjected to the lowest CSS stress(T1)had significantly(P<0.05)lower contents of Ca and Fe and higher contents of P and Zn compared to those grown in CK.Leaf Cu content did not significantly(P>0.05)differ across all CSS treatments.Leaf contents of most elements among CSS treatments(except for Na and Zn in T1at day 60 and K in T2at day 90)significantly(P<0.05)differed from those in CK at both days 60 and 90.At day 90,only plants in T4had significantly(P<0.05)higher leaf P content than that in CK,and no significant(P>0.05)differences were observed between CK and the other three CSS treatments.Both leaf Cu and P uptake were more adversely affected by CSS at earlier stages(days 30 and 60)than those at the later stage(day 90)of growth(P<0.05).
With increasing CSS levels,the TFroot-stemvalues generally decreased at day 30 for Na,Mg,P,and Zn,at day 60 for Mg,Fe,Mn,and Cu,and at day 90 for Na,Mg,Cu,and Zn(Table II).In contrast,the TFroot-stemvalues increased at day 30 for Ca,Fe,Mn,and Cu,at day 90 for P,Fe,and Mn,and at all three growth stages for K.The TFstem-leafvalues decreased at day 30 for Cu,at days 60 and 90 for P and Mn,and at day 60 for Fe and Zn,while increased at days 60 and 90 for Cu,and at all three stages for Na.Correlation analysis indicated that leaf Cu content was negatively correlated with leaf P,Mg,and Zn contents,whereas positive correlations were observed among leaf Na,P,and Mg contents,and also of leaf K content to Zn,Ca,and Mn contents(P<0.05,Table SII,see Supplementary Material for Table SII).The critical CSS threshold was determined to be at T1or T2based on the relationship between CSS and leaf nutrients or TF values(Tables I and II),implying that CSS levels between T1and T2could be optimal for tobacco growth.
Stress physiologyand leaf hormone status
Increased CSS generally led to increased leaf MDA,except for T1at day 90(Fig.1).Leaf Pro content exhibited an inverted“U”trend with increasing CSS levels,reaching the highest value in T2,subsequently declining in both T3and T4.Compared to CK,both MDA and Pro(except for day 90)in T1 significantly increased by 3.3%–17.6%(P<0.05),implying that the CSS level in T1effectively activated the stress response of tobacco seedlings.Moreover,leaf Pro content decreased at days 60 and 90 in comparison with day 30.Correlation analysis showed that the leaf Pro content was significantly correlated with K,P,Mn,Cu,and Zn,whereas the MDA content was only correlated with K(P<0.05)(Table II).
Composite soil salinity differentially regulated the leaf hormonal status of the plants,depending on the CSS levels and sampling time points(Fig.1).The ABA concentration of leaves increased significantly at all CSS levels for the three growth points(except for T4at day 90)compared with CK.With an increase in CSS levels,both IAA andthe hormone/ZN ratio showed an increasing trend at day 30,whereas the opposite trend occurred at day 90,i.e.,the CSS level increased,but both IAA and hormone/ZN ratio continuously declined.At day 30,the leaf ZN level was not affected by T1,T2,and T3;however,it dropped significantly(P<0.05)in T4.At day 60,ZN level remained unchanged across all treatments.At day 90,the ZN level was not affected by the lowest CSS(T1),whereas it was significantly(P<0.05)enhanced in T2–T4treatments.
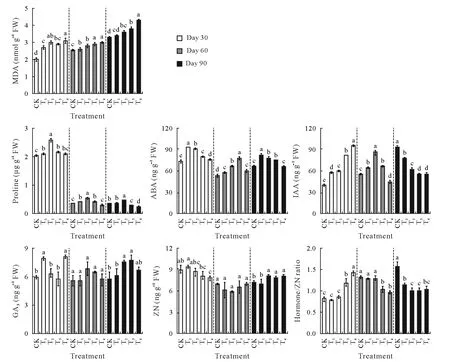
Fig.1 Contents of anti-adversity metabolites,including malondialdehyde(MDA)and proline,and hormones,including abscisic acid(ABA),indole acetic acid(IAA),gibberellic acid(GA3),and zeatin nucleoside(ZN),of tobacco leaves in the greenhouse experiment with five composite soil salinity(CSS)levels at days 30,60,and 90 after transplanting.Error bars are standard errors of the means(n=3).For the same time point,bars with different letters are significantly different at P<0.05 according to the Duncan’s multiple range test.CK=control with basal CSS level;T1,T2,T3,T4=treatments with CSS levels 3,6,9,and 12 times the basal CSS level,respectively;FW=fresh weight;hormone/ZN ratio=(ABA+IAA+GA3)/ZN ratio.
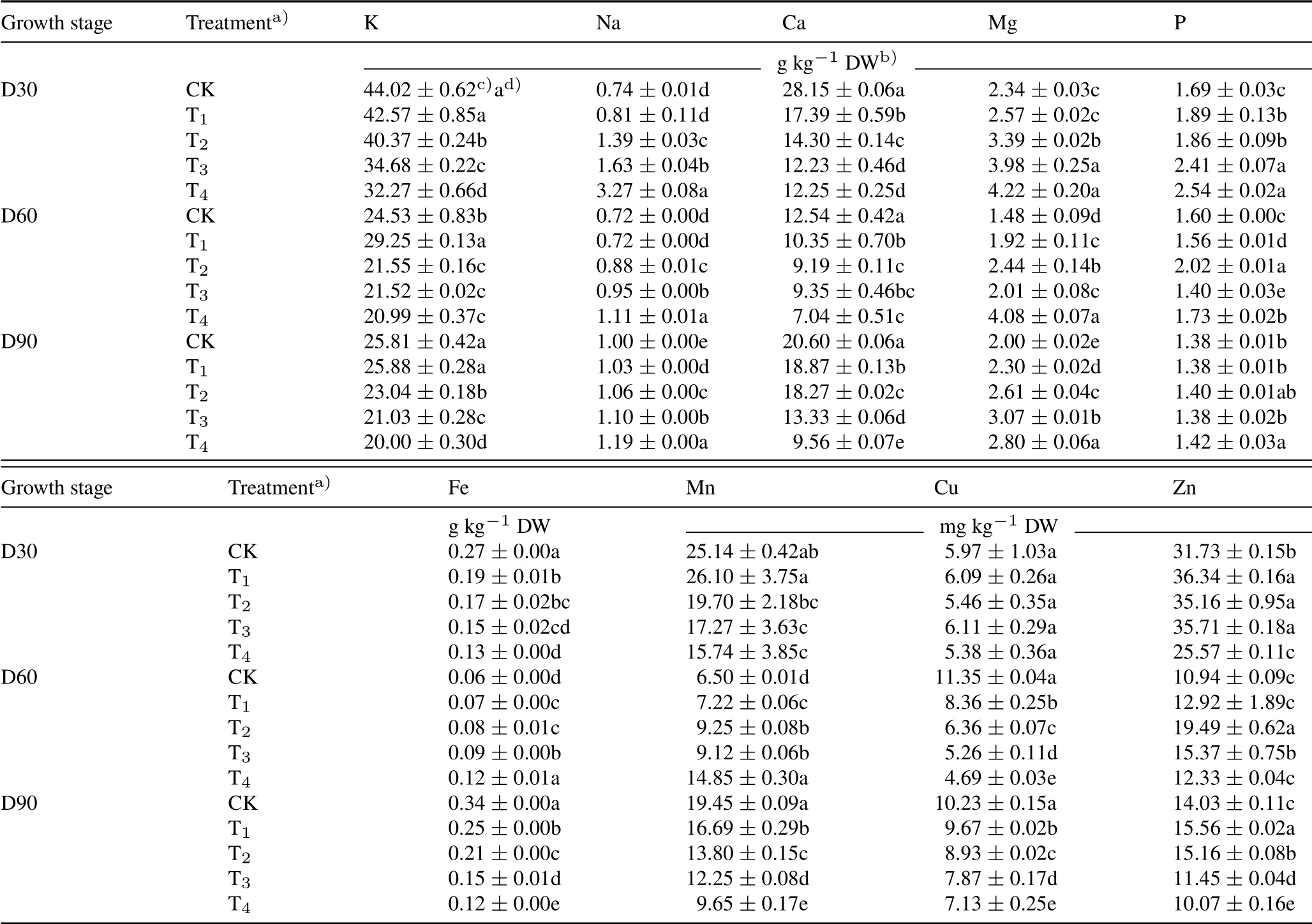
TABLE IContents of leaf K,Na,Ca,Mg,P,Fe,Mn,Cu,and Zn of tobacco in the greenhouse experiment with five composite soil salinity(CSS)levels at days 30(D30),60(D60),and 90(D90)after transplanting
Photosynthesis of leaves
Photosynthetic parameters showed various response profiles upon exposure to different CSS levels(Fig.2).Moderate(T1)and high(T4)levels of CSS led to contrasting responses of tobacco plants.Considering the parameters at day 90,an indication of long-term recovery of plants from the CSS stress,higher levels of Tr,Gs,Pn,and chlorophyll were observed in T1in comparison with CK,whereas all these parameters were negatively affected by the highest CSS treatment.The in-between CSS levels(T2and T3)were also in transitory states towards the lowest photosynthesis level observed for T4,implying that the moderate CSS treatment(T1)stimulated the photosynthetic activity of plants,and the activity progressively declined as CSS increased.At day 90,Gs,Pn,and SPAD values were significantly(P<0.05)higher in T1than those in both CK and T2treatments,suggesting T1as the optimal CSS level with growth-stimulating effects,beyond which the plant photosynthetic performance diminished.
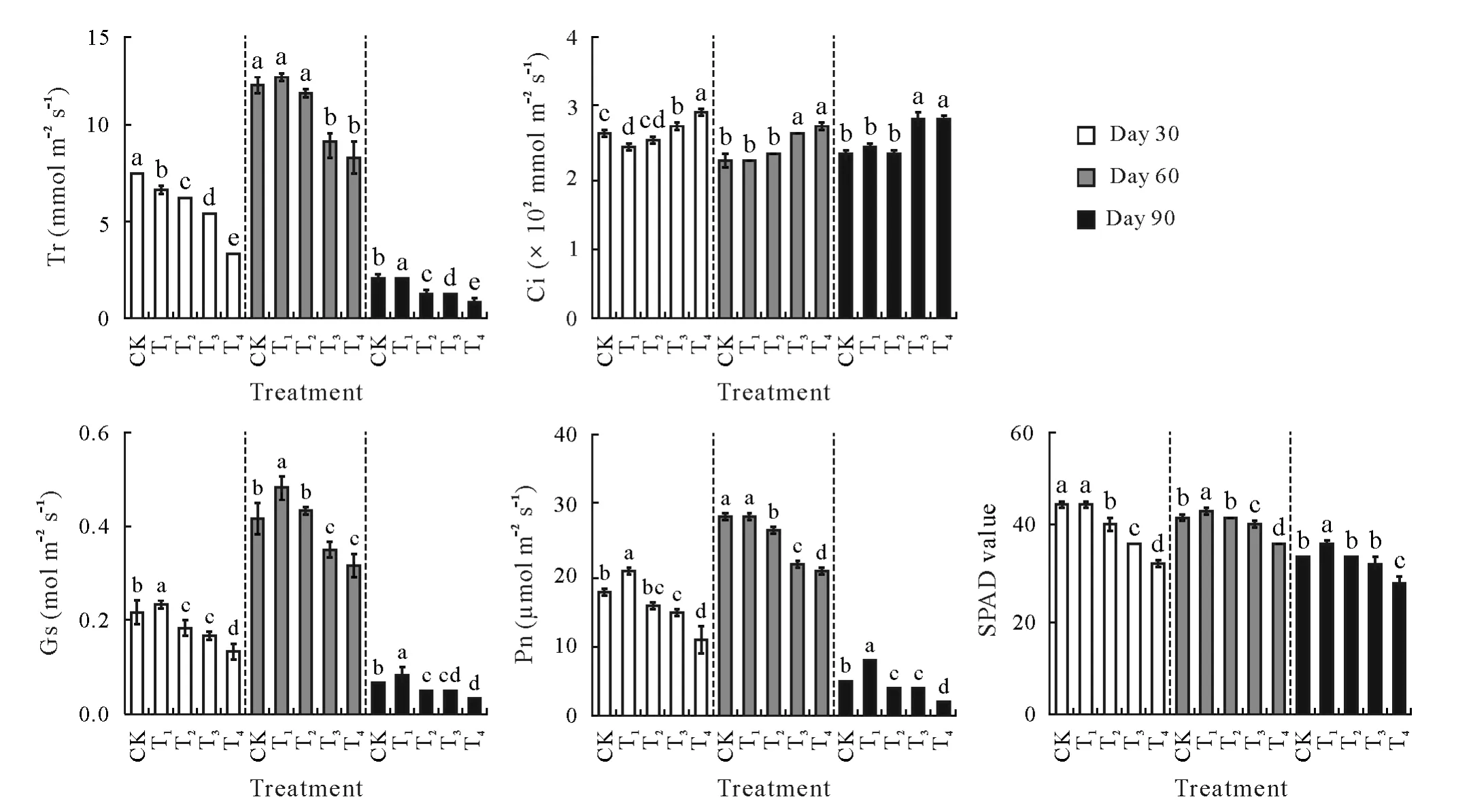
Fig.2 Photosynthetic physiology parameters of tobacco leaves in the greenhouse experiment with five composite soil salinity(CSS)levels at days 30,60,and 90 after transplanting.Error bars are standard errors of the means(n=3).For the same time point,bars with different letters are significantly different at P<0.05 according to the Duncan’s multiple range test.CK=control with basal CSS level;T1,T2,T3,T4=treatments with CSS levels 3,6,9,and 12 times the basal CSS level,respectively.Tr=transpiration rate;Ci=intercellular CO2 concentration;Gs=stomatal conductance;Pn=net photosynthetic rate;SPAD=soil and plant analyzer development.
Correlation analysis revealed positive correlations among Tr,Gs,Pn,and SPAD values,whereas negative correlations were observed of Ci to Tr,Gs,Pn,and SPAD values(Tables SII–SV,see Supplementary Material for Tables SII–SV).In general,leaf Na content was negatively correlated withTr,Gs,Pn,and SPAD values,while the opposite trend was observed for K,suggesting that a sufficient K level could be beneficial for CSS stress tolerance in tobacco plants.
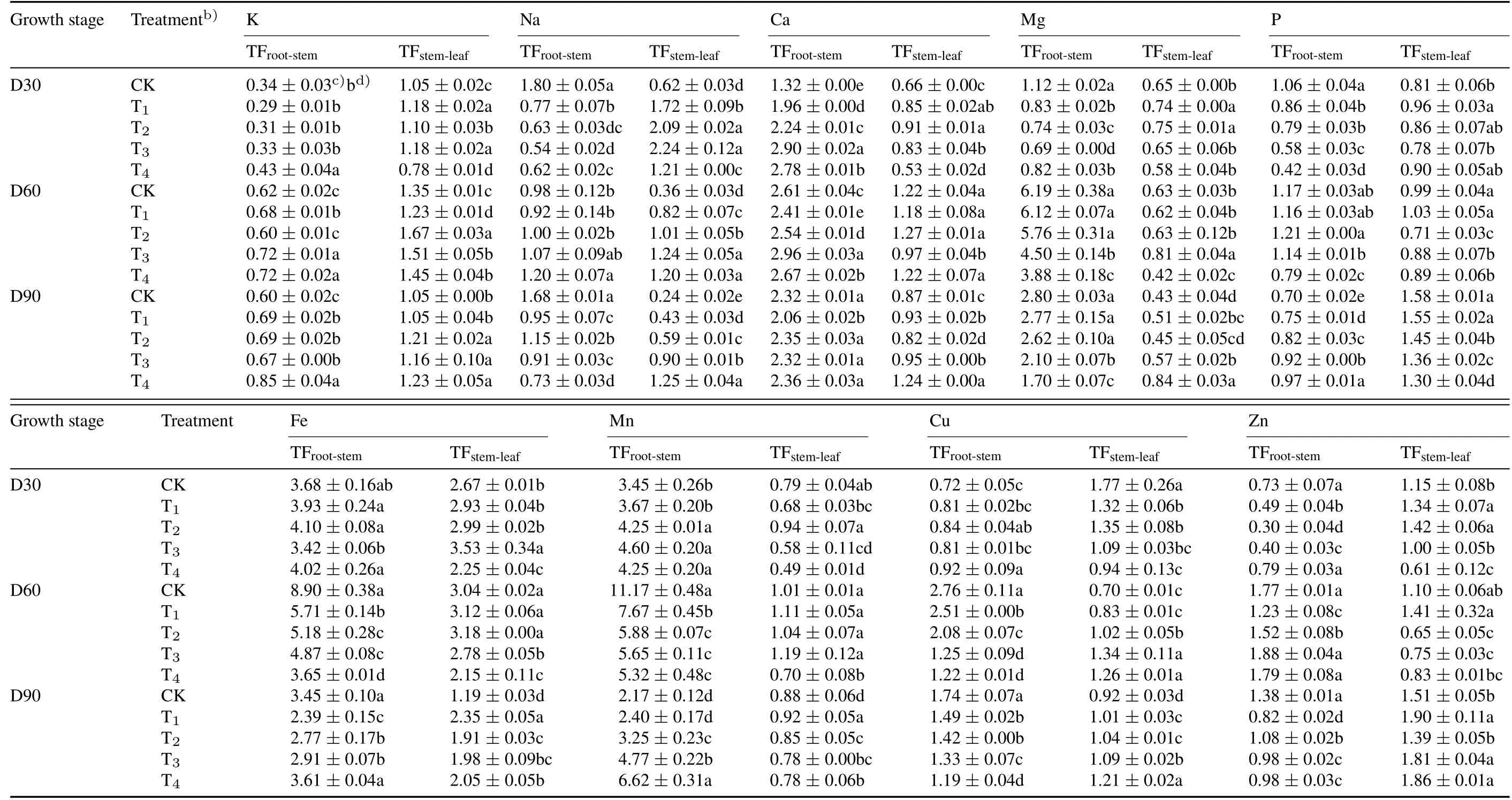
TABLE IITranslocation factors(TFs)a)for mineral elements between different tissues(root,stem,and leaf)of tobacco in the greenhouse experiment with fvie composite soil salinity(CSS)levels at days 30(D30),60(D60),and 90(D90)after transplanting
Plant growth
With the increasing levels of CSS,the total dry biomass of tobacco plants had an inverted“U”trend,i.e.,at all three time points,plants under the moderate CSS level(T1)had the highest total biomass,and plants in T4had the lowest biomass(Fig.3).Similar trends were also observed for root,stem,and leaf biomass(except for root biomass in T1at day 60).However,no significant differences were found in the fraction of the total biomass in each tissue among the CSS treatments(Fig.S1,see Supplementary Material for Fig.S1).Thus,CSS significantly affected tobacco biomass,but not the biomass distribution across different tissues.
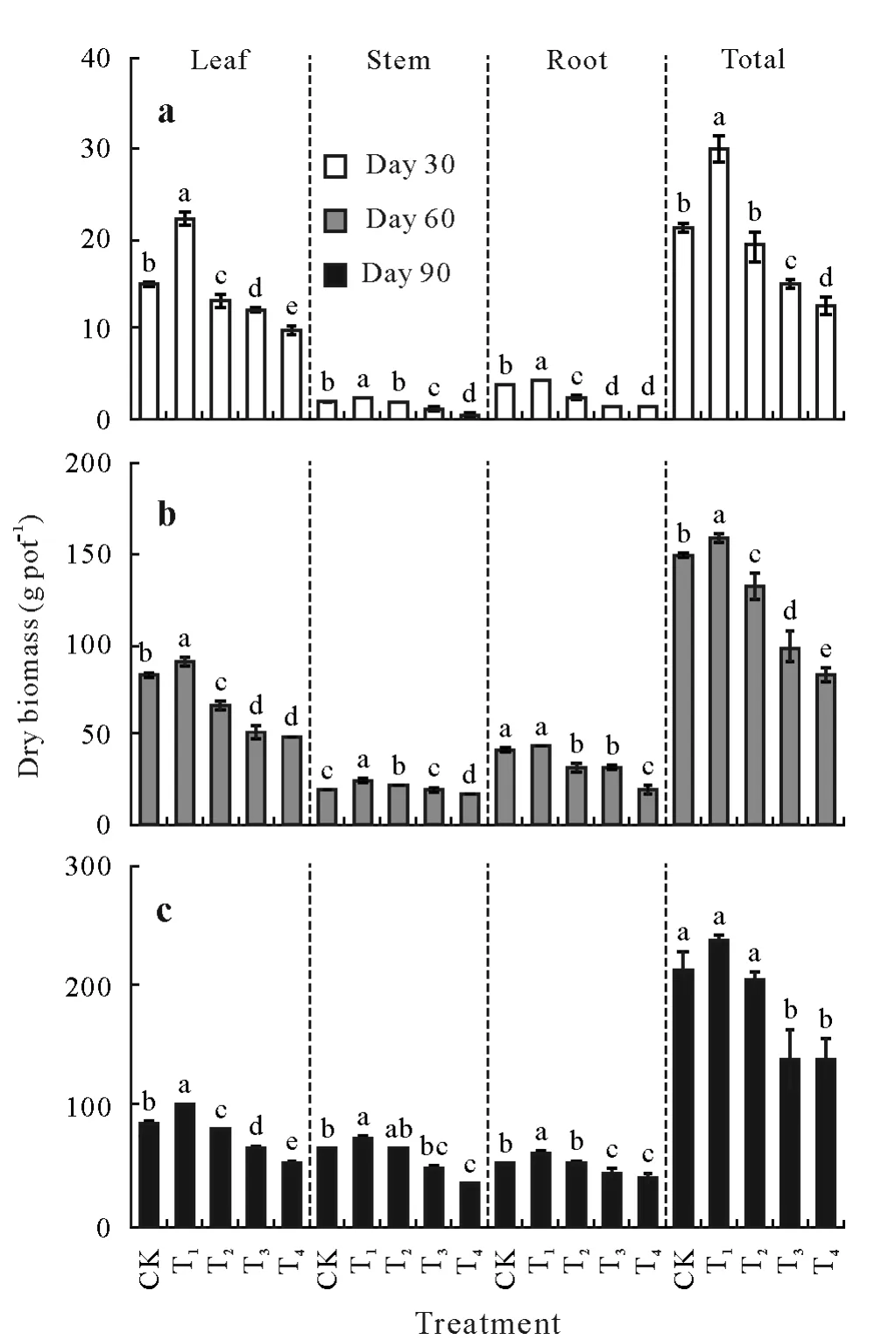
Fig.3 Dry biomass of tobacco plants in the greenhouse experiment with five composite soil salinity(CSS)levels at days 30(a),60(b),and 90 after transplanting.Error bars are standard errors of the means(n=3).For the same time point,bars with different letters are significantly different at P<0.05 according to the Duncan’s multiple range test.CK=control with basal CSS level;T1,T2,T3,T4=treatments with CSS levels 3,6,9,and 12 times the basal CSS level,respectively.
The height,stem girth,and leaf growth of tobacco plants generally decreased with increasing CSS levels(Fig.4).Plant height,stem girth,and length and width of the largest leaf(LLL and WLL,respectively)significantly(P<0.05)decreased at both days 30 and 60(except for LLL at day 60)in T3and T4compared to CK.Significant(P<0.05)differences were also detected for both plant height and LLL values between T2and CK at day 30,for plant height between T2and CK at day 60,and for stem girth between T1and CK at both days 30 and 60.No significant(P>0.05)differences were observed in LLL value among all CSS treatments at both days 60 and 90.For the number of available leaves,there was no significant(P>0.05)difference among CK,T1,T2,and T3at day 30,among all CSS treatments at day 60,and among CK,T2,T3,and T4at day 90.Therefore,tobacco growth was more sensitive to CSS at the early growth stage,and the estimated threshold values for healthy tobacco growth were between the T1and T2treatments.
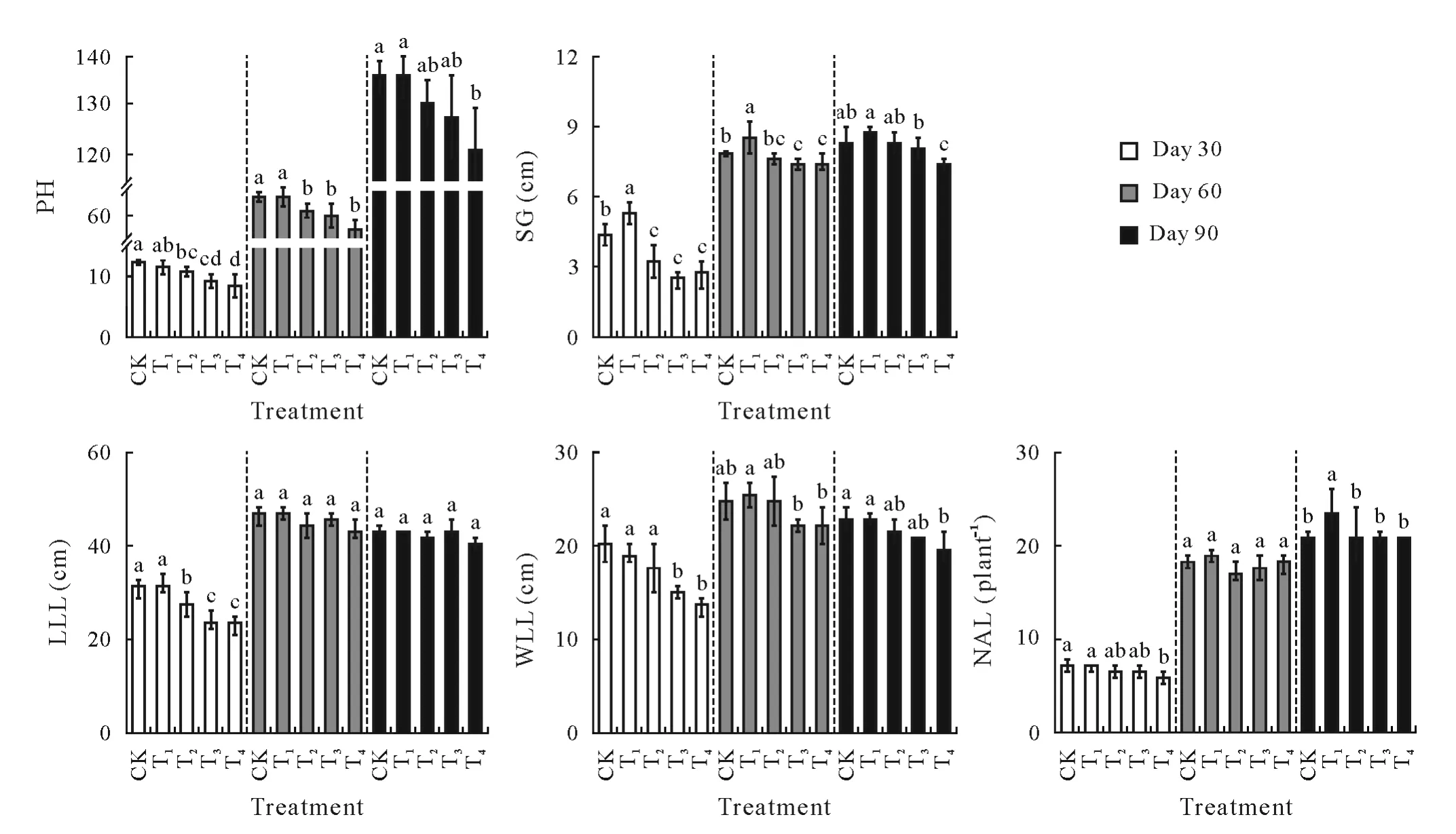
Fig.4 Growth attributes of tobacco plants in the greenhouse experiment with five composite soil salinity(CSS)levels at days 30,60,and 90 after transplanting.Error bars are standard errors of the means(n=3).For the same time point,bars with different letters are significantly different at P<0.05 according to the Duncan’s multiple range test.CK=control with basal CSS level;T1,T2,T3,T4=treatments with CSS levels 3,6,9,and 12 times the basal CSS level,respectively.PH=plant height;SG=stem girth;LLL and WLL=length and width of the largest leaf,respectively;NAL=number of available leaves.
To quantify the effect of CSS level on the growth of tobacco plants,a path-coefficient analysis was performed.Based on the 22 factors listed in Tables III and SII,the factors(X)relevant to the dry biomass of tobacco leaves at days 30,60,90,and the whole growing period(Y30,Y60,Y90,andYwhole,respectively)were selected and used for the stepwise regression analysis(Table SVI,see Supplementary Material for Table SVI)as follows:
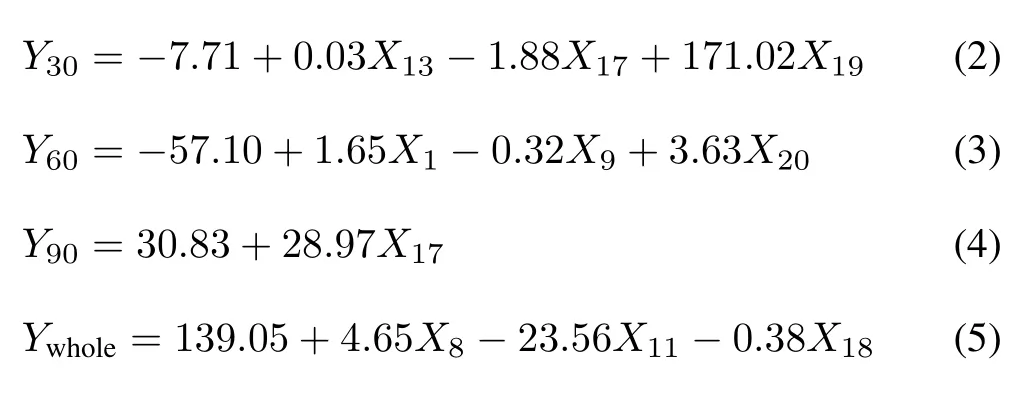
whereX13is IAA,X17is Tr,X19is Gs,X1is K,X9is Zn,X20is Pn,X8is Cu,X11is proline,andX18is Ci.
Direct and indirect effects and total effects of the selected parameters on the dry biomass of tobacco leaves were calculated using the path coefficient analysis(Table III).At day 30,X19(Gs)had the highest total effect(0.94)and direct effect(1.65),followed byX17(Tr,0.69 and−0.65,respectively),andX13(IAA,−0.63 and 0.15,respectively).The indirect effect was the highest forX17(1.34),followed byX13(−0.78)andX19(Gs,−0.71).At day 60,X1(K,0.88)andX20(Pn,0.97)showed the highest total effect,with the highest direct effect forX20(0.73)and indirect effect forX1(0.57).At day 90,onlyX17had a total effect(0.98)and a direct effect(0.98).The analysis also indicated that several factors could affect the dry biomass of tobacco leaves at different stages.Across the whole growing period,photosynthetic factors such as Tr,Gs,and Pn played a vital role in the biomass accumulation of tobacco leaves under simulated CSS conditions.
Positive correlations were observed among LLL,WLL,the number of available leaves,and dry biomass of tobacco leaves based on the data collected throughout the growing period(Table SII).The three factors,Cu(X8),Pro(X11),and Ci(X18),with their total effects of 0.78,−0.87,and−0.58,direct effects of 0.30,−0.66,and−0.25,and indirect effects of 0.49,−0.22,and−0.32,respectively(Table III),were found to be the key factors controlling leaf biomass production in tobacco plants under composite salt stress.
DISCUSSION
Excessive uptake of Na and Cu can adversely affect plant growth(Deinleinet al.,2014;Negrãoet al.,2017;Wang JJet al.,2019;Nomanet al.,2021),but a relatively high uptake of K,P,and Fe promotes the growth of plants,especially tobacco(Gaoet al.,2015;Shrivastava and Kumar,2015;Yeet al.,2017).In this study,CSS caused reductions in the TFstem-leafof P and Fe,while a reverse trend was observed for K at days 60 and 90(Table II).Moreover,both leaf Cu and P uptake were more adversely affected by CSS at the earlier growth stage(day 30)than at later stages.Plant nutritional responses result from ion competitive and synergistic interactions and changes in ion transfer pathways.The K+and Na+cations share several transporters because of their similar structural and chemical properties(Benitoet al.,2014;Zhaoet al.,2021).High Na+content in soil limits water uptake and the absorption of nutrients in plants(Zhao and Lin,2020;Zhaoet al.,2021),while accumulation of K+in plant cells could relieve the toxic effects of excess Na+(Shrivastava and Kumar,2015;Negrãoet al.,2017;Jinet al.,2018;Sánchez-Barrenaet al.,2020).Here,inward and outward K+transport channels were presumably activated under high CSS levels,reflected as increased TFroot-stemof K and decreased TFroot-stemof Na(Table II),ameliorating the toxicity of excessive Na+uptake.At the early stage(day 30),the TFroot-stemof Fe and Cu were elevated,which could also reduce Na+toxicity.Changes in ion transfer pathways are another possible detoxification mechanism.Although plants subjected to CSS stress had decreased TFroot-stemof Na at day 30 and Cu at days 60 and 90,the TFstem-leafof Na,Cu,and K increased at both days 60 and 90.This implied that tobacco plants altered their ion transport pathway at different growth stages as a self-protective response against CSS,which does not necessarily provide sufficient protection for stressed plants,i.e.,the self-protection ability would depend on the stress level.A high K+/Na+ratio could be an index of salt tolerance(Assahaet al.,2017).Regulation of K+in plant cells has been reported to be the most critical mechanism for maintaining normal ion content and cytoplasmic function,leading to improved salinity tolerance(Zhang and Shi,2013;Wang Let al.,2018).In addition,there are different abilities for K and Na transportation at different stages of plants(Wang T Tet al.,2018;Huanget al.,2020).High-affinity K transporter 1(HKT1),one of the most important transporters of Na+,can transport Na+in aboveground parts to phloem and Na+in roots to xylem(Li N Net al.,2019).At the early stages of salt stress,the expression ofSvHKT1;1in the roots ofSporobolus virginicusincreased,promoting the absorption of Na+and its transport to the aboveground part(Kawakamiet al.,2020).In the later period,salt stress inhibited the growth and development of lateral roots in Arabidopsis,affecting ion absorption by roots(Julkowskaet al.,2017).In addition,a long-term salt stress may disturb the free radical generation system and destroy cell membrane structure,thus affecting ion transporters(Julkowska and Testerink,2015).
Usually,high salinity is detrimental to the structure and functionality of plasma membranes,germination efficiency,plant growth,flowering,and may also cause early leaf senescence(Shrivastava and Kumar,2015;Wang Let al.,2018;Khatri and Rathore,2019;Nolanet al.,2020).Although the overall plant responses to salinity are well established(Daliakopouloset al.,2016;Jinet al.,2018;Hayeset al.,2019;Zhaoet al.,2021),detailed quantitative responses have been rarely explored.In this study,the path-coefficient analysis revealed that Cu,Pro,and Ci were key factors controlling leaf biomass accumulation in tobacco plants under CSS stress.Proline acts as an osmolyte and maintains the turgidity of cells against osmotic pressure(Husenet al.,2016;Peret al.,2017;Huanget al.,2020).Previously,an enhanced Pro level was shown to elevate the salinity tolerance of tobacco plants(De la Torre-Gonzálezet al.,2018;Li JHet al.,2019;Wang H Yet al.,2019).The Cu is an essential micronutrient involved in numerous metabolic processes in all photosynthetic organisms(Ilyaset al.,2015;Nakajimaet al.,2015;Lin and Jin,2018).Nakajimaet al.(2015)reported that an increase in leaf Cu content would enhance the chlorophyll concentration and photosynthetic productivity per unit biomass of the lichenStereocaulonjaponicum.Ilyaset al.(2015)found that Cu foliar spray caused a significant improvement in Ci,Tr,Gs,chlorophyll,fruit yield,and quality in hybrid citrus(Kinnow).However,excessive Cu could potentially destroy the photosynthetic systems and inhibit plant growth(Nakajimaet al.,2015;Lin and Jin,2018;Wang J Jet al.,2019).In this study,CSS significantly decreased Cu transfer from root to stem and leaf Cu content(Tables I and II),consequently lowering Tr,Gs,Pn,SPAD values,and plant dry biomass(Fig.2).This
finding implied that a sufficient leaf Cu content was essential for healthy tobacco growth,and a moderate dose of Cu,as a foliar spray,might mitigate the toxic effects of salt stress in tobacco plants.Similar findings have been found,and the Cu produce(biogenic Cu nanoparticles)is recommended to alleviate salt stress in maize plants(Nomanet al.,2021).However,the related mechanisms in tobacco need to be studied further.
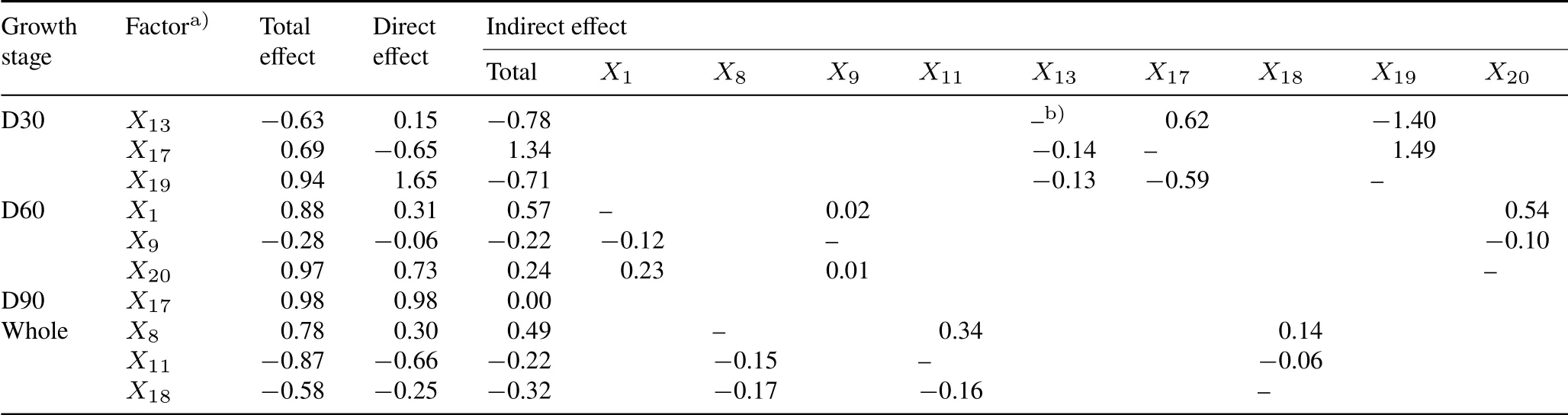
TABLE IIIDirect and indirect effects and total effects calculated using path coefficient analysis of selected factors on dry biomass of tobacco leaves in the greenhouse experiment with five composite soil salinity levels at days 30(D30),60(D60),and 90(D90)after transplanting
Proline is an elicitor of several defense responses,such as salt and water stresses,and plays a crucial role in osmotic adjustment,scavenging of reactive oxygen species,stabilizing membranes and proteins,carrying cell signals,and regulating gene expression(Kaur and Asthir,2015;Benitezet al.,2016;Kamranet al.,2020).Exogenous addition of Pro could mitigate the damage caused by salt stress and maintain the uptake of K,Ca,P,and N to enhance plant growth(Hayatet al.,2012;Huanget al.,2020;Zhaoet al.,2021).Increased leaf Pro concentration in tobacco plants under T1and T2at days 30 and 60 could be a plant defense mechanism to withstand CSS(Fig.1).Short-term salt stress can decrease IAA in plant leaves(Liuet al.,1998;Sunet al.,2013).Indole acetic acid,synthesized through tryptophan-dependent or-independent pathways,is the main phytoauxin alleviating the adverse effects of salt stress(Javidet al.,2011;Husenet al.,2016;Khanet al.,2021).In corn(Zea maysL.),exogenous IAA application considerably increased leaf Na content and decreased leaf K content(Kayaet al.,2013),which is similar to our observations at the early tobacco growth stage(Table SIII).Moreover,the leaf Na content was positively correlated with the hormone IAA(Table SII).The IAA levels fluctuated considerably at different time points and under different CSS levels(Tables SIII–SV),suggesting that IAA plays different roles in various plant response stages to CSS.Thus,leaf Pro could be an important factor and monitored in time for early warning CSS levels,further for rapidly taking measures for stable yield of tobacco.In addition,it should be elucidated how leaf IAA responds and regulates tobacco growth under high CSS levels,which could provide more guidance for tobacco production in CSS regions.
CONCLUSIONS
Tobacco growth and physiological responses were associated with both the level of CSS and the growth period.Compared with most study cases using NaCl,this study could reflect the relationship between soil salinity and tobacco growth more accurately based on the ion composition of soil salinity investigated widely.The possible thresholds of CSS(3 and 6 times the basal CSS level),more vulnerable time(day 30 after transplanting),and the key physiological factor(Pro)were found,which can assist with tobacco production under CSS stress.Moreover,this study acted as a pioneer suggesting use of Cu to withstand salt stress for tobacco production.However,the related mechanisms between Cu and CSS stress should be studied further,and it is necessary to obtain quantitative parameters for Cu application under CSS stress.
ACKNOWLEDGEMENTS
This research was funded by the Key Laboratory for Tobacco Cultivation of Tobacco Industry of China(No.30800665),the Marine Science and Technology Innovation Fund of Jiangsu Provincial Department of Natural Resources,China(No.JSZRHYKJ202003),the Scientific and Technological Innovation Fund of Jiangsu Provincial Department of Science and Technology,China(No.BE2022304),and Luoyang Tobacco Company of China(No.LYKJ201501).Authors would like to thank Haichao Guo from the Noble Research Institute,USA for revising the manuscript,and editors and anonymous reviewers for their valuable comments on improving the clarity and quality of this manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the EditorTrade-offevaluation using carbon dioxide equivalent and hazard index of a paddy soil with application of organic liquid fertilizer
- Reduced tillage with residue retention improves soil labile carbon pools and carbon lability and management indices in a seven-year trial with wheat-mung bean-rice rotation
- Soil texture affects the conversion factor of electrical conductivity from 1:5 soil-water to saturated paste extracts
- Comparative analysis of planted and unplanted controls for assessment of rhizosphere priming effect
- Sugarcane bagasse amendment mitigates nutrient leaching from a mineral soil under tropical conditions
- Effective alleviation of Cd stress to microbial communities in mining reclamation soils by thiourea-modified biochar amendment
