Comparative analysis of planted and unplanted controls for assessment of rhizosphere priming effect
2022-12-14JianLIandPerBENGTSON
Jian LI and Per BENGTSON∗
1Departmentof Biology,Microbial EcologyGroup,Lund University,Sölvegatan 37,Lund 223 62(Sweden)
2KeyLaboratoryof Urban Environmentand Health,Institute of Urban Environment,Chinese Academyof Sciences,1799 Jimei Road,Xiamen 361021(China)
ABSTRACT The rhizosphere priming effect(RPE)is increasingly being considered to be an important regulator of soil organic matter(SOM)decomposition and nutrient turnover,with potential importance for the global CO2 budget.As a result,studies on the RPE have rapidly increased in number over the last few years.Most of these experiments have been performed using unplanted soil as the control,which could potentially lead to incorrect assessment of the RPE.Therefore,we performed a greenhouse experiment to investigate how the choice of control(i.e.,unplanted control and planted control)influenced the quantification of RPE on SOM decomposition and gross nitrogen(N)mineralization,and to link this to differences in microbial and abiotic soil properties between the two controls.In the planted control,planted seedlings were cut at soil surface 5 d before measurement of the RPE.The RPE on SOM decomposition was positive in pine soil and almost 2-fold higher when calculated from the planted control than from the unplanted control.In spruce soil,a negative RPE on SOM decomposition was found when calculated from the planted control,while the RPE was positive when calculated from the unplanted control.No RPE on gross N mineralization was found when calculated from the planted control,while a positive RPE of more than 100%was found when calculated from the unplanted control.The microbial biomass and growth rate were lower,while the inorganic N content was higher in the unplanted control than in the planted control.The microbial community composition and potential enzyme activity in the planted treatment and planted control were similar,but they differed significantly from those in the unplanted control.The results showed that the RPE varied widely depending on the choice of control;thus,we suggest that a planted control,in which the aboveground plant parts are removed only a few days before the measurement of RPE,should be used as the control when elucidating the RPE on belowground C and N cycling responses to environmental change.
KeyWords:enzyme activity,gross nitrogen mineralization,microbial community composition,nitrogen availability,soil organic matter decomposition
INTRODUCTION
The rhizosphere priming effect(RPE),defined as the stimulation or suppression of soil organic matter(SOM)decomposition in response to labile root carbon(C)input(Kuzyakovetal.,2000),is becoming increasingly recognized as a key factor in the regulation of soil C and nitrogen(N)cycling(Bengtsonetal.,2012;Chengetal.,2014).The RPE can increase SOM decomposition by up to 380%,and there are also reports of RPE contributing to a 50%reduction of SOM decomposition(Chengetal.,2014).
There are several possible reasons why the RPE varies,but most are linked to N availability.The microbial Nmining hypothesis proposes that microbes in the rhizosphere utilize C-rich rhizodeposits to“mine”N out of SOM(i.e.,to mineralize organic N or depolymerize proteins and other organic N sources)when inorganic N limits microbial growth and activity,resulting in accelerated SOM decomposition and a positive RPE(Kuzyakovetal.,2000).On the other hand,competition between roots and soil microorganisms for available N may suppress microbial growth and activity when N availability is limited(Månssonetal.,2009),resulting in reduced SOM decomposition(Kuzyakov,2002;Kuzyakov and Xu,2013).This mechanism has been suggested as a possible reason for negative RPEs,as the input of labile C in root exudates might aggravate the competition(Kuzyakov and Xu,2013;Lietal.,2021).
It has also been suggested that negative RPE can occur at high N availability,since a preferential use of root C exudates by the microbial decomposer community might reduce SOM decomposition under such conditions,resulting in negative RPE(Kuzyakovetal.,2000).Therefore,N availability seems to be a key regulator of the RPE(Luietal.,2018;Lietal.,2021),with negative RPE occurring under extremely N-limiting and N-rich conditions,while positive RPE at intermediate N contents(Kuzyakovetal.,2000;Lietal.,2020).
Most studies on the RPE have compared SOM-C mineralization in planted and unplanted soil.Interpretationof the results from such studies is complicated by the fact that the concentration of dissolved nutrients available for microbial uptake,particularly inorganic N,increases over time in unplanted soil(Schereretal.,1992).Furthermore,the microbial community composition,biomass,and activity in unplanted soil also change over time(Petersen and Klug,1994),and the pool of SOM that can be primed(in terms of amount and quality)is likely to differ between planted and unplanted soil.Compared to unplanted soil,observations of higher C and N mineralization rates in the presence of living plant roots are,therefore,not necessarily a result of the RPE.Such patterns may also be the result of diminishing C and N mineralization rates in the unplanted control,caused by the accumulation of inorganic N,depletion of available C,and changes in the microbial community composition and activity during the incubation period.Furthermore,the definition of“decompose”is“to separate into constituent parts or elements or simpler compounds”(Merriam-Webster.com Dictionary,2021).Therefore,measurements of C or N mineralization rate can not necessarily reflect or correlate to the total microbial SOM decomposition occurring at any given time(Ehtesham and Bengtson,2017).Decomposition also results in the production of organic compounds,and it appears that the rate-limiting step of SOM decomposition is the depolymerization of SOM into dissolved organic compounds that can be taken up and metabolized by soil microbial community(Bengtson and Bengtsson,2007).Measurements of the total SOM decomposition into compounds available for microbial uptake and metabolization might,therefore,provide a more accurate estimation of the RPE than measurements of SOM-C mineralization.
Here,we propose and evaluate an alternative control that was designed to minimize the differences in abiotic and biological soil properties between the control and treatment,thereby isolating the direct effect of root exudates(i.e.,the RPE)on soil C and N cycling rates.The experiment was performed using pine and spruce seedlings and two different controls(an unplanted control and a planted control).In the planted control,the seedlings were cut at soil surface 5 d before measurement of the RPE.The aims were to investigate how the choice of control influenced calculations of the RPE and that if variations in the calculated RPE can be linked to differences in soil N availability,enzyme activities,and microbial community composition and biomass between the two controls.
MATERIALS AND METHODS
Soil collection and experimental design
Soils(sandy loam)were collected from a Norway spruce(Picea abies)forest and a Scots pine(Pinus sylvestris)forest in southern Sweden.The soil samples were freshly sieved through a 5-mm mesh,mixed in a ratio of 4:1(spruce:pine),and stored at 4◦C for 6 d before the seedlings were planted.The pH of the mixed soil was 4.4,SOM content was 63 g kg−1,inorganic N content was 9.8µg g−1dry weight(DW)soil,total N content was 1.65 mg g−1DW soil,and C:N ratio was 20.3.
Five 2-year-old Norway spruce or Scots pine seedlings were planted in each pot(top diameter of 18 cm,a bottom diameter of 14 cm,and a depth of 16 cm)containing 1 250 g of freshly mixed soil.There were a total of 20 pots with pine seedlings,20 pots with spruce seedlings,and 8 pots without any seedlings.The unplanted pots were used as the unplanted control(UCK).The pots were then kept in a greenhouse supplemented with halogen lamps,at a temperature of 22◦C and a light-dark cycle of 12 h.The soil was adjusted to 50%water-holding capacity(WHC)and maintained at this level by watering the seedlings twice per week.After a 6-month cultivation period,half of the planted pots were randomly selected,and seedlings were cut at the soil surface.These pots are hereafter referred to as the planted controls(PCK).Seedlings in the remaining pots were left intact and are referred to as the planted treatments(PT).The appropriate time between cutting the seedlings and commencement of the measurements of RPE were evaluated in a preliminary experiment described below.On the 1st and 4th days after cutting the seedlings,all pots(48 in total)were watered with 150 mL of 2 atom%deuterium(D)water to estimate the D incorporated into fungal and bacterial biomarker phospholipid fatty acids(PLFAs).On the 5th day after cutting,a solution containing15NH4Cl(0.2µg N g−1soil,25 atom%15N)was injected into the soil at ten locations,to allow to determine gross N mineralization and assimilation rates(Lietal.,2021).Half of the pots were destructively harvested within 2 h after the addition of15N and the rest of the pots were harvested a day later.A part of the collected fresh soil was used for inorganic N extraction,analysis of bacterial and fungal biomass C production,and measurement of potential enzyme activity.The remaining soil was freezedried and used for PLFA analysis and determination of15N content by elemental analyzer(EA)-isotope ratio mass spectrometry(IRMS).The13C and15N contents of dried plant materials(roots and shoots)were also determined using EA-IRMS.Gross N assimilation was partitioned into plant and microbial assimilation of N,based on the fraction of15N recovered from plant roots,plant shoots,and SOM(Lietal.,2021).
Evaluation of appropriate time of cutting seedlings
A separate experiment was performed before the main experiment,to evaluate the appropriate time between cutting the seedlings and commencement of the measurements of RPE.The experimental settings and incubation conditions were identical to those used in the main experiment.Seedlingswere placed in a 5.4-L plexiglass chamber(length×width×height:30 cm×30 cm×60 cm),fitted with a gas-tight lid with a septum(one pot in each chamber)and labeled with13CO2by injecting 20 mL13CO2(99 atom%13C)into the chamber.After completion of the13CO2pulse labeling,the chambers were flushed with standardized laboratory air.The net CO2flux and stable C isotope value(δ13C)of CO2were measured for 6 d.After 6 d,the seedlings were cut at the soil surface and the pots were returned to the chambers for post-cutting measurements of the net CO2flux andδ13C of CO2.A Picarro G2201-i analyzer(Picarro Inc.,USA)was connected to the chambers using PTFE tubes and used to measure the concentrations of12CO2and13CO2.The analyzer was equipped with two 16-port manifolds that enabled recycling and automatic shifting between the chambers.Two minutes of automatic flushing with CO2-free laboratory air was performed between measurements,to remove residual air from the previous measurement in the tubing and analyzer cavity.
Laboratoryand data analyses
Gross N cycling rates were determined using the15Npool dilution method(Davidsonetal.,1991).15N-enriched NH4Cl(0.2 µg N g−1soil,25 atom%15N)was injected into the soil at 10 different positions.Half of the replicate pots were harvested within 2 h and the rest 24 h later.The soil was then transferred into a urine beaker and 1.0 mol L−1KCl was added.The beakers were shaken for 2 h,and the solution was then filtered through a GF/Ffilter paper into a new set of beakers.An NH3trap consisting of an acidified filter disk placed between two strips of PTFE tape was placed in each beaker.The beakers were then placed on a horizontal shaker for 5 d,after which the traps were removed,and the filter discs were dried in a desiccator.The contents of14N and15N in the traps were determined by IRMS at a stable isotope facility(Thermo Scientific Inc.,Germany)at the Department of Biology of Lund University,Sweden.Gross N mineralization and NH+4and NO−3assimilation rates were calculated from the change in contents of14N and15N in NH+4,NO−3,and organic N,using the FLUAZ model(Maryetal.,1998).Plant and microbial assimilation of N were separated by the proportion of15N recovered in plants and microorganisms,respectively(Lietal.,2021).We did not observe an enrichment of15N in NO−3during the assay,suggesting that autotrophic nitrification did not occur.Therefore,the nitrification rate was set to a fixed value of zero in the calculations.Likewise,we assumed that no denitrification,NH3volatilization,or recycling of microbial15N to NH+4occurred due to oxygenated conditions,low pH,and short duration of the assay,respectively.
To account for variability in the measured variables and uncertainty in the assumed variables(microbial C:N and C use efficiency)used to calculate SOM decomposition and the RPE,a probabilistic risk analysis was performed in@RISK 7.6(Palisade Corporation,USA)(Bengtsonetal.,2012).The SOM decomposition rate was estimated from the gross inorganic N assimilation rate(Bengtsonetal.,2012),and the RPE was calculated from the differences in SOM decomposition(or gross N mineralization)between PT and UCK or PCK.
Microbial C assimilation(Cass)was first calculated from microbial N assimilation using Eq.1:

where Nassis the total N assimilated by plants and microorganisms as calculated using the FLAUZ model;f15Nmicis the fraction of the total15N assimilated by microorganisms;and C:Nmicis the C:N ratio of soil microorganisms.The C:Nmicvalue was set to a maximum of 10.6(representing a microbial community dominated by fungi)and a minimum of 6.6(representing a microbial community dominated by bacteria),with an average value of 8.2.These values represent the average microbial C:N ratio in terrestrial forest ecosystems(Cleveland and Liptzin,2007).The SOM decomposition rate(SOMdec)was then calculated according to Eq.2:

where CUE is the microbial C use efficiency.The CUE was assumed to vary between 0.3 and 0.5(mean=0.4,standard deviation=0.05),which is within the range found for fungi and bacteria in forest soil(Bengtson and Bengtsson,2007;Spohnetal.,2016).Several studies have reported the CUE below 0.3(Freyetal.,2001;Tiemann and Billings,2011).As an underestimation of the CUE would lead to an overestimation of SOM decomposition in the presence of plants,a lower limit of 0.3 was considered to be appropriate for this study.
The RPE on SOM decomposition(RPESOM,%)was then calculated as the difference in SOM decomposition between PT and UCK or PCK,according to Eq.3:

where SOMUCKorPCKis the SOM decomposition in UCK or PCK and SOMPTis the SOM decomposition in PT.
The RPE on N mineralization(RPEN,%)was calculated as the difference in gross N mineralization between PT and UCK or PCK(Eq.4).
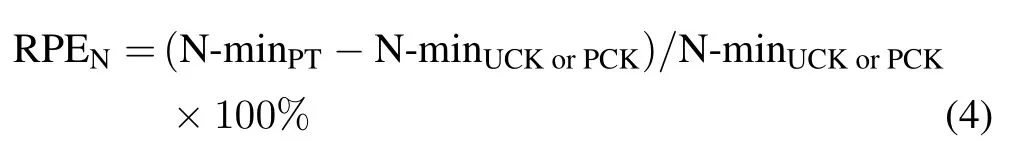
where N-minPTis the gross N mineralization in PT andN-minUCKorPCKis the gross N mineralization in UCK or PCK.
Bacterial C production was estimated using the3Hleucine incorporation method(Bååth,1994).Briefly,1.0 g fresh soil and 20 mL distilled water were placed into a centrifuge tube and vortexed.After centrifugation,1.5 mL of the suspension was transferred into an Eppendorf vial.And then,a diluted3H-leucine solution(containing 2 µL3Hleucine,2µL non-radioactive leucine,and 11µL distilled water)was added to the Eppendorf vial.After 2 h of incubation,trichloroacetic acid(TCA)was added to the solution.The washing procedure and subsequent measurement of radioactivity were performed according to Bååth(2001).The amount of3H-leucine in the samples was measured using a Tri-Carb 2910TR liquid scintillation counter(Perkin-Elmer Life Sciences,USA).The bacterial C production(Cbac,ng g−1DW soil d−1)was then calculated using the following equations:
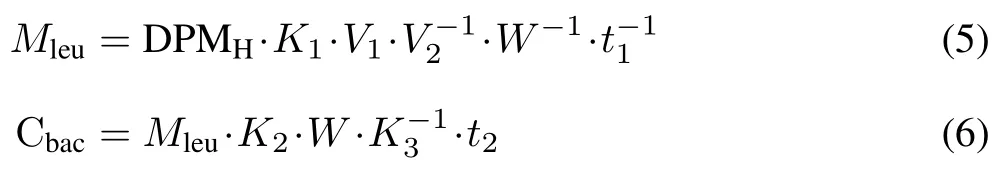
whereMleuis the amount of3H-leucine incorporated into bacteria(pmol g−1DW soil h−1);DPMHis the disintegrations per minute of the measured[3H]-radioactivity;K1andK2are the conversion factors used to convert leucine into biomass C,equal to 9.476 4×10−5and 3.1,respectively;K3is the extraction efficiency for bacteria(Rousk and Bååth,2007);V1is the volume of the supernatant taken from the centrifuge tube;V2is the volume of the soil solution in the centrifuge tube;Wis the dry weight of soil;t1is the incubation time;andt2is 24 h.
Fungal C production was estimated using the14C-acetate incorporation method(Bååth,2001).Briefly,1 g fresh soil was placed in a test tube,and then a diluted14C-acetate solution(1.95 mL water,20µL14C-acetate solution,and 30µL non-radioactive acetate solution)was added.The samples were then incubated at room temperature.After 4 h,5%formalin was added to terminate the incubation.Ergosterol was extracted,separated,and quantified using high-performance liquid chromatography(HPLC)equipped with a UV detector(Rousk and Bååth,2007).The radioactive fraction was retrieved with a fraction collector and quantified using a scintillation counter(Perkin-Elmer Life Sciences).Fungal C production(Cfun,ng g−1DW soil d−1)was calculated using the following equations:

whereMaceis the amount of14C-acetate incorporated into ergosterol(pmol g−1DW soil h−1);DPMCis the disintegrations per minute of the measured[14C]-radioactivity;K1andK2are the conversion factors used to convert acetate into biomass C,equal to 0.124 233 103 and 2.5,respectively(Rousk and Bååth,2007);Wis the dry weight of soil;t1is the incubation time;andt2is 24 h.
The PLFAs were extracted from freeze-dried soil and converted to fatty acid methyl esters(FAMEs)following Lietal.(2020).Briefly,lipids were extracted from freezedried soil and then loaded on silica-based sorbent cartridges,and the PLFA fraction was isolated.Methyl nonadecanoate(C19:0,0.023 mg mL−1),as an internal standard,was added to the fraction containing the PLFAs,which were then transmethylated to their corresponding FAMEs.The concentration and isotopic composition of the FAMEs were determined at a stable isotope facility at Lund University.Fatty acids i15:0,a15:0,i16:0,16:1ω7,18:1ω7,10 Me18:0,and cy19:0 were chosen to indicate bacteria,whereas fatty acid 18:2ω6,9 was used to indicate fungi(Churchlandetal.,2013).Microbial D incorporation was calculated based on the D atom%excess in individual PLFAs(Lietal.,2020).
We also measured the potential activities of 6 extracellular hydrolases(C-targeting:α-glucosidase,β-glucosidase,β-xylosidase,and cellobiohydrolase;N-targeting:Nacetyl-β-D-glucosaminidase and leucine-aminopeptidase)and 2 oxidases(phenoloxidase and peroxidase)by using a combination of fluorometric and photometric assays(Marxetal.,2001;Saiya-Corketal.,2002;Wildetal.,2019).Briefly,fresh soil was placed in a container with sodium acetate buffer.Soil suspensions and fluorescence-labeled substrates(Sigma Aldrich,USA)were then added,and hydrolytic enzyme activities were analyzed using an FLUOstar Omega(BMG Labtech,Germany).Potential oxidative enzyme activities were measured in soil suspensions mixed withL-3,4-dihydroxyphenylalanine(DOPA,Sigma Aldrich)substrate,and measured photometrically before and after the incubation.Potential oxidative enzyme activities were determined from the difference in absorbance between two time points,and are expressed in nmol DOPA g−1DW soil h−1.
Statistical analysis
Statistical tests were performed using STATISTICA version 13(Dell Inc.,USA).The data were tested for normality and homogeneity of variance before analysis.The effects of treatment on soil and microbial properties were tested by one-way analysis of variance,followed by Tukey’s honestly significant differencepost-hoccomparisons.Differences among treatments were considered significant atP<0.05.Principal component analysis was performed to investigate the effect of treatment on microbial community composition and potential enzyme activities.Treatment differences in SOM decomposition and the RPE were evaluated by testing if the 85% confidence interval overlapped or not(Paytonetal.,2000,2003).
RESULTS AND DISCUSSION
Evaluation of appropriate time of cutting seedlings
In the preliminary experiment aimed at evaluating the appropriate time between cutting the seedlings and commencement of the measurements of RPE,a strong negative net CO2flux during13CO2labeling showed that the seedlings actively assimilated the labeled13C(Fig.1).After 6 d,the net CO2flux remained constant,while theδ13C decreased,probably due to reduced respiration of the fixed13C by roots and root-associated microorganisms.Soil CO2efflux was high(24.6µmol h−1)during the first day when the seedlings were cut,and gradually decreased to stable levels(10.0µmol h−1)on day 5(Fig.1).These results suggest that the decomposition of roots did not induce a priming effect for up to 6 d after the seedlings were cut.Shahzadetal.(2015)reported that the priming effect can occur if the time between the cutting of seedlings and measurements of C and N turnover is too long.The estimated RPE caused by root exudation was,therefore,not confounded by the priming effect caused by decomposing roots in the planted control.Theδ13C of CO2increased from 24.5‰(on day 1 after cutting the seedlings)to 47.6‰(on day 4)and then decreased to 44.0‰(on day 6)(Fig.1).The decrease in the CO2flux that occurred 4 d after cutting the seedlings thus coincided with the stabilization ofδ13C of CO2.Since the roots were left undisturbed after cutting,we interpreted the high efflux of13C-enriched CO2as the occurrence of residual root respiration,up to 3 d after the seedlings were cut.Based on these findings,it can be concluded that a 5-d period between cutting the seedlings and the measurements of C and N turnover was sufficient to allow root activities to cease,but not so long when a priming effect from the decomposition of roots was induced.
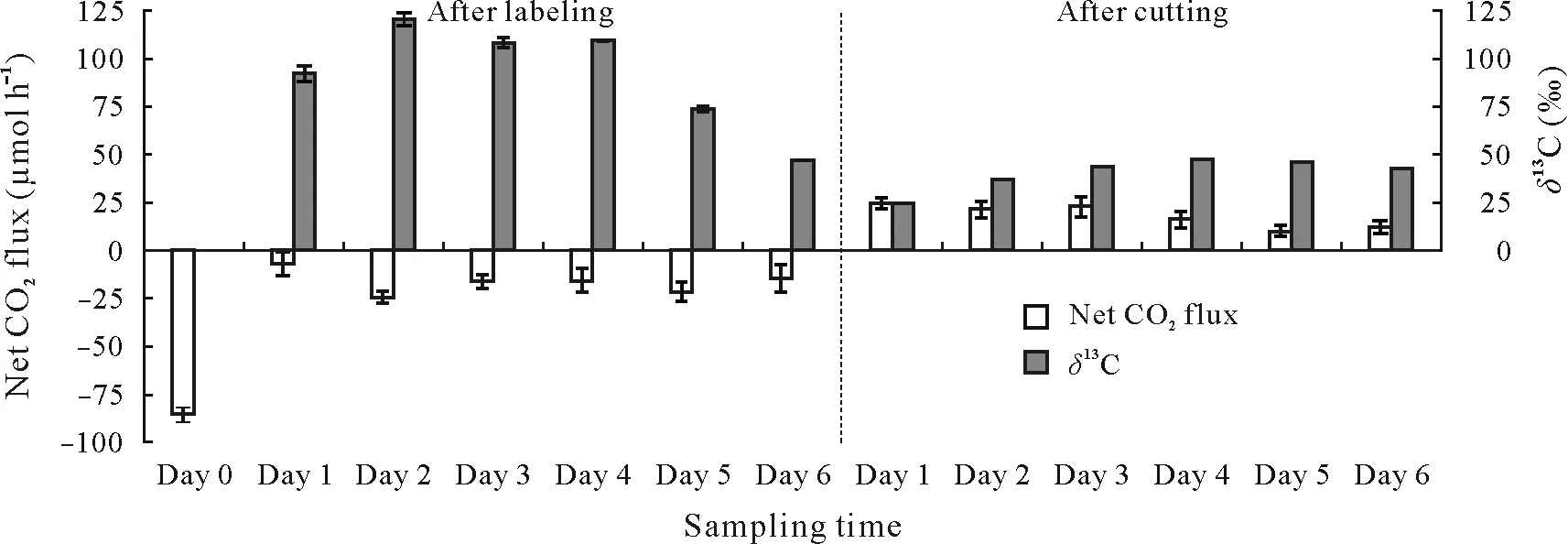
Fig.1 Net CO2 flux and stable C isotope value(δ13C)of respired CO2 6 d after 13CO2 labeling of intact seedlings and 6 d after cutting the seedlings.Time points for which the error bars(representing 85%confidence intervals)do not overlap are significantly different.
RPEon SOM decomposition and N mineralization calculated from two controls
The RPE varied considerably,depending on the control used for the calculations.The RPE on SOM decomposition was 23.3%and−18.5%in the pine and spruce soils,respectively,when calculated from PCK;it was 14.0%and 21.0%,respectively,when calculated from UCK(Fig.2).When the RPE on gross N mineralization was calculated from PCK,no significant RPE was observed(i.e.,the RPE did not differ significantly from zero).In contrast,when the RPE on gross N mineralization was calculated from UCK,a strong positive RPE on gross N mineralization was detected in both pine(120%)and spruce(190%)soils(Fig.2).The difference between the two types of controls was due to much lower gross N mineralization rates in UCK than in PCK(Table I).These results demonstrate that the choice of control has a decisive influence on the reported RPE.

Fig.2 Rhizosphere priming effect(RPE)on soil organic matter(SOM)decomposition(RPESOM)and on N mineralization(RPEN)for the planted treatment with intact seedlings,calculated from the planted control(PCK)and unplanted control(UCK)using Eqs.3 and 4,respectively.In PCK,pine or spruce seedlings were cut at soil surface 5 d before the measurement of RPE.Error bars represent the standard errors.Significant differences,assessed by testing for overlaps between 85%confidence intervals,are indicated by different letters.
Variations of soil N and microbial communityaffecting RPE
Nitrogen availability has been suggested to be a key regulator of the RPE(Dijkstraetal.,2013;Chengetal.,2014).In the absence of plants,variations in N availability may also have a pronounced effect on SOM decomposition,because inorganic N influences both the production and activity of enzymes such as peroxidases and phenol oxidases(Galloetal.,2004;Zhou and Zhang,2014).Inorganic N content was observed 74%–188% higher in UCK than in PCK,while inorganic N in PCK was comparable to their corresponding PT(Fig.3).It cannot be excluded that the positive RPE found in the spruce soil calculated from UCK was an artifact of high inorganic N contents reducing SOM decomposition in UCK.Accordingly,it is well-established that high N availability commonly results in reduced SOM-C mineralization rates(Riggs and Hobbie,2016;Lietal.,2017;Liuetal.,2018).The significant positive RPE on gross N mineralization calculated from UCK was also most likely caused by the higher inorganic N content in UCK relative to PCK.High N availability is known to reduce the expression of genes involved in microbial N acquisition(Merrick and Edwards,1995),leading to reduced gross N mineralization rates.However,it should be noted that we only measuredthe gross N mineralization of a single day.Further research on temporal variations in the gross N mineralization rate in an unplanted control is needed to better understand how N availability influences the RPE when it is estimated based on an unplanted control.
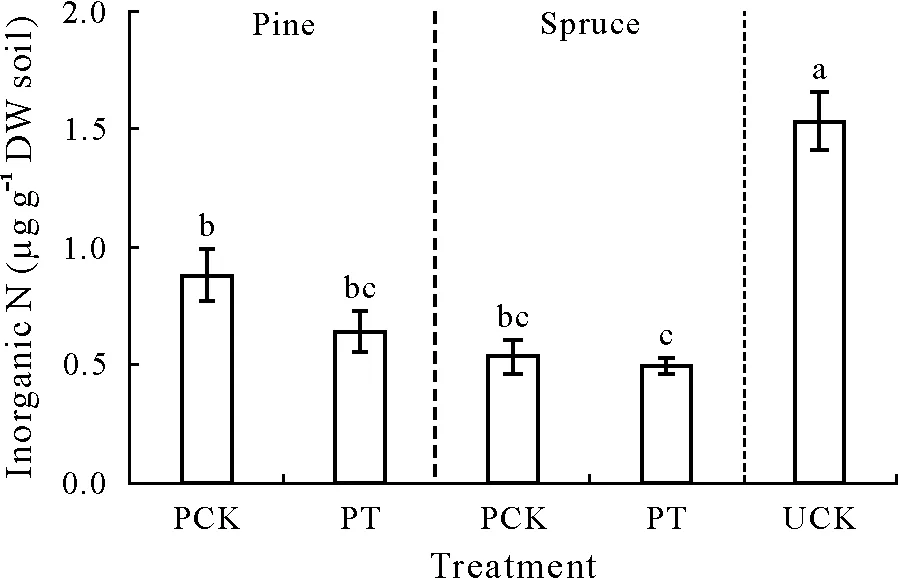
Fig.3 Soil inorganic N content in the planted control(PCK),unplanted control(UCK),and the planted treatment with intact seedlings(PT).In PCK,pine or spruce seedlings were cut at soil surface 5 d before the measurement of rhizosphere priming effect.Vertical bars denote the standard errors of means(n=10 in PCK and PT,n=8 in UCK).Significant differences are indicated by different letters,as assessed by a one-way analysis of variance,followed by the Tukey’s honestly significant difference test at P<0.05.DW=dry weight.
Variations in SOM-C and gross N mineralization rates have been linked to variations in microbial biomass(Zamanetal.,1999;Thiessenetal.,2013),activity(Bengtssonetal.,2003;Blagodatskyetal.,2010),community composition(Högbergetal.,2007;Xuetal.,2015),and enzyme production(Zamanetal.,1999;Xuetal.,2015).Our results showed that most of these microbial properties differed between the two types of controls.Microbial biomass was lower in UCK than in PCK,although the effect was only significant between UCK and the PCK of pine(Fig.4).The microbial community was dominated by bacteria to a much greater extent in UCK(Fig.4).Furthermore,the measurements of leucine incorporation into bacteria,acetate incorporation into fungi,and D incorporation into fungal and bacterial PLFAs consistently showed that fungal and bacterial growth was lower in UCK than in PCK;even if the differences between UCK and PCK of spruce were not always significant(Table I).
Principal component analysis of the relative abundance of PLFAs in the controls and planted treatments further showed that the microbial community composition in UCK was distinctly different from those in PCK and PT(Fig.5a).In contrast,similar microbial communities were found in PCK and corresponding PT.The potential activities of oxidative and C-and N-acquiring hydrolytic enzymes also differed between the two types of control,while there was no difference in the potential enzyme activity between UCK and their planted counterpart(Fig.5b).However,there were larger differences in potential enzyme activity between the pine and spruce soils than between the spruce soils and the soils in UCK,while the greatest differences in the RPE between the two controls were observed.This suggests that themeasurements of potential enzyme activities are of limited value for predicting the RPE.
Keyregulators of RPE
Variations in soil N availability and microbial biomass,activity,and community composition between PCK and UCK strongly influence our understanding and evaluation of the RPE,particularly because N availability and microbial community attributes are considered as key regulators of the RPE(Kuzyakov,2002;Cheng,2009).Our findings are in line with previous observations,which suggest that storage of soil at ambient temperatures results in changes in microbial biomass(Westetal.,1986),community composition(Petersen and Klug,1994),and activity(Stotzkyetal.,1962;Wangetal.,2015),and consequently in soil enzyme concentrations(Dadenkoetal.,2009;Wangetal.,2015),inorganic N content,and C and N mineralization rates(Mann,1986;Turner and Romero,2009).Observations of higher C and N cycling rates in the presence of living plant roots than in their absence,are therefore,not necessarily a result of the microbial SOM decomposition activities stimulated by roots and root exudates;they are equally likely a result of the diminishing C and N turnover rates in UCK.In otherwords,the use of unplanted controls risks the overestimation of RPE,potentially complicating and distorting our understanding of the RPE,unless it is interpreted with care.In contrast,the inorganic N content(Fig.3),microbial biomass(Fig.4),microbial community composition(Fig.5a),and potential enzyme activities(Fig.5b)were as well-preserved in PCK when compared to PT.This suggests that we could successfully distinguish the RPE caused by root exudates from other root effects on SOM decomposition rate.As the RPE is defined as the stimulation or suppression of SOM decomposition in response to labile root C input,a planted control is preferable when quantifying the RPE.
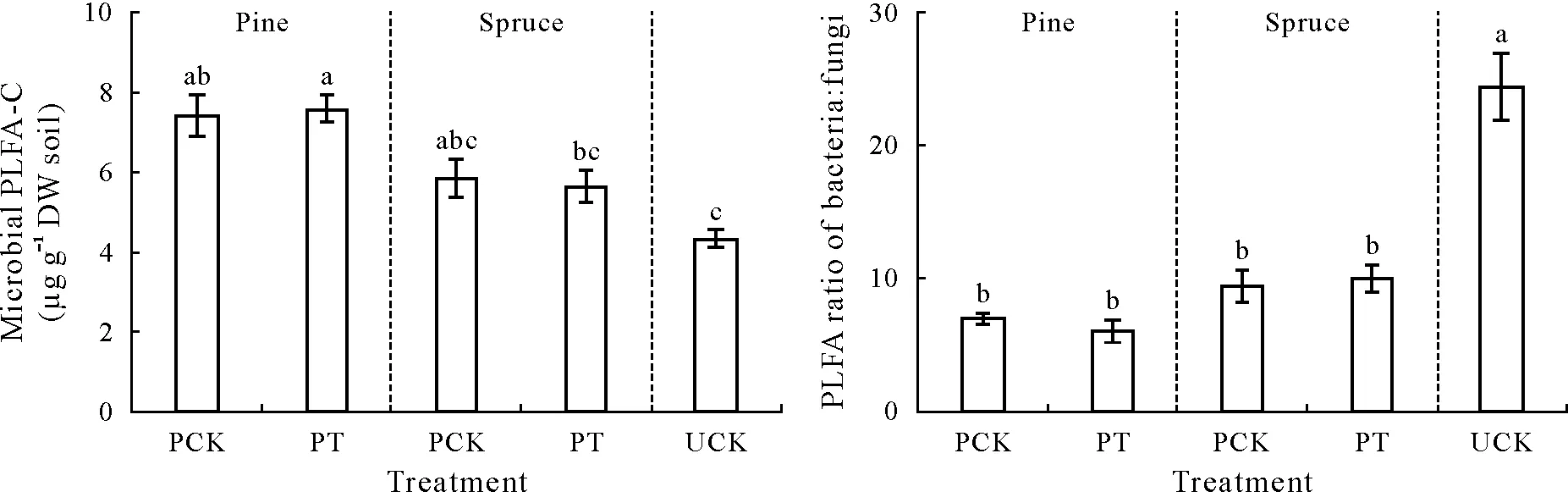
Fig.4 Content of microbial phospholipid fatty acid(PLFA)-C and PLFA ratio of bacteria:fungi in the planted control(PCK),unplanted control(UCK),and the planted treatment with intact seedlings(PT).In PCK,pine or spruce seedlings were cut at soil surface 5 d before the measurement of rhizosphere priming effect.Vertical bars denote the standard errors of means(n=10 in PCK and PT,n=8 in UCK).Significant differences are indicated by different letters,as assessed by a one-way analysis of variance,followed by the Tukey’s honestly significant difference test at P<0.05.DW=dry weight.
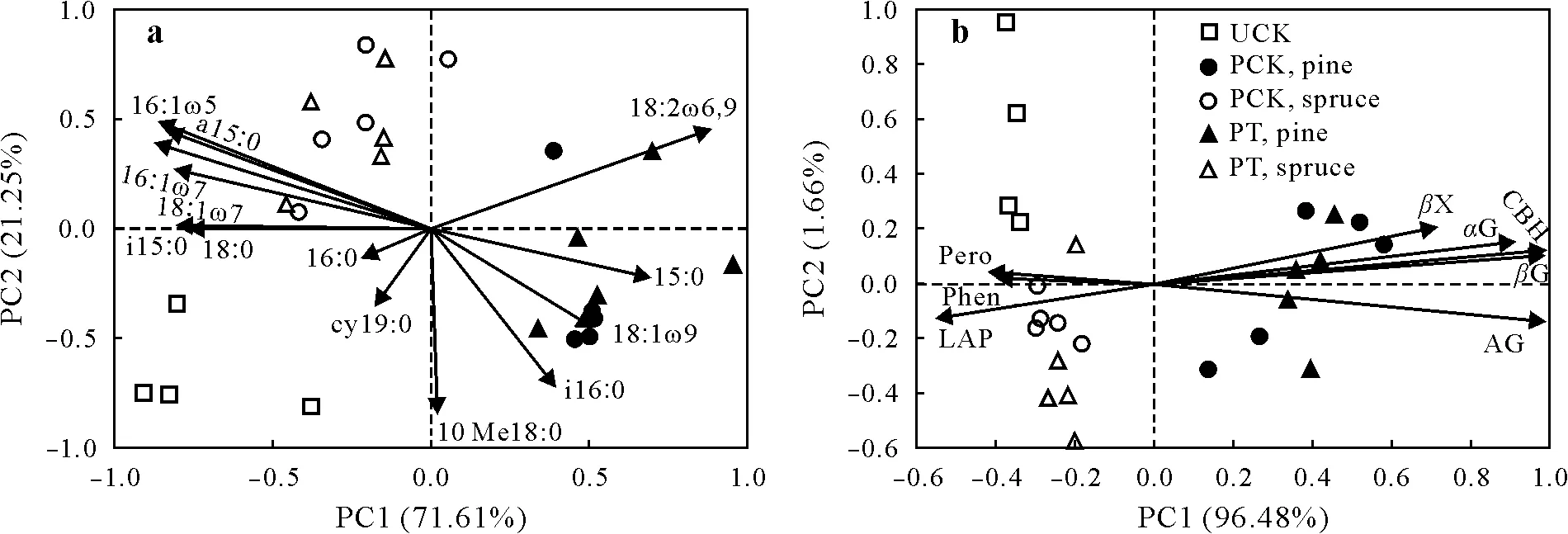
Fig.5 Principal component(PC)analyses of relative abundance of phospholipid fatty acids(a)and potential enzyme activities(b)in the planted control(PCK),unplanted control(UCK),and the planted treatment with intact seedlings(PT).In PCK,pine or spruce seedlings were cut at soil surface 5 d before the measurement of rhizosphere priming effect.αG=α-glucosidase;βG=β-glucosidase;βX=β-xylosidase;CBH=cellobiohydrolase;AG=N-acetyl-β-D-glucosaminidase;LAP=leucine-aminopeptidase;Phen=phenoloxidase;Pero=peroxidase.
The method proposed herein for quantifying the RPE has its own set of limitations.Most importantly,it only provides a snapshot of the RPE at the time of the experiment.The approach suggested here is also not suitable for plant species that continue to release root exudates after mowing,since the roots could still exude C,inducing an RPE effect even after the aboveground plant parts are cut off.If environmental variables that significantly affect the RPE exhibit high temporal variation and the aim is to quantify the cumulative RPE during a growth season,repeated experiments that capture such temporal variations need to be performed to give reliable estimates.If the aim is to estimate soil C losses or changes in soil C turnover times caused by the RPE,continuous labeling with13C depleted CO2(Pauschetal.,2016)or observations of C3-to-C4 vegetation changes(Kumaretal.,2016)might be more appropriate methods to use.It should also be noted that our experiment was limited to one soil type and two tree species,and the findings cannot be extrapolated to other soil types and plant species without further investigation.
CONCLUSIONS
It has been stated that rhizospheric effects on SOM decomposition are omnipresent in terrestrial ecosystems and that the SOM decomposition rate found in root exclusion experiments and unplanted soil incubations should be considered unrealistic(Cheng,2009).Even so,most experiments assessing the RPE have been conducted using plant-free soils as controls.Nonetheless,such experiments may produce valuable data,depending on the purpose of the study.However,we conclude that the planted control proposed here should be considered if an experiment aims to gain a mechanistic understanding of how the plant responses to the environment translate into altered priming of SOM decomposition and belowground C and N cycling and to reliably quantify the RPE,independent of the reduction in SOM decomposition that might occur in an unplanted control.
ACKNOWLEDGMENTS
This work was funded by the Swedish Research Council(No.2016-04710,2016),the Knut and Alice Wallenberg Foundation,Sweden(No.2013.0073),and China Postdoctoral Science Foundation(No.2021M703135).Jian LI was supported by the Chinese Scholarship Council.Saeed Alaei and Moyan Zhou are acknowledged for their assistance in fields,laboratories,and greenhouses.
杂志排行
Pedosphere的其它文章
- Effect of indaziflam on microbial activity and nitrogen cycling processes in an orchard soil
- Spectral indices measured with proximal sensing using canopy reflectance sensor,chlorophyll meter and leaf color chart for in-season grain yield prediction of basmati rice
- Effect of Zn binding to phytate and humic substances on its uptake by wheat(Triticum durum L.)as affected by carbonates and Fe oxides
- Occurrence of polycyclic aromatic hydrocarbons in the estuarine sediments of the Taihu Lake and their associated toxic effects on aquatic organisms
- Contribution of arbuscular mycorrhizal fungi and soil amendments to remediation of a heavy metal-contaminated soil using sweet sorghum
- Nitrogen slow-release behavior of oxamide granules in two different types of paddy soils
