Rapid differentiation of simple saccharides based on cluster ions by paper spray tandem mass spectrometry
2022-12-07WngminHuTinyiLiYuleiYngShnshnJiMeiZhng
Wngmin Hu, Tinyi Li, Yulei Yng, Shnshn Ji, Mei Zhng,∗
a School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing 102488, China
b National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China
Keywords:Simple saccharide Rapid structure differentiation Paper spray Tandem mass spectrometry
ABSTRACT Simple saccharides have a variety of biological functions, but their structural diversity and inherent structural features pose a major challenge for rapid analysis.In this work, we developed a derivative-free and ion mobility-free method for the rapid analysis of monosaccharides and disaccharides using paper spray tandem mass spectrometry.Trimeric cluster ions consisting of saccharide analytes, ligands and transition metal ions are used as precursor ions.We defined the R-value as the ratio of the intensity of the product ion that loses one molecule of ligand over the intensity of the product ion that loses one molecule of saccharide via collision induced dissociation (CID).The species and conformation of simple saccharides can be easily differentiated by calculating this R-value.With the capability of directly analyzing clinical samples using paper spray ionization, our method can be used to rapidly quantify the molar ratio of galactose to glucose in dried plasma samples to aid in the diagnosis of galactosemia.The analytical strategy provided herein has good potential to be applied to a wide range of saccharide analysis applications in the future.
Saccharides, commonly known as carbohydrates or sugars, are defined as optically active polyhydroxy aldehydes or polyhydroxy ketones or substances which give these on hydrolysis are termed as saccharides.Complex and diverse saccharides are ubiquitous to almost all cells in nature and are essential for all life forms, where they play major metabolic, structural and physical roles in biological systems.For instance, saccharides can provide energy to cells and serve as structural components for plant cell walls.By conjugating with proteins and lipids, saccharides are widely involved in intracellular transport, cell adhesion, cell-cell interactions [1–5].The saccharide molecule has an extreme structural diversity, with an almost infinite number of isomers based on different carbonyl positions, the number of carbon atoms in the sugar ring and their chiral configuration.The D- or L-configuration is determined by the orientation of the asymmetric carbon atom farthest from the carbonyl group.In sugar analysis, it is necessary to determine each monosaccharide unit and its stereochemistry, as well as the isomeric configuration of the glycosidic bonds in the oligosaccharide chain, the linkage positions, and the order of the individual monosaccharides in the oligosaccharide.
The common methods used for saccharide structure analysis mainly include nuclear magnetic resonance (NMR), chromatography and mass spectrometry (MS).Higher dimensional NMR is a powerful tool to obtain detailed structure information of saccharides [6–9].However, NMR analysis often requires milligram-level purified samples, which implies high requirements for both sample size and sample purity.Chromatographic analysis combined with the use of standard saccharides as controls is also a common method for differentiation of saccharides.Previous reports include the use of gas chromatography (GC) [10–12], high performance liquid chromatography (HPLC) [13–16] and capillary electrophoresis(CE) [17–20].However, chromatographic methods also suffer from many limitations, mainly due to the inherent structural characteristics of saccharides that often require an additional pre-column derivatization step prior to GC or LC injection to obtain better detection or better separation [10,21].Additional derivatization is not only time-consuming, but can also lead to greater errors and complications in analytical operations.MS has been widely used for structural analysis of saccharides owing to its high sensitivity, high resolution and small sample size [22], and it also can be considered as a detector in tandem with chromatography.
Despite the significant advantages of MS, its application in the field of saccharide analysis still remains two challenges: low ionization efficiency due to the high hydrophilicity and polarity of saccharides; and a vast number of possible isomers due to stereochemistry, conformational differences, monosaccharide composition and linkage sites.A great deal of work has been done by many analytical chemists, and as a result, much progress has been made in the field of saccharide MS in recent years.For instance,matrix assisted laser desorption ionization (MALDI)-MS, including MALDI-based MS imaging, has been used for the analysis of many simple saccharides [23,24].For saccharide analysis using electrospray ionization (ESI)-MS, Gao’s group distinguished seven saccharide isomers by one-step radical-induced dissociation in MS analysis based on their synthesized free-radical-induced dissociation reagent Me-FRAGS [25].Kondaet al.distinguished 1,3-linked disaccharides with different stereochemistry and anomeric conformations by tandem MS combined with18O-labeling [26].Taken together, tandem MS analysis for simple saccharides is still often used with additional steps of chemical reactions such as derivatization, free radical reactions or labeling.What’s more, the ion mobility mass spectrometry (IM-MS) has been developed in recent years as a commercial instrument that can analyze saccharide isomers [27,28].In IM-MS analysis, the ion “drift time” is measured along with its mass-to-charge (m/z) [29].The drift time can be used to calculate the rotationally averaged collision cross section(CCS) of an ion in a specific drift gas, which is correlated only to the shape of the ion.This provides additional information on the size and shape of the ion, which can help to distinguish saccharide isomers [30].However, if sizes of isomer-ions are too small, such as monosaccharide or disaccharide ions, the differences reflected in their CCSs are too small to be used to distinguish them.That was why Clemmer’s group used the CCS of cluster ions, containing particular monosaccharides, as the basis for differentiating the 16 common monosaccharides in the IM-MS analysis [30].The cluster ion formed by one monosaccharide and ligands coordinated to a transition metal increases the size of the ion, thus also increasing the variability of their CCSs.
In fact, cluster ions have long been used for chiral analysis in MS [31], and the methodology has been applied to analysis of chiral amino acids [32,33],α-hydroxyacids [34], chiral drugs [35,36].In particular, Cooks’s group reported an ESI-MS/MS method for the quantitative analysis of chiral monosaccharides using cluster ions[37].Modified amino acids were used as chiral ligands and defined the ratio of the product ion to the intensity of the precursor ion of the monosaccharide analyte as the "R-value".However, the reference only reported that monosaccharides can be analyzed using this method, and did not mention the application to disaccharide differentiation.
In this study, the calculation method of “R-value” was modified as the ratio between two product ions and expanded the involvement of precursor-product ion pairs (we used two pairs), enabling this method to be applied to disaccharide analysis.Furthermore,paper spray MS was applied for analysis, thus enabling direct and rapid analysis of clinical dried blood spot samples.Paper spray MS has been shown to allow rapid analysis of oligosaccharides in complex matrices [38].In the current method, the trimeric cluster ions[M(L)2(S)–H]+or [M(L)(S)2–H]+were used as the precursor ions,which are formed from each saccharide (S) with a metal ion (M)and the chiral amino acid as the ligand (L).Each precursor ion may lose either a molecule of ligand or a molecule of saccharide to generate product ionsviacollision induced dissociation (CID).TheRvalue was defined as the ratio of the intensity of the product ion that loses one molecule of ligand over the intensity of the product ion that loses one molecule of saccharide.Here, we described identification of common simple saccharides (including monosaccharides and disaccharides) byR-values as the basis for differentiation.The method established does not require derivatization nor labeling and can differentiate simple saccharides by tandem MS without the need of ion mobility.The analytical strategy provided in this paper combines the advantages of rapid detection capability of paper spray and high sensitivity of mass spectrometry, and is expected to be applied in the future for rapid detection of simple saccharides in clinical samples, or for high-throughput analysis of other glycan biomarkers.
Chemicals are all purchased from Sigma-Aldrich (St.Louis, MO)unless otherwise specified, including saccharides, amino acids, inorganic salts, methanol, formic acid and ultrapure water.Monosaccharides: D-Glucose (D-Glc), L-Glucose (L-Glc), D-Galactose (D-Gal),D-Mannose (D-Man), D-Fructose (D-Fru).Disaccharides: D-Lactose(D-Lac), D-Sucrose (D-Suc), D-Lactulose (D-Lact), D-Cellobiose (DCel), D-Melibiose (D-Mel), D-Trehalose (D-Tre), D-Maltose (D-Mal),D-Turanose (D-Tur).Amino acid: L-Proline (L-Pro), L-Phenylalanine(L-Phe), L-Glutamic acid (L-Glu), L-Histidine (L-His),N-Fmoc-LProline (N-Fmoc-L-Pro).Inorganic salts: Nikel chloride (NiCl2),nickel nitrate [Ni(NO3)2] and copper chloride (CuCl2).The ultrafiltration tubes were purchased from Millipore (Cat.#UFC800396, 3 kD cutoff; Burlington, MA).
All MS experiments were performed using a commercial Q-Trap mass spectrometer (Q-Trap 6500, Sciex, USA).PS-MS method was used for almost analytical experiments unless otherwise stated.The analysis of prepared solutions followed the general PS-MS procedure; dried plasma spot samples were analyzed using the reported method with minor changes [39].Generally, we prepared standard solutions by dissolving each saccharide analyte in MeOH:H2O = 1:1 (v/v) to a final concentration of 0.2 mmol/L.The sample (2 μL of each prepared sample solution; 400 μL of plasma for making a dried plasma spot) was loaded directly onto a triangular section of chromatography paper (about 7 mm base width and 7 mm height).Spray voltage was set at 4.5 kV analyzed in positive mode, and 8 μL of MeOH solution containing 0.1% formic acid was used as the elution solvent.For plasma analysis, we first added 10 μL of a mixed solution of ligand and metal ions onto the dried plasma spot and waited 1 min for sufficient formation of cluster ions; subsequently applied eluent solvent and high voltage for paper spray analysis.The best collision energy (CE) of CID fragmentation for each analyte in MS/MS analysis was optimized individually.A standard curve was made for the quantitative analysis of plasma samples.We used plasma that had been ultrafiltered to remove small molecules as the matrix and spiked with 5.0 mmol/L glucose and a range of different concentrations of galactose as samples for making the standard curve.The galactose concentrations covered from 0.05 to 5.0 mmol/L, resulting in molar ratios of D-Gal/D-Glc equal to 0.01, 0.05, 0.1, 0.2, 0.5 and 1.0, respectively.Samples were loaded onto the chromatography paper required for PS-MS analysis and left to dry before use.
Dilution experiments were carried out using the same mass spectrometer equipped with an ESI ion source.Samples were injected into the ESI sourceviaa syringe pump at a flow rate of 2 μL/min.ESI conditions were set as follows: positive mode;ion source voltage, 5 kV; capillary temperature, 150 °C; and curtain gas, 20 °C.The ESI-MS sample was made as a mixture: the sugar analyte, the ligand and the metal ion were co-dissolved in MeOH:H2O = 1:1 (v/v), and the concentrations of the components were described in the specific experiments.
In this work, the trimeric cluster ions [M(L)2(S)–H]+or/and[M(L)(S)2–H]+were used as the precursor ions, in which “S” refers to a particular saccharide, “M” refers to a metal ion, and “L” refers to the chiral amino acid ligand.As shown in Scheme 1, the precursor cluster ion A [M(L)2(S)–H]+can produce two typical product ionsviaCID, which are the product ion [M(L)(S)–H]+as losing a ligand and the product ion [M(L)2–H]+as losing a saccharide;while and the precursor cluster ion B [M(L)(S)2–H]+can produce two typical product ionsviaCID, which are the product ion[M(S)2–H]+as losing a ligand and the product ion [M(L)(S)–H]+as losing a saccharide.According to our definition of theR-value,theRA-value is equal to the intensity ratio of the two product ions [M(L)(S)−H]+/[M(L)2−H]+, produced by the parent cluster ion[M(L)2(S)−H]+; while theRB-value is equal to the intensity ratio of the two product ions [M(S)2−H]+/[M(L)(S)−H]+, produced by the parent cluster ion [M(L)(S)2−H]+.Different monosaccharides and disaccharides were used to investigate the applicability of the developed method (Scheme 2).We found it convenient to useR-values (RA-values alone orRA- in combination withRB-values)to differentiate the conformation or species of simple saccharides.R-values can even be used to some extent to quantify the proportion of monosaccharide components in a mixture.Therefore, it is possible to develop useful applications for saccharide analysis based onR-values in rapid clinical detection.
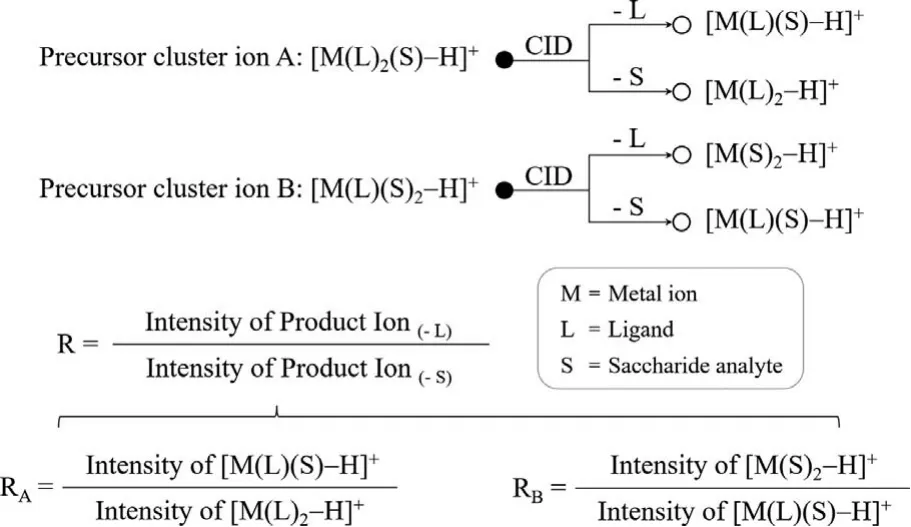
Scheme 1 .Fragmentation of precursor cluster ions and definition of R-values.
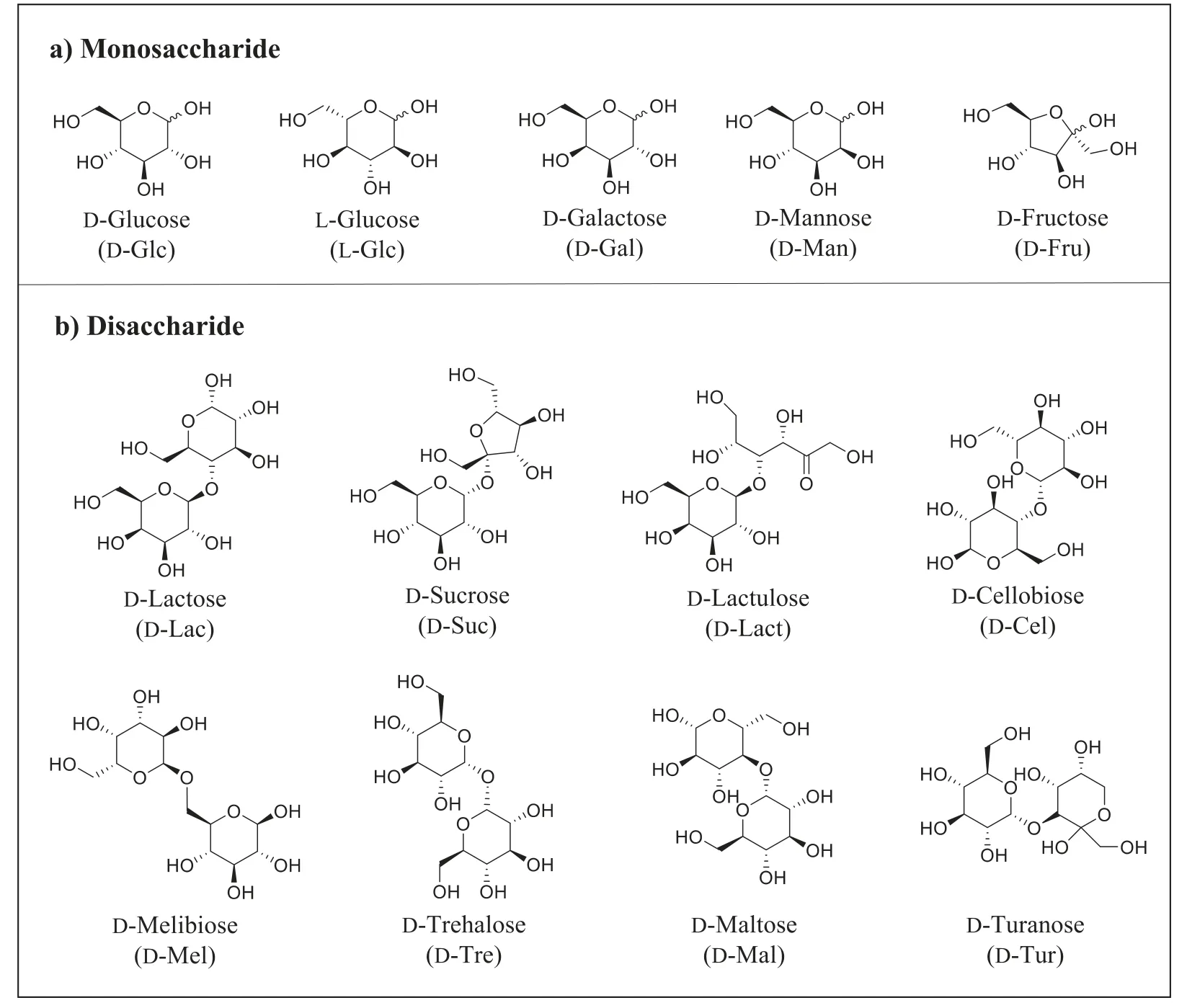
Scheme 2 .Structures of the simple saccharides analytes in this paper: a) Monosaccharide analytes: different monosaccharide species (D-Glc, D-Gal, D-Man and D-Fru), as well as different configurations of one monosaccharide (D- and L-Glc); b) disaccharide analytes: eight isomers with different monosaccharide compositions, linkage sites and α/β conformations (D-Lac, D-Suc, D-Lact, D-Cel, D-Mel, D-Tre, D-Mal and D-Tur).
The cluster ion formed by one monosaccharide is mainly an A-type precursor cluster ion [M(L)2(S)−H]+, and theRA-value is able to differentiate the D-/L-configuration of the monosaccharide.We used glucose (Glc) as an example, and Fig.1 shows the MS/MS CID spectra of D-Glc and L-Glc.Their respective precursor ions are bothm/z911 ([NiII(N-Fmoc-L-Pro)2(Glc)–H]+), and the precursor cluster ionm/z911 can produce the two typical product ionsm/z574 ([NiII(N-Fmoc-L-Pro)(Glc)–H]+) andm/z731 ([NiII(N-Fmoc-L-Pro)2–H]+)viaCID.TheRA-value is equal to the ratio of the intensity ofm/z574 to that ofm/z731.RA-value of D-Glc is 0.769 which is less than 1 (m/z574 peak is lower thanm/z731 peak in MS/MS spectrum), while theRA-value of L-Glc is 1.156 which is greater than 1 (m/z574 peak is higher thanm/z731 peak in MS/MS spectrum).By calculatingRA-values we can easily distinguish between D-Glc and L-Glc.
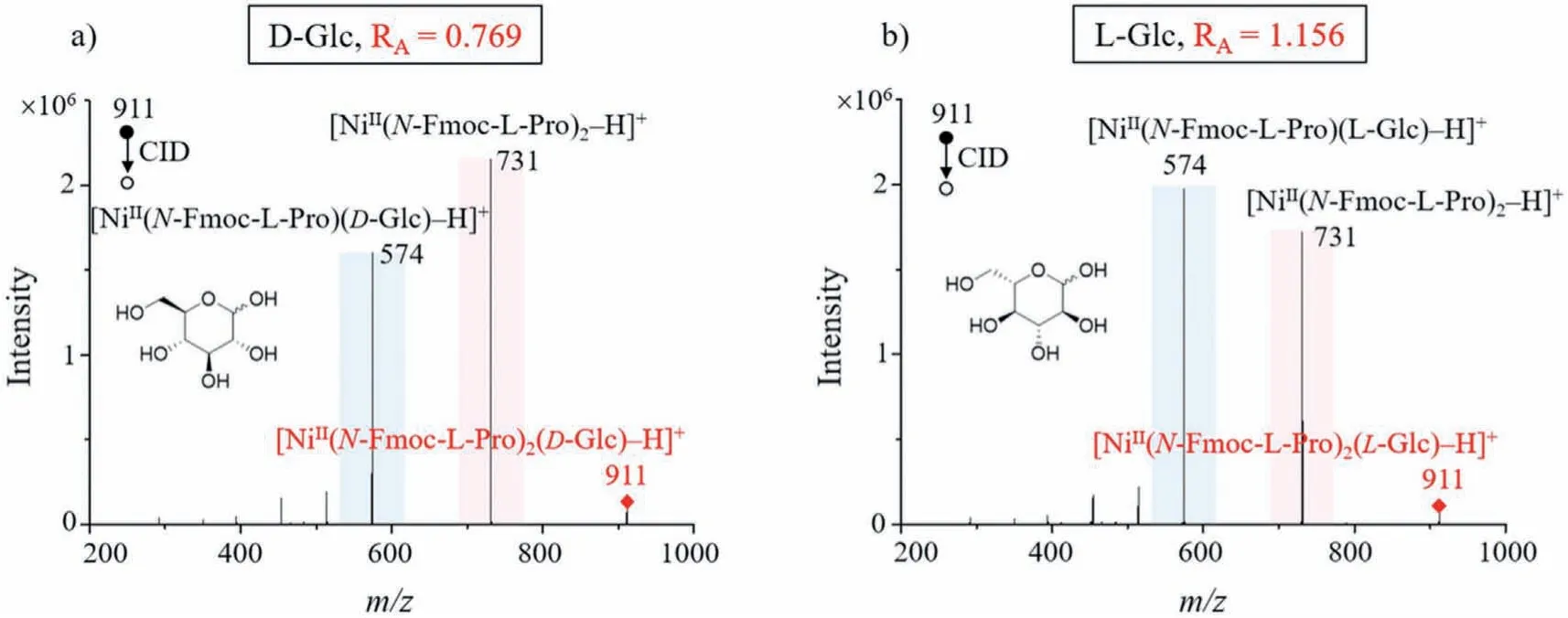
Fig.1 .MS/MS CID spectra of D-Glc (a) and L-Glc (b), and glucose with different configurations can be easily differentiated based on their RA-values.
To achieve better differentiation of monosaccharide species,we optimized the metal ions and amino acid ligands that form the cluster ions.In previous reports on the use of cluster ions to differentiate chiral compounds, researchers have confirmed that Cu2+and Ni2+ions performed better by investigating numerous transition metals [37].Therefore, we made an optimized selection between Cu2+and Ni2+ions in the present study.Meanwhile, we optimized chiral amino acids as ligands among L-Pro, L-Phe, L-Glu, L-His andN-Fmoc-L-Pro.After optimization,NiCl2andN-Fmoc-L-Pro were chosen for the monosaccharide experiments.In addition, the CE for CID fragmentation was also optimized and the experimental reproducibility was confirmed.The relevant detailed descriptions are given in the Supporting information.
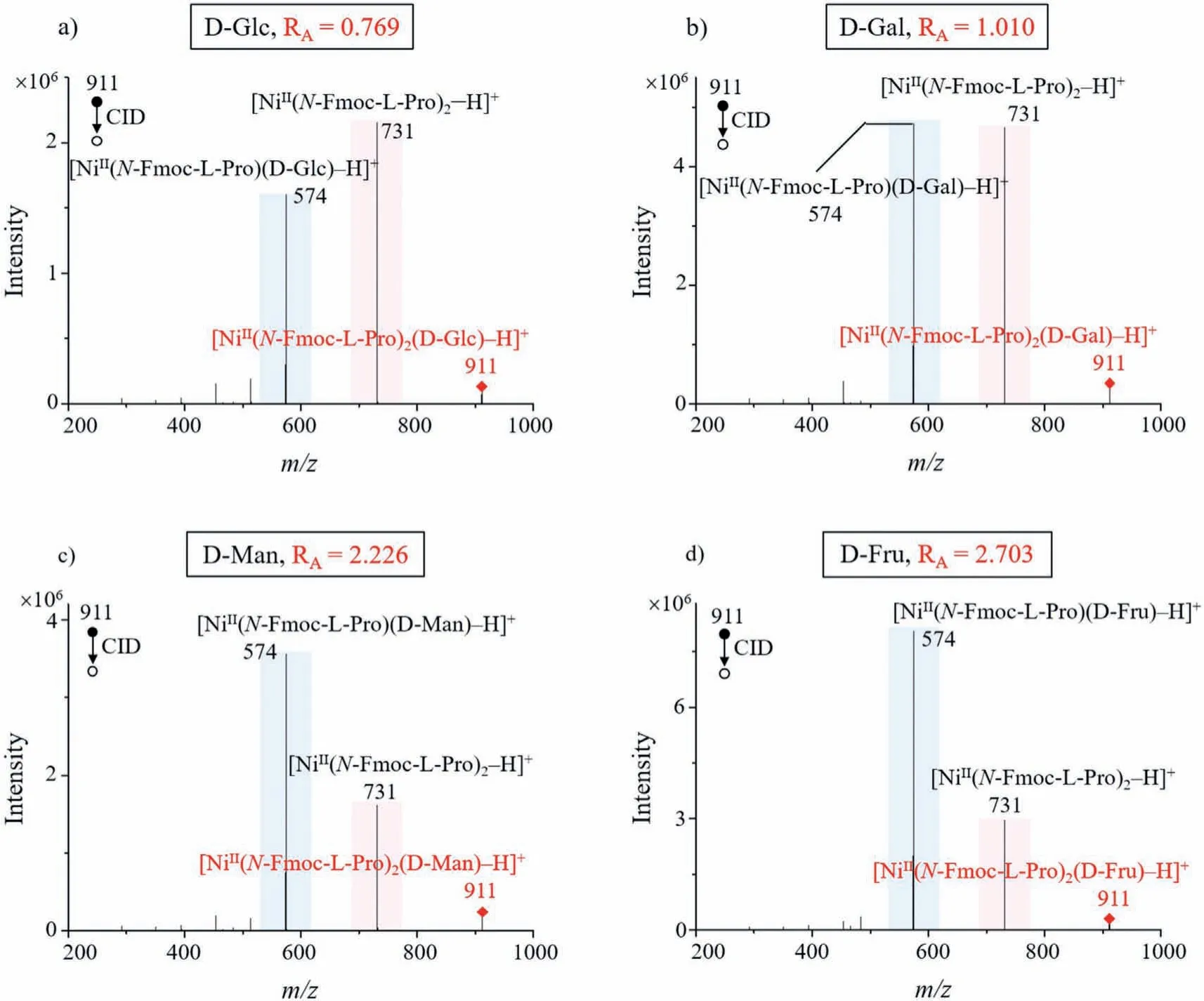
Fig.2 .MS/MS CID spectra of four common monosaccharide cluster ions: (a) D-Glc, (b) D-Gal, (c) D-Man and (d) D-Fru.Different monosaccharide species can be easily differentiated based on their RA-values.
With using the optimized metal ion Ni2+and ligandN-Fmoc-LPro for monosaccharide analysis, we investigated the ability ofRAvalues to differentiate monosaccharide species.For this purpose,we used four common monosaccharides (D-Glc, D-Gal, D-Man and D-Fru) for the analysis, fixing them all in the D-configuration, and their MS/MS CID spectra are shown in Figs.2a–d.
Similarly, theRA-value is equal to the ratio of the intensity ofm/z574 ([NiII(N-Fmoc-L-Pro)(monosaccharide)–H]+) to that ofm/z731 ([NiII(N-Fmoc-L-Pro)2–H]+), so theRA-values corresponding to D-Glc, D-Gal, D-Man, and D-Fru are around 0.769, 1.010, 2.226,and 2.703, respectively.TheRA-values of different monosaccharide species are sufficiently different, so we can use theRA-values to distinguish them.In addition, we also observed that a sugar cluster can be more easily ionized than the sugar itself (Fig.S6 in Supporting information).Therefore, using cluster ions as analytes is able to solve the problem of low sensitivity in sugar MS detection.
Disaccharides can form multiple cluster ions, unlike monosaccharides that form almost only one cluster ion [M(L)2(S)−H]+.For instance, Fig.3 shows the MS and the MS/MS CID spectra of DLac cluster ions as an example.We can observe both cluster ion A([CuII(L-Pro)2(D-Lac)–H]+) and cluster ion B ([CuII(L-Pro)(D-Lac)2–H]+) in the MS spectrum (Fig.3a).In MS/MS spectra of cluster ions A or B,RA-values orRB-values can be calculated based on the product ions obtained from the precursor cluster ionsviaCID fragmentation (Figs.3b and c).Just as monosaccharide clusters needed to be optimized, we used a similar approach to optimize the metal ions and chiral amino acid ligands comprised in the disaccharide cluster ions and found Cu2+and L-Pro to be the best choices for disaccharide analysis.
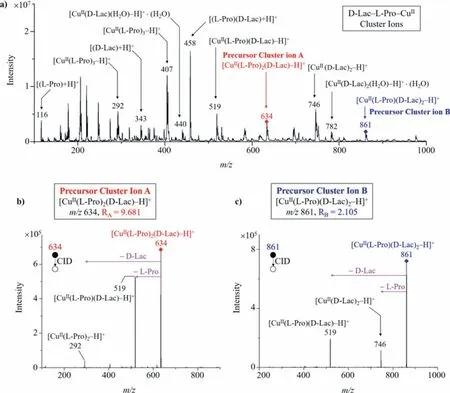
Fig.3 .MS and the MS/MS CID spectra of D-Lac cluster ions: (a) Both cluster ion A ([CuII(L-Pro)2(D-Lac)–H]+) and cluster ion B ([CuII(D-Pro)(D-Lac)2–H]+) can be observed in the MS spectrum; (b) CID fragmentation of cluster ion A and the calculation of RA-value; (c) CID fragmentation of cluster ion B and the calculation of RB-value.
According to our definition of theR-value,RA-value should be calculated as the ratio of the intensity of product ionm/z292 ([CuII(L-Pro)2–H]+) to that of product ionm/z519 ([CuII(LPro)(disaccharide)–H]+), produced from the precursor cluster ion Am/z634 ([CuII(L-Pro)2(disaccharide)–H]+); while theRB-value should be calculated as the ratio of the intensity of product ionm/z746 ([CuII(disaccharide)2–H]+) to that of product ionm/z519([CuII(L-Pro)(disaccharide)–H]+), produced from the precursor cluster ion Bm/z861 ([CuII(L-Pro)(disaccharide)2–H]+).For some disaccharides, theirRA-values were different enough to differentiate them, such as D-Lac (RA= 9.681), D-Suc (RA= 0.701), D-Lact(RA= 25.574), D-Cel (RA= 1.753), D-Mel (RA= 2.925) and D-Tre(RA= 0.045).The MS/MS CID spectra of these six disaccharides were shown in Figs.4a–f.
In the case of disaccharides that cannot be differentiated byRAvalues alone, such as D-Mal and D-Tur whoseRA-values are both∼2.5 (Figs.5a and b), we can then determine them easily by usingRA-values in combination withRB-values.As shown in Fig.5c and d, theRB-value of D-Mal is approximately zero (N/A), which is due to the fact that precursor cluster ion [CuII(L-Pro)(D-Mal)2–H]+undergoes CID fragmentation to produce almost no product ion [CuII(D-Mal)2–H]+, and therefore the intensity of the product ion [CuII(D-Mal)2–H]+is very low; while theRB-value of D-Tur is around 3.745.The difference between theRB-values of D-Mal and D-Tur is very large, so they can be easily differentiated.
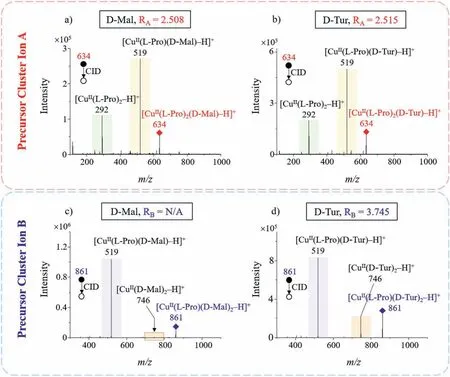
Fig.5 .Differentiation of disaccharides D-Mal and D-Tur using their RA-values in combination with RB-values: RA-values of (a) D-Mal and (b) D-Tur are both around 2.5, so they cannot be differentiated by their RA-values alone; (c) D-Mal and (d) D-Tur have different RB-values, so they cannot be differentiated by RA-values in combination with RB-values.
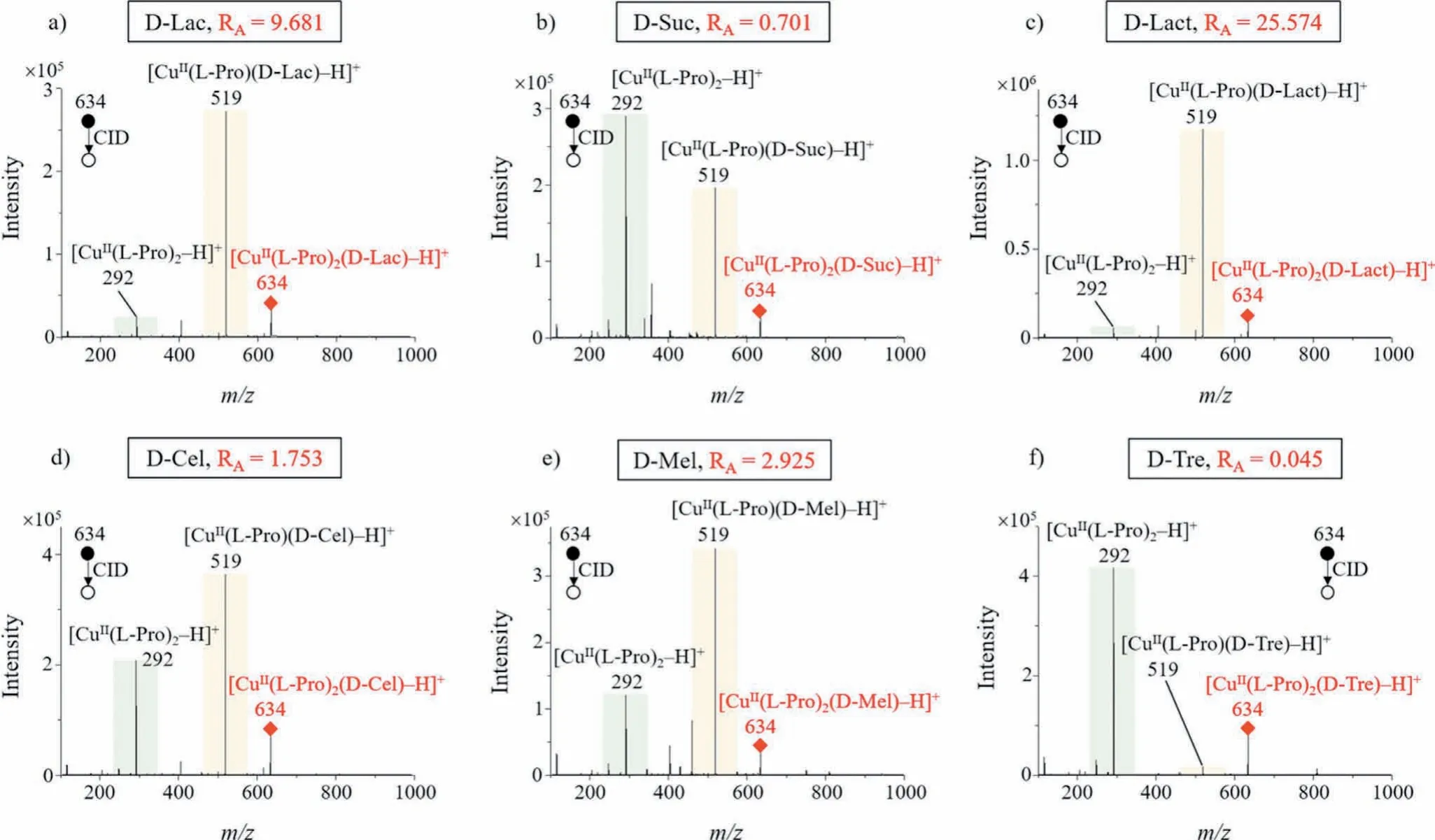
Fig.4 .MS/MS CID spectra of six common disaccharide cluster ions: (a) D-Lac, (b) D-Suc, (c) D-Lact, (d) D-Cel, (e) D-Mel and (f) D-Tre, and they can be differentiated by their RA-values alone.
A disaccharide is formed by two monosaccharides linked by a glycosidic-bond, so the differentiation of disaccharide isomers needs to be discussed based on their monosaccharide composition, linkage site, and glycosidic-bond conformation.The six different cases of disaccharide isomers are summarized in Table 1.We investigated these cases by comparative experiments, and all detailed results were given in Fig.S7 (Supporting information).The differentiation of disaccharides can be achieved very easily on the basis ofRA-values alone in the first five cases (case #1–5).In the sixth case, the three disaccharides D-Suc/D-Mel/D-Tre could be determined byRA-values alone, while the differentiation of D-Mal from D-Tur required the use ofRA-values in combination withRBvalues.Overall, we could achieve the differentiation of common disaccharides based onR-values.
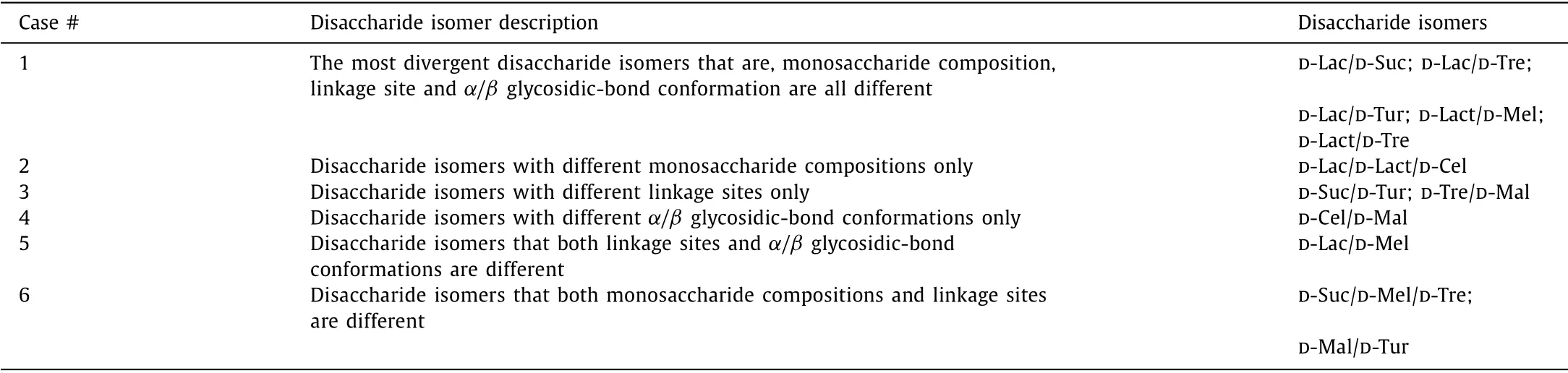
Table 1 Differentiation of disaccharide isomers in six different cases.
Cluster ions are formed by the coordination of saccharides and ligands with transition metal ions.The intensity ratios of the fragment ions produced by the cluster ionsviaCID should only be related to the molar ratio of the cluster components.Therefore,we used ESI-MS/MS to analyze prepared sample solutions in molar ratios of different components as a verification.First, we investigated the effect of preparing solutions in different ratios of saccharides and ligands onR-values, in excess of both.The concentration of NiCl2was fixed while excess D-Glc andN-Fmoc-L-Pro, or D-Gal andN-Fmoc-L-Pro were added at molar ratios of 1:1, 1:2, 2:1,1:3 and 3:1.We performed MS/MS analysis of the above solutions and investigated theRA-values of the cluster ions [NiII(N-Fmoc-L-Pro)2(D-Glc)–H]+and [NiII(N-Fmoc-L-Pro)2(D-Gal)–H]+for each condition.The experimental results showed that theRA-values remained constant regardless of the degree of saccharide and ligand excess (Fig.6a).This indicates that the cluster ions formed by the coordination of saccharide and ligand with Ni2+have a fixed composition ratio, which is determined by the coordination ability of Ni.Next, we investigated whether theR-value of the mixed sugar cluster ions changes as the solution is continuously diluted.We mixed D-Glc with D-Gal as an example of a saccharide mixture.Solutions were prepared in proportion to the molar ratio of forming the cluster ion [NiII(N-Fmoc-L-Pro)2(saccharide)–H]+(saccharide:ligand:metal = 2:2:1, molar ratio).We tested three saccharide mixtures with molar ratios of 1:1, 1:3 and 3:1 for D-Glc and DGal, respectively.The initial concentrations of all three saccharide mixtures were 0.2 mmol/L and were diluted 2-fold, 5-fold and 10-fold for ESI-MS/MS analysis, respectively.The experimental results showed that the mixed saccharides had almost stableRA-values and were almost unaffected by dilution (Fig.6b).In addition, we also investigated the complexation reactivity of metal ions, ligands,and saccharides in low concentrations.Differentiation of D-Glc and D-Gal was used as an example.When saccharides, the ligand and the metal ion are diluted to 0.2 μmol/L, 0.2 μmol/L and 0.1 μmol/L,respectively and then mixed, the corresponding cluster ions could still be formed rapidly and theirRA-values obtained by CID fragmentation remained constant (Fig.S8 in Supporting information).Although the different saccharide components in the sugar mixture compete for coordination with the metal, a fixed proportion of the mixture components has a stable coordination tendency and thus will have a relatively stable and constantR-value.Furthermore, since we useR-values to differentiate saccharides, it is necessary to investigate the convincing ranges ofR-values.Table S1(Supporting information) summarizes the 95% confidence intervals for all measurements.
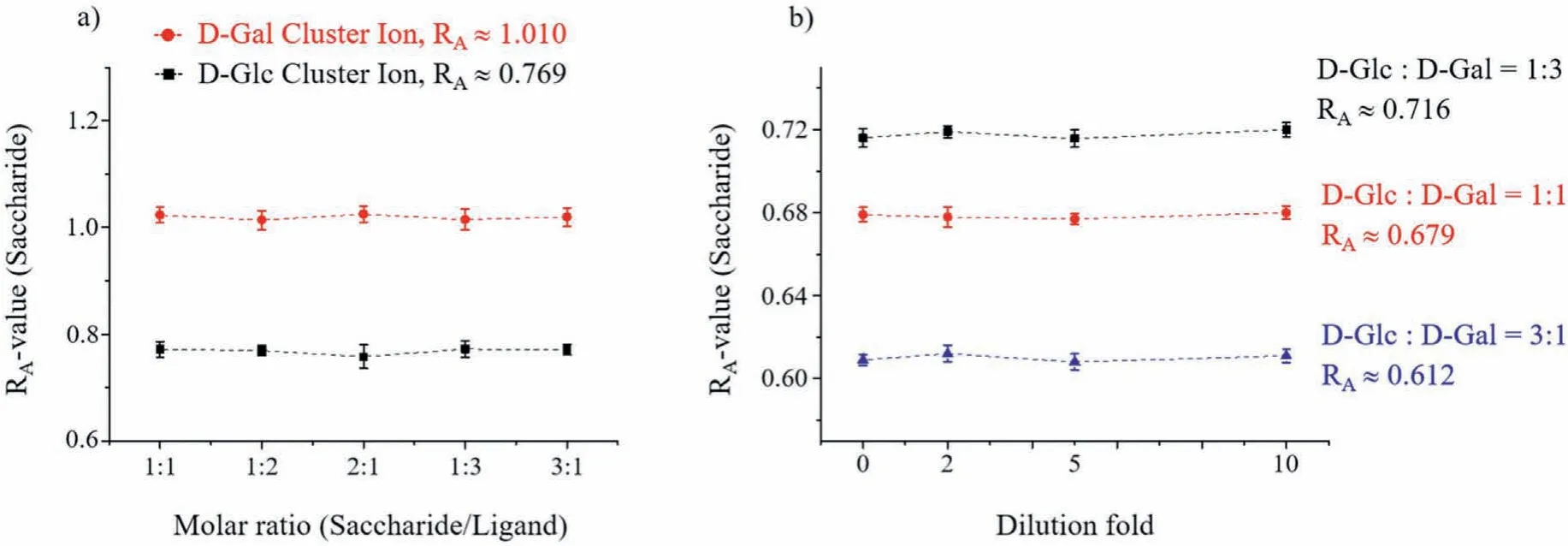
Fig.6 .The degree of excess of sugar and ligand (a) and the dilution of the solution (b) do not change the R-values.
The stability of theR-values of the cluster ions allows our method to be developed for potential practical applications, such as rapid clinical detections.For example, our method can be used to quantify galactose and glucose in the plasma to aid in the diagnosis of galactosemia.Galactosemia is an autosomal recessive inborn error of metabolism caused by a deficiency of the enzyme galactose-1-phosphate uridyltransferase, leading to galactose accumulation [40].Galactose accumulation induces in newborns many symptoms, such as liver disease, cataracts, and sepsis leading to death if untreated.Neonatal screening is developed and applied in many countries using several methods to detect galactose or its derived product accumulation in blood or urine [41,42].
Clinically, when the galactose concentration in the blood of a neonate is greater than 1.1 mmol/L, it indicates the possibility of galactosemia.Also, we have already known that the normal neonatal blood glucose range is 2.6–7.0 mmol/L.The standard curves of D-Gal/D-Glc molar ratio versusRA-values (both taken as natural logarithms) covered a wide range from health to disease with acceptable linearity (correction factorr2= 0.9972) (Fig.7).We spiked different proportions of glucose and galactose in the background matrix to simulate plasma samples from healthy and galactosemic neonates, respectively.For future clinical detection,we believe that dried whole blood spots can be directly used.However, in this study, dried plasma spots were used instead of dried whole blood spots for achieving accurate mixing of glucose and galactose to prepare the corresponding simulated samples.By calculating theRA-values, we can approximately determine whether the molar ratio of D-Gal/D-Glc is in the healthy or diseased range.For the ambiguous range between health and disease,more information is needed to make a judgment.This uncertainty is largely determined by the fact that blood glucose in healthy individuals is a range rather than a fixed value.Our method is very useful in rapidly determining typical health or disease samples.
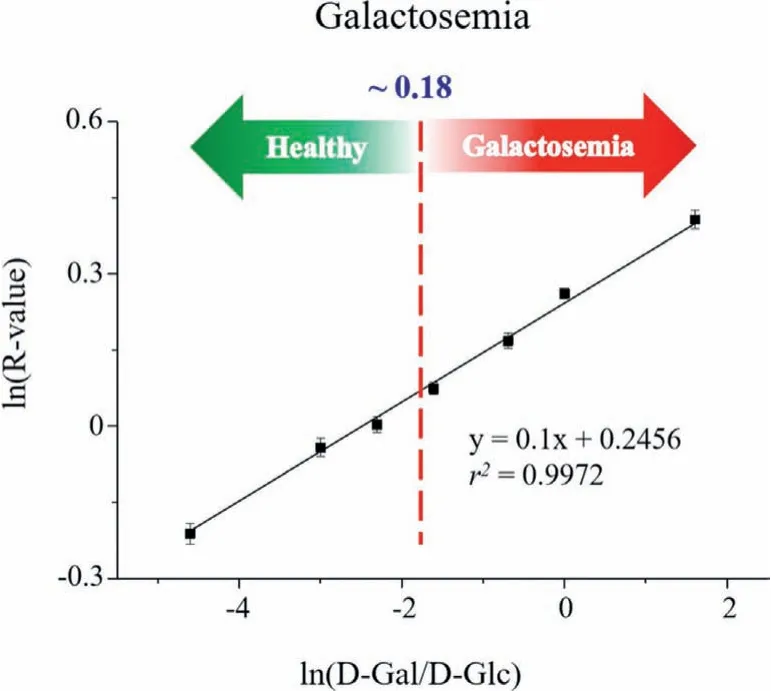
Fig.7 .Rapid quantification of galactose to glucose molar ratio in dried plasma to aid in the rapid diagnosis of galactosemia.
In summary, a derivative and ion mobility-free method was developed for the rapid qualitative and quantitative analysis of simple saccharides using paper spray tandem mass spectrometry.We defined theR-value in terms of the ratio of the intensity of the product ions obtained by CID from the precursor ion of the saccharide cluster.The different species and conformations of saccharides can be easily differentiated based on theirR-values.This method would be a good solution to the problem of difficult identification of isomers and low sensitivity of saccharides MS analysis.Experimental conditions have been optimized, and the method reproducibility has been investigated.An example of the application of this method for the rapid quantification of galactose to glucose molar ratio in dried plasma was also demonstrated to aid in the rapid diagnosis of galactosemia.This method could be applied to a wide range of saccharide analysis applications in the future.
Declaration of competing interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by NNSF China (No.82072247) and Research Projects of Beijing University of Chinese Medicine (Nos.2021-JYB-XJSJJ-001, XJYS21005 and 2021-SYJS-007).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.026.
杂志排行
Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
