Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
2022-12-07ZihenShangguanXingzhongYuanLongboJiangYanlanZhaoLeiQinXuerongZhouYanWuJiaWeiChewHouWang
Zihen Shangguan, Xingzhong Yuan,∗, Longbo Jiang, Yanlan Zhao, Lei Qin,Xuerong Zhou, Yan Wu, Jia Wei Chew, Hou Wang,∗
a College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
b Key Laboratory of Environment Biology and Pollution Control, Hunan University, Ministry of Education, Changsha 410082, China
c College of Resources and Environment, Hunan Agricultural University, Changsha 410128, China
d School of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore 637459, Singapore
Keywords:Zeolite-based catalysis Methodology External field Degradation Useful chemical regeneration
ABSTRACT Wastewater treatment and reclamation from wastewater are essential for the sustainable use of water resource.Zeolite-based heterogeneous catalysis shows great potential in circumventing the current limitations on pollutant removal and transformation to useful chemicals, inspiring advancements towards practical water treatment.This paper summarizes the methods for synthesizing zeolite-based catalyst,and the corresponding advantages and disadvantages.In comparison with traditional Fenton-like reaction, the superiority of zeolite-based catalysis lies in less sludge, wide pH range and easy recyclability.Accordingly, applications of zeolite-based Fenton-like catalysis (ZFCs) in pollutant removal and reclamation of wastewater were reviewed.Emphasis was placed on the methodological strategies in improving ZFCs, including the combination of external driving force (e.g., photocatalysis or electrochemistry), as well as the introduction of various transition metals into zeolite-based catalyst.Possible challenges and future perspectives for ZFCs were proposed.
1.Introduction
The problems of water pollution caused by the discharge of various organic compounds (e.g., organic dyes, pharmaceuticals, pesticides, steroid estrogens, and personal care products) have become more serious and attracted public attention [1–5].Different technologies have been applied to water treatment, which can be divided into biological, physical, and chemical methods.The biological treatment (such as activated sludge and membrane bioreactor) used the metabolic function of microorganisms to degrade and mineralize the organic pollutants [6,7].However, it has limitations such as being prone to sludge expansion and long pretreatment cycles, and difficulty to treat wastewater with poor biodegradability [8–10].The single physical treatment technology (such as membrane filtration, flotation, and sedimentation) only separates the pollutants from the wastewater, which cannot remove them completely and needs to be further processed [10,11].By contrast, chemical technology (such as Advanced oxidation processes(AOPs), neutralization, and coagulation) can be used as an efficient technology to treat wastewater containing non-degradable organic compounds [9,10].Advanced oxidation processes (AOPs) have been widely used to treat non-degradable and biologically toxic pollutants because it has high catalytic efficiency and is environmentally friendly [12–20].As a kind of AOP, Fenton catalysis has unique advantages including strong oxidizing ability, low initial cost, flexible operation and non-selective destruction to organic pollutants [21].The classical homogeneous Fenton process involves the reaction of Fe(II) with hydrogen peroxide (H2O2) under acidic solution condition to generate highly reactive hydroxyl radicals (•OH), oxidizing Fe(II) to Fe(III).Then,•OH can react with organic compounds in a rapid free radical chain reaction, non-selectively mineralizing or decomposing organic compounds, and generating harmless products such as CO2, H2O or mineral salts [9,22,23].
However, the classical homogeneous Fenton-like reaction has limitations such as narrow reaction pH range (normally 2–3), difficulty in separating and recycling, secondary pollution, and poor stability of catalyst [24,25].Heterogeneous Fenton-like catalysis can not only minimize the production of iron-containing sludge and reduce recycling costs, but it can also be carried out at a mild pH[26–28].The widespread use of heterogeneous Fenton-like catalysis for water treatment has been focused on multiple times and the latest studies on this topic have been outlined in Table S1(Supporting information) [29–38].Although heterogeneous catalysts showed excellent catalytic activity (e.g., metal-organic frameworks (MOFs), nano zero-valent iron), their relatively high cost could become a barrier to their large-scale application in actual wastewater treatment.The relatively inexpensive carrier materials might be a better choice.The existing heterogeneous carrier materials include various solid materials such as clays, silicas, carbon materials and zeolites [39–41].Zeolites have been widely studied as heterogeneous carrier materials due to the significant adsorption capacity [42,43].It can concentrate the organic molecules near the catalytic active center by enhanced adsorption, thereby increasing the degradation efficiency [44,45].Besides, they are relatively inexpensive, easily available, and can reduce sludge formation [46–49].
Conventional zeolite is a crystalline aluminosilicate material built with SiO4and AlO4tetrahedrons [50,51].The basic unit of the zeolite structure is the neutral SiO4tetrahedrons.When Si4+is replaced by Al3+, the framework is introduced a net negative charge and needs to be compensated by extra exchangeable cations, such as H+and metal cations, which contributes to form strong Brønsted acid sites [52,53].In addition, these cations are loose and the cation exchange capacities can be up to several milliequivalents per kilogram [54], because the porous structure formed by rings of SiO4and AlO4tetrahedrons connected through the oxygen ions at the common vertex can provide large internal surface areas.The Brønsted acid sites and cation exchange properties make the zeolite ideal as a Fenton catalyst or catalytic support.Furthermore, even if•OH radicals are involved, zeolites are not oxidized and remain stable, which makes them a feasible alternative for long-lasting, recyclable catalysts in oxidative conditions[50,55].According to the International Zeolite Association Structure Commission (IZA-SC), zeolite can be divided according to the type of framework and given unique three-letter framework type codes.255 framework type codes have been assigned to date by IZA-SC.Among them, LTA, FAU, MFI, MOR framework types of zeolites are currently most studied in AOPs.And it is well-known that zeolite Y belongs to the FAU type and ZSM-5 belongs to the MFI type.They are both widely used zeolite-based catalysts in Fenton-like chemical industries.Zeolite-based catalysis exhibit excellent prospects in AOPs to treat various refractory organic contaminants.
Due to the inherent advantages, significant efforts have been made to advance zeolite-based catalysis [47,51,52,56].Martinezet al.introduced the new trends of zeolite-based catalysts with respect to tailoring active sites through artificial design, controlled preparation and operation visualization [52].Yanget al.focused on the zeolite-based composite photocatalysts for removing organic contaminants in wastewater and air [51].Specifically for wastewater treatment, we propose a type of cascade system, named zeolite-based Fenton-like catalysis (ZFCs), which involves zeolitebased catalyst, Fenton-like reaction (e.g., radical evolution) and pollutant transformation.It brings together multiple disciplines spanning materials, chemistry, water and environmental protection to expand the current knowledge on zeolite catalysts, advance our understanding of existing Fenton-like reaction systems, and connect fundamental catalysis insights with practical wastewater treatment.This is expected to serve as a timely comprehensive review on zeolite-based Fenton-like catalysis applied to wastewater treatment.
The objective of this review is thus to critically summarize the recent advances in ZFCs for application in pollutant removal and water reclamation.At first, several types of methods for synthesizing zeolite-based catalysts for Fenton-like process are summarized,including impregnation, ion-exchange and hydrothermal method.Subsequently, the performance of the traditional ZFCs for wastewater treatment and reclamation are discussed, highlighting the crucial operating parameters.Furthermore, methodological strategies to enhance the catalytic efficiency of ZFCs are introduced, such as integration of photocatalysis or electrochemistry with ZFCs, as well as introducing various transition metals into the zeolite-based catalyst.Lastly, existing challenges and future efforts for zeolite-based heterogeneous catalysis are presented.
2.Synthesis of catalyst in zeolite-based Fenton-like catalysis
The reactive performance of ZFCs is closely related to the preparation method of the catalyst.Transition metals/or their compounds are embedded into zeolites to make full use of catalytic functionality and lower the usage amount.The transition metals/or their compounds immobilized into the zeolites act as the main active species to activate H2O2in ZFCs.The different synthetic methods of zeolite-based catalysts could have an impact on the dispersion of metal species, thereby affecting Fenton-like catalytic properties, stability, and the sustainable utilization of zeolite catalysts.Thus, three kinds of common methods for preparing zeolitebased Fenton-like catalysts from the operation, cost, dispersion of metal species, and stability in this section are analyzed and summarized.The specific synthesis conditions of catalysts in zeolitebased Fenton-like catalysis are shown in Table S2 (Supporting information).Three kinds of common methods used for the synthesis of zeolite-based Fenton-like catalysts are outlined in this section(Fig.1 and Table S3 in Supporting information) [57–60].
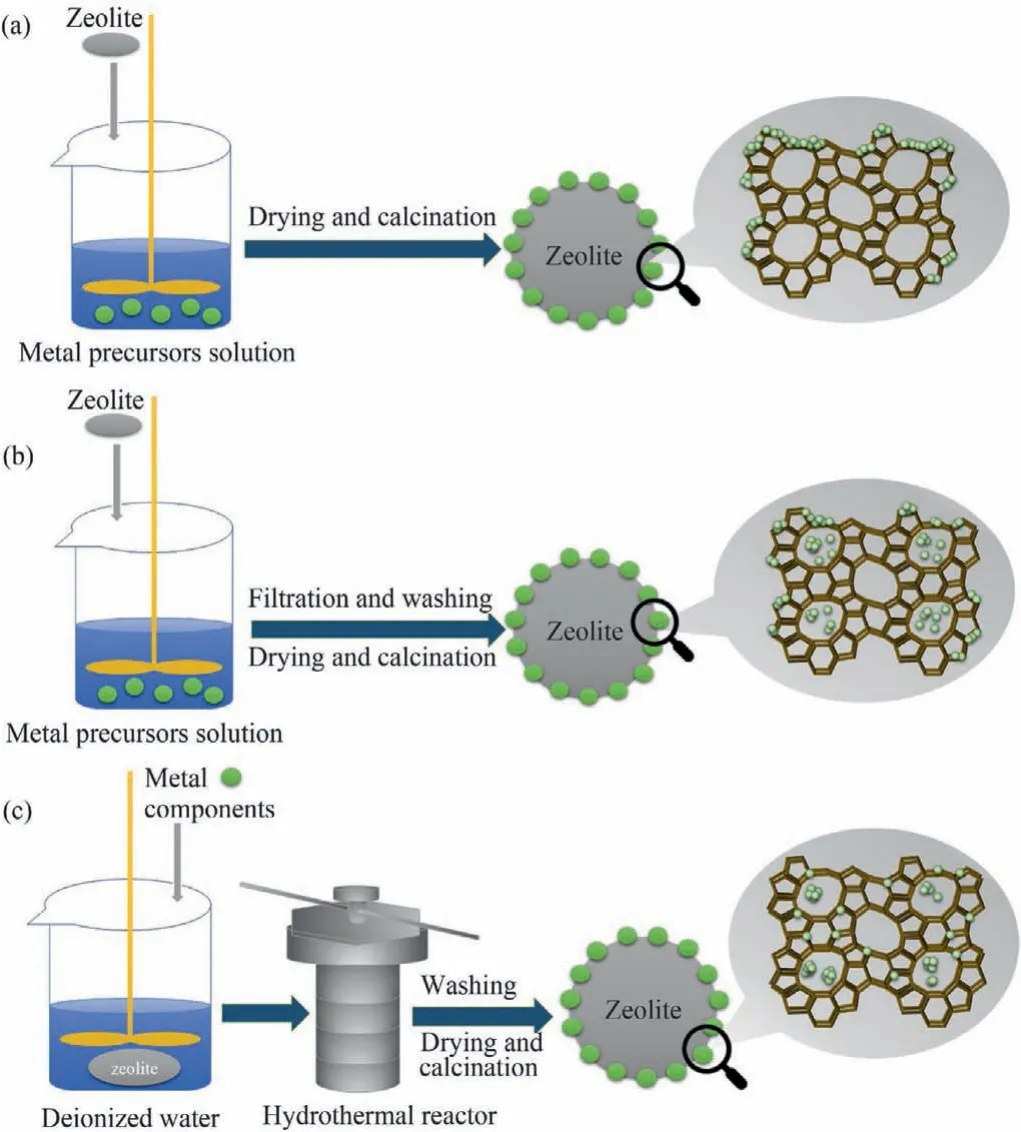
Fig.1 .Synthesis of zeolite-based Fenton-like catalysts by (a) impregnation, (b) ionexchange and (c) hydrothermal methods.
2.1.Impregnation methods
Impregnation can effectively realize that metal species are supported on the zeolite.Compared with single metal oxides or natural metal-containing materials, supported catalysts not only have a considerable degradation efficiency but also have an improvement in leaching resistance under acidic conditions [61,62].Thus,impregnation is a common methodology in the preparation of Fenton-like catalysts.And it is also a common methodology for preparing zeolite-based Fenton-like catalysts because of the porous properties of zeolite.The types of impregnations include wet impregnation and incipient wetness impregnation.Wet impregnation refers to the use of an excessive volume of precursor solution relative to the pore volume of zeolite.On the other hand, incipient wetness impregnation can also be called capillary impregnation,which means that the volume of precursor solution is equal to or slightly larger than the pore volume of zeolite [58,63].The principle of impregnation method is that the active components can penetrate into the inner surface of the zeolite [58,60], as in the uptake of the liquid into the pores of the zeolite through capillary pressure [58,64].The active components can then be deposited onto the internal and external surfaces of the zeolite after the solvent evaporates, so the impregnation method generally requires no additional filtration and washing, and thus eases the loading of metal species [60].Zahraniet al.used the wet impregnation method to synthesize Fe-ZSM-5 nano catalyst by ZSM-5 and iron ions [57].ZSM-5 was added into the FeSO4solution and then the Fe was uniformly deposited on ZSM-5 by evaporating the water.As the increasing of the impregnated Fe amount, the accumulated Fe ions shield the active sites, which ultimately decreased its catalytic activity in electro-Fenton reaction.Yanet al.synthesized Fe-ZSM-5 with good crystal structures by the incipient wetness impregnation[65].However, the total pore volume and BET-measured surface area of the Fe-ZSM-5 catalyst were reduced compared with ZSM-5.The presence of iron oxide could cause pores blockage, thereby effectively reducing the specific surface area of Fe-ZSM-5.It was also found that the iron leaching concentration slightly increased after Fe-ZSM-5 was used 3 times in 27 h.
It could be thought that in the preparation of zeolite-based Fenton-like catalysts, although the impregnation method is easy to operate and low cost in the preparation of zeolite-based Fentonlike catalysts, it cannot achieve a good dispersion of the active sites and prevent metal ions leaching.Zeolite-based catalysts prepared by the impregnation method are generally loaded with metal species on the outer surface of zeolite crystals [66].Thus, the impregnation method inevitably causes the agglomeration of particles and metal ions leaching (Table S3).Further research should be concentrated on how to increase the dispersion of the active sites, prevent metal ions from leaching and tightly anchor the active species on the zeolites.
2.2.Ion-exchange methods
The ion-exchange method has been widely used to synthesize transition metal ions doped zeolite-based Fenton catalysts because of the strong cation exchange capacities of zeolite.The principle of ion-exchange is that the metal ions replace the exchangeable cations in the channels and cages [66].Ion exchange includes solid-state ion exchange and wet ion exchange.Solid-state ion exchange involves mechanically mixing and milling the solid metal salt with the zeolite powder, which is then dried, washed, and calcined.As for the wet ion-exchange method, it is similar to the impregnation method, but requires additional filtration and washing.
Compared with the solid ion exchange method, the zeolitebased Fenton-like catalysts synthesized by the wet ion-exchange have relatively higher dispersion of the metals.Romero-Sáeet al.synthesized Fe-ZSM-5 catalysts by different routes, namely, wet and solid-state ion exchange as well as impregnation [67].After Fe doping, the crystal properties of zeolites were maintained without any structural changes.Among the methods, the wet ion exchange gave catalysts (average size of 10 nm) with higher number as well as dispersion of Fe2O3nanoparticles (Fig.2a).The sample synthesized by impregnation had similar sizes of about 10 nm but a poorer dispersion of Fe2O3(Fig.2b), while that synthesized by solid-state ion exchange formed aggregates (Fig.2c).Thus, compared with other methods, the catalysts synthesized by wet ion exchange had better catalytic behavior in Fenton-like reaction.Wuet al.used wet ion exchange to synthesize Cu-ZSM-5 membrane catalysts, which had more original Cu species and lattice oxygen [68].It was found that the Cu leaching rate reached about 30% for the optimal Cu-ZSM-5 with a Cu loading of only 1.91 wt%.
It is acknowledged that zeolite-based catalysts could obtain more dispersed active metal components by wet ion exchange than wet impregnation.Thus, the wet ion exchange method could improve the contact efficiency and reduce the metal ions leaching.According to the reported results of Wuet al., using wet ion exchange to prepare Cu-ZSM-5 zeolite membrane catalysts coating on paper-like sintered stainless fibers (PSSF) had more Cu species and lattice oxygen than thatviaincipient wetness impregnation [69].The PSSF (Fig.2d) with a three-dimensional network structure and accessible porosity could improve the growth of ZSM-5 membrane and mass transfer [70].The continuous and dense ZSM-5 membrane with a thickness of about 5 μm covered the steel fibers well(Fig.2d).After ion-exchange and impregnation, the ZSM-5 membrane structure was preserved well with no major damage.However, the dispersibility of CuO particles synthesized by these two methods on the surface of catalysts was different.The Cu species could be dispersed well into the framework of ZSM-5viawet ionexchange, but a lot of CuO particles accumulated on the ZSM-5 membrane after impregnation, which might block the channels of the zeolite.Besides, the maximum Cu ion leaching concentration of the catalysts prepared by wet ion exchange was significantly lower (40 ppm) than the catalysts prepared by the impregnation method (180 ppm).As for wet ion exchange, Cu ions fixed in the zeolite channels was relatively stable.In contrast to impregnation,the CuO on the outer surface of zeolite crystals was easily washed by reacted solution under acidic conditions in Fenton-like reaction.

Fig.2 .(a) TEM image of the Fe-ZSM-5 zeolites prepared by wet ion exchange.(b) TEM image of the Fe-ZSM-5 zeolites prepared by impregnation.(c) TEM image of the Fe-ZSM-5 zeolites prepared by solid state ion exchange.(a–c) Reprinted with permission [67], Copyright 2016, Elsevier.(d) SEM images of supports and Cu-ZSM-5/PSSF catalysts.Reprinted with permission [69], Copyright 2020, Elsevier.
Compared with the impregnation method, the ion-exchange method can not only load the metal species on the outer surface of zeolite crystals, but also inside the channels and cages [66].So, the ion exchange method can show higher dispersibility compared to the impregnation method [69].Thus, in the synthesis of catalysts in ZFCs, using ion-exchange method could provide relatively high surface area to increase the contact efficiency.But its limitations are that it requires high solvent volume, has high metal content in the residual solvent, and the step needs to be repeated many times in the solution containing the active components until suffi-cient load is obtained [60].Furthermore, the active species loaded on zeolite-based catalysts were possible to re-exchange by other cations in practical industrial wastewater [61].
Advantages of the impregnation and wet ion exchange methods include easy operating, good combination of the metal components with the zeolite, and preservation of the original framework structure of the zeolite.However, problems of loss of active centers are encountered in the preparation process, as shown in Table S3.For instance, due to the movement of catalytically active components to the outer surface, the concentration in the inner surfaces is reduced such that even the carrier is not covered.The impregnation or ion exchange methods may reduce the external and internal specific surface area of zeolite, decrease the micropore volume,leach out metal ions and cause loss of active centers.Therefore, the longevity and regeneration of the catalyst synthesizedviathe impregnation method as well as the ion-exchange method should be further studied to improve the Fenton-like catalytic performance.
2.3.Hydrothermal methods
In order to solve the leaching and agglomeration of metal species in the Fenton-like process, hydrothermal methods could achieve metal encapsulation or introduction in the framework of zeolite (higher resistance to metal ions leaching and fulfill better sustainable utilization of zeolite-based Fenton-like catalysts).Hydrothermal methods have been applied to synthesize zeolite-based catalysts, which involves mixing the metal precursor and zeolite or active seeding gel of zeolite, then reacting in a hydrothermal reactor at a certain temperature for a certain time (more than 24 h),after which the obtained samples were filtered, washed, dried and calcinated [71,72].The zeolite-based catalysts synthesized by the impregnation method or the ion-exchange method exhibit poor stability because of the weak interaction between the catalysts and support materials in an acid medium.By contrast, hydrothermal synthesis is able to prevent the loss of active centers, caused by the metal component doped in the zeolite during the crystallization of the zeolite, thereby making the synthesized zeolite-based catalysts more stable [61,72].Encapsulating metal oxide nanoparticles in zeoliteviahydrothermal methods has been studied.Luoet al.used a hydrothermal method to disperse and encapsulate iron oxide nanoparticles in ZSM-5 [73].Diaoet al.designed and synthesized a catalyst with Fe2O3nanoparticles encapsulated in the pores of meso–ZSM-5 zeoliteviaa hydrothermal method [74].The encapsulated structure was important because it can restrict the leaching and sintering of iron oxide nanoparticles.The Fe-leaching amount of Fe@MZ5 to be only 3% of that for the Fe-oxide impregnated ZSM-5 (Fe/Z5).
Framework metal species in zeolite-based catalysts synthesized by hydrothermal methods have also been studied due to benefits of good dispersion and stability.Copper-doped hierarchical micro/mesoporous zeolite ZSM-5viaa simple one-pot hydrothermal synthesis method (Fig.S1 in Supporting information) was prepared by Zhouet al.[71].Negatively charged zeolite sub-nano crystals could be bound with the positively charged cetyltrimethylammonium chloride micelles.Through the co-assembly of these micelles and inorganic substances, unique Cu-ZSM-5 with hierarchical mesoporous structure could be synthesized after simply calcining in the air.Due to the partial decomposition of local structure-directing agents during the calcining process, plenty of Cu(I) species could be producedviathein-situreduction of Cu(II)species.The simple one-pot hydrothermal method is very conducive to the high dispersion of Cu species, which are the active components in zeolite-based catalysts, into the matrix.Denget al.synthesized framework Fe-doped zeolites with Mobil-Five structure such as FeTS-1, FeS-1, and FeZSM-5viaone-pot hydrothermal synthesis method [72], resulting in well-crystallized MFI structure and successful introduction of Fe species.Among these zeolite catalysts, FeZSM-5 showed a larger surface area and negative zeta potential, so it had higher Fenton-like catalytic activity.Zhu and coworkers used different organic iron salts as precursors and ethylene glycol (EG) as a reducing promoter to synthesize hierarchical Fe-ZSM-5 nanorod assembly microspheresviaa hydrothermal method [61].The synthesized catalysts have abundant highly dispersed and valency-controlled framework iron species.Compared with the direct use of Fe2+precursor (ferrous gluconate), more low-valence Fe species could be preserved when using Fe3+precursor (ferric citrate) through the reducing/chelating properties of EG.The zeolite catalyst with Fe3+salt as a precursor and with the aid of EG has the best catalytic performance, thus indicating that adding reductive organic compounds during the hydrothermal process is beneficial for making the Fe species stabilized by chelation or reduced in Fe(II) form in Fenton-like process.
The active metal components on the catalyst prepared by the hydrothermal method can be encapsulated in the channels and cages of zeolite or even incorporated into the zeolite framework to form framework metal ions [66].This makes the metal component and the zeolite bond firmer, and thereby the catalyst synthesizedviathe hydrothermal synthesis method has a better stability.The hydrothermal method can be considered as a more stable and efficient method to prepare zeolite-based Fenton catalyst.Moreover, the addition of reductive organic compounds is able to boost the reduction of Fe(III) to Fe(II) in the Fenton-like process.However, the disadvantages of hydrothermal synthesis, namely, timeconsuming due to the slow crystallization, need to be overcome(Table S3).
2.4.Other methods
For preparing zeolite-based heterogeneous Fenton-like catalysts, there are other methods, such as solvothermal microwaveassisted and sol-gel.The solvothermal microwave-assisted synthesis method is such that a specific microwave generator is used during the hydrothermal synthesis process to emit high-frequency microwave radiation into the reaction, which enhances the collisions among the reactant molecules and thereby accelerates the crystallization of the zeolite-based catalyst.In the study of Leet al.,the commercial carbon felt substrate with Fe-rich Mobil-Five zeolite nanoparticles was synthesizedviasolvothermal microwaveassisted synthesis [75].The zeolite nano-seeds distributed uniformly on the surface of the commercial carbon felt, resulting in an increase of more than 600 times of the specific surface area.And this optimized electrode could induce more dissolved oxygen to generate higher quantity of H2O2in electro-Fenton process.As for the sol-gel method, for example, Farhadiet al.used titanium prop oxide to form the clear sol by hydrolysis and polycondensation reactions, and added zeolite into the clear sol [76].Then, the liquid sol was transformed to the viscous gel phase to form a zeolite-titanate catalystviadrying and calcination.The titanium dioxide nanoparticles were successfully loaded onto the zeolite material, and the photo-Fenton catalytic activity was improved because the unique microporous structure of the zeolite in the liquid sol can control the hydrolysis rate, thereby improving the dispersibility of titanium dioxide nanoparticles [51].There are few studies that used the sol-gel method or solvothermal microwaveassisted method to synthesize zeolite-based Fenton-like catalysts.These promising methods, which can make the active components more dispersed and stable on the catalysts, should be developed and applied to the synthesis of catalysts in ZFCs.
3.Traditional zeolite-based Fenton-like catalysis
The traditional ZFCs applied in the degradation of organic contaminants can be divided into two steps, namely, adsorption and Fenton-like oxidation (Fig.3).As shown in Fig.3, the organic compound is adsorbed and diffused into the framework of zeolite,then achieved degradationviaoxidation.And the key properties of zeolite-based catalyst about how to increase adsorption of organic contaminants and facilitate mass transfer and promote Fenton-like oxidation will be emphasized in Section 3.1.And the metal-based zeolite catalysis can generate high-activity reactive oxygen species(ROS) in Fenton-like reaction.Active metal components doped on zeolite are generally Fe and/or Cu.The specific mechanisms of Fe and Cu-based zeolite heterogeneous catalysisviaFenton-like process will be discussed in Sections 3.2 and 3.3, respectively.
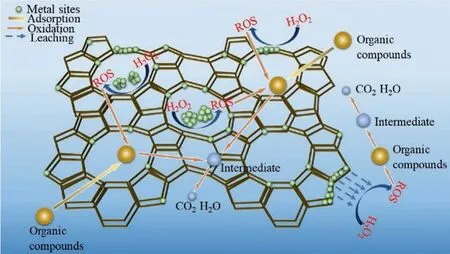
Fig.3 .Schematic diagram depicting the adsorption and Fenton-like oxidation by zeolite-based catalysts.
Pollutant removal and reclamation in wastewater are essential for environmental remediation and resource sustainability [77,78].In this section, the typical applications of traditional ZFCs to degrade toxic organic pollutants or selectively convert them to useful intermediates or other high-value chemicals are also summarized.The relevant reaction conditions, as well as removal effectiveness of organic pollutants and the effectiveness of the selective conversion into useful chemicals, are summarized in Table S4 (Supporting information) [79–84].
3.1.The role of zeolite in Fenton/Fenton-like reaction
For the first step of adsorption, the organic contaminants are adsorbed on the outer or internal surface of the zeolite-based catalysts.Different types of zeolites have different structural properties (e.g., pore opening and pore space) and surface hydrophobicity.They have effect on the adsorption of organic contaminants [50].First, the pore opening size of zeolites determines the mass transfer, including diffusion and accessibility of organic contaminants during adsorption process [50,51].When the diameter of the organic contaminant is bigger than the pore opening size of the zeolite-based catalyst, the organic molecule can be adsorbed only on the outer surface of the catalyst.When the diameter of the organic contaminant is smaller than the pore-opening size of the zeolite-based catalyst, the organic molecule can be adsorbed on both the internal and outer surfaces of the catalyst[51].In this case, the steric hindrance can be ignored, and the organic contaminants are easy to diffuse inside the zeolite framework (Fig.3).For instance, it has been reported that low molecular weight (relatively small molecular size) organic substrates (e.g.,ethanol, 1,1-dimethylhydrazine) in conventional heterogeneous Fe-ZSM5/H2O2catalysis were oxidized and mineralized faster compared with homogeneous catalysis [85].This may be attributed to that the molecular size of these organic compounds is smaller than the pore opening size of ZSM-5 (5.1×5.5 ˚A, 5.3×5.6 ˚A), which made the adsorption of organic compounds onto the internal surface of zeolite.This enhanced the interaction between the organic substrates and the•OH generated from the Fe-containing catalytic active sites on the zeolite surface.As for organic compounds of high molecular weight (relatively high molecular size,such as lignin), the mineralization degree was lower in such conventional heterogeneous Fe-ZSM-5/H2O2Fenton-like system.Because the pore-opening sizes of zeolites limited the adsorption and diffusion of the high molecular weight organic compounds, thereby causing excessive spacing between the active sites and adsorbed organic compounds [50,86].To improve the oxidation or mineralization of large molecules, the specific surface area accessible by large molecules could be increased and the distance between the adsorbed large molecules and the metal sites could be reduced.Sashkinaet al.prepared hierarchical zeolite Fe-ZSM-5, which had additional meso–/macro- porosity that notably reduced the nontargeted oxygen evolution from H2O2and increased the oxidation of large organic molecules by•OH [87].According to Zhouet al., hierarchically porous Cu(I)-ZSM-5 was used in cyclohexane oxidation [71], which could provide the fast diffusion pathway for reactant/product molecules and made framework copper sites(Cu+/Cu2+) highly dispersed.It gave conversion rates of as high as 28% and selectivity of cyclohexanone and cyclohexanol of above 93%.Besides, the pore-opening size of the zeolite was similar to the diameter of the pollutant molecule, which contributes positively to the adsorption [50].According to close-fit theory, tightly fitting pores may cause strong interactions between organic contaminants and zeolite-based catalysts [88].For example, Yanget al.deposited Fe2O3particles on zeolite Y, mordenite and ZSM-5, and used these zeolite-based Fenton catalysts to remove phenol.The pure zeolite Y, mordenite, and ZSM-5 respectively could achieve about 40%, 10% and 80% of phenol removal because of the different adsorption ability of the zeolites.ZSM-5 performed the best,because of the similar sizes of phenol and the pore-opening size of ZSM-5 [89].Second, the surface hydrophobicity of zeolite could prevent water clusters from blocking the pores, which could supply more pores and higher surface areas for organic compounds diffusion and adsorption [88,90].The type and the pore space of zeolite could also have an impact on hydrophobicity and mass transportation.For example, Yanget al.found that ZSM-5 had better adsorption of phenol due to a relatively more hydrophobic surface compared to zeolite Y and mordenite [89].That was because the smaller restricted pore space of ZSM-5 inhibited the interactions of water molecules.
For the second step of Fenton-like oxidation, the adsorbed organic contaminants are degraded or mineralizedviaheterogeneous Fenton oxidation.In the heterogeneous Fenton oxidation, the generation of ROS highly depends on the interfaces.The active metal sites over the interfaces of catalysts can react with H2O2to generate high-activity ROS.As shown in Fig.3, the metal species immobilized on the zeolite by three ways (supported, encapsulated,and introduced into the framework of zeolite, respectively) are the main active species in Fenton-like reaction.The catalysts used for heterogeneous Fenton process are generally required to have excellent stability in the catalysis process, effectively preventing metal species from leaching and achieving sustainable utilization.Using zeolites with regular pore structure and high specific surface areas are chosen as the carrier for anchoring metal species in the Fenton process.For example, Ghaffariet al.prepared Fe-immobilized materials (i.e., Fe-ZSM-5 and Fe-silica) to degrade phenol [91].Compared with the relatively high Fe leaching of amorphous Fe-silica,the Fe-ZSM-5 showed better stability with low Fe leaching, which was ascribed to Fe being uniformly distributed in the framework and channels of zeolite.Sashkinaet al.studied the catalytic stability of Fe sites in hierarchical Fe-ZSM-5 [92], which is a mixture of ZSM-5 and amorphous silica.The Fe species were enclosed in zeolite mesopores or located on the external surface of amorphous silica globules.The Fe sites of the former were more stable, while the Fe species of the latter were unstable and prone to leaching.Note that zeolite with no amorphous silica had no Fe leaching.Thus, the porous structure of zeolite encouraged the stability of Fe sites during the Fenton reaction.However, it is also possible that the ROS was generatedviaa homogeneous Fenton mechanism inducing by metal ions leached from the zeolite-based catalysts [93].Thus, in order to verify whether the mechanism of ZFCs includes a homogeneous catalytic mechanism, it is necessary to remove the solid catalyst and then perform a homogeneous Fenton experiment.These highly ROS subsequently degrade the absorbed organic contaminants into useful intermediates or completely mineralize to CO2and H2O.Moreover, the acid Brønsted sites in the zeolite can provide an acidic environment for Fenton-like oxidation of organic compounds.Hacket al.found that the proton broke away from the Brønsted acid site of H-ZSM-5 and existed as an excess proton in the water cluster, which were bonded to the deprotonated Brønsted acid siteviahydrogen bond interactions [53].Cihano˘gluet al.found that, in the Fenton-like oxidation of acetic acid, as the Brønsted acidity of the zeolite-based catalyst increased,the COD removal increased [94].Yanget al.found that zeolite Y with strong Brønsted acidity could promote the production rate of•OH, enhance reduction of Fe(III) to Fe(II) species, as well as negate the need for additional acid in the Fenton-like system to degrade phenol [89].
3.2.Iron-based heterogeneous catalysis
Among transition metals, iron is generally considered the preferred choice based on high efficiency, economic and environmental considerations and thus widely used for heterogeneous Fentonlike processes [39,95-99].Lin and Gurol proposed an iron-based heterogeneous Fenton-like reaction mechanism based on surface complex chemistry, which has been widely accepted, as shown in Eqs.1–4 [100].Thus, in ZFCs, the key to ROS, produced by heterogeneous catalysis and oxidizes organic contaminants, are the solid interface iron (≡Fe) sites over the zeolite-based catalyst.
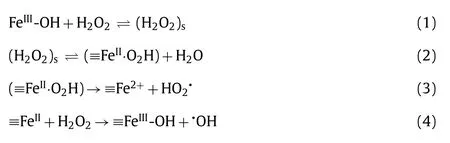
The traditional ZFCs can be used for the degradation of aromatic compounds and dyes.According to Yanget al., zeolite Y with Fe loading of 9% (FeY-9) had excellent catalytic performance (90%of phenol conversion) [89], which was ascribed to the dual functional properties of high surface Brønsted acidity and strong dispersion of octahedral Fe(III) in 5 nm Fe2O3nano particles for efficient decomposition of H2O2.At first, zeolite-based catalyst absorbed phenol either on the surface or in the micropores.The redox cycle of Fe2+/Fe3+occurred synchronously on the zeolite surface, which was initiated by the reaction between Fe species and H2O2to produce•OH (Fig.S2a in Supporting information).The Fe species were Fe2O3nano particles deposited on zeolite surface.They had good dispersion that provided enough active sites because of the interaction between Fe species and “α-oxygen” sites.The synthesized Fe-PANI/Zeolite was used for Acid Red G degradation by Shiet al.[101] The proposed mechanism is shown in Fig.S2b (Supporting information).The surface of negatively charged natural zeolite adsorbed positively charged aniline molecules to form a thin layer to inhibit the agglomeration of polyaniline.Fe species doped in polyaniline mainly interacted with N atoms on the quinoid rings.Specifically, the ≡Fe3+could be reduced to≡Fe2+by the –NH– in polyaniline through the electron transfer process, and the –NH– lost an electron and combined with a Cl−to form an amino salt group.≡Fe2+is the representative active site for the decomposition of H2O2on the catalyst surface, which could be oxidized into ≡Fe3+and the•OH was generated with H2O2decomposition to oxidize the Acid Red G for decolorization.The leached iron hardly contributed to the decolorization of Acid Red G at pH 6viahomogeneous test, which further proved that the heterogeneous catalyst was in responsible for the decolorization of Acid Red G in this system.It achieved the degradation almost 100%.
In addition to aromatic compounds and dyes, pharmaceutical wastewater has attracted much attention recently due to its damage to ecology and human health [102,103].The presence of pharmaceutical compounds can significantly affect the growth of microorganisms, fishes, seaweeds, and various fungal species [104].Because of the generation of non-selective•OH, ZFCs could be applied to eliminate the complex structures of pharmaceuticals.Velichkovaet al.found that Fe-ZSM5 was active in heterogeneous Fenton oxidation of paracetamol and could maintain its catalytic activity in a continuous process [105].Compared to maghemite Fenton-like oxidation system with the same order-of-magnitude Fe concentrations, the overall mineralization yield of paracetamol over Fe-ZSM5 could achieve above 50% at high H2O2excess.
Fe-ZSM5 was reported to be active in the heterogeneous Fenton oxidation of ibuprofen [106], giving 88% of ibuprofen removal and 27% of TOC removal under optimal conditions.Lanet al.exploited a heterogeneous Fenton hollow-fiber membrane reactor micro-sized iron-zeolite catalyst, Fe-ZSM5, to degrade ibuprofen [107].Under this experimental condition, the fouling tendency of the membrane was not influenced by the aging of the membrane because of contact with Fenton solution, resulting in almost 100% removal of ibuprofen.Actually, the homogeneous mechanism induced by Fe ions leached from the zeolite-based catalyst could make a contribution to generating ROS to degrade organic compounds.
Phenol hydroxylationviaFenton pathways with H2O2as an oxidant to produce useful intermediates dihydroxy benzenes (DHBs)has been widely reported [74,108,109].Catechol (CAT) and hydroquinone (HQ) are DHBs, which are useful chemicals widely used in synthetic perfume, chemical pesticides and photographic chemicals[110,111].There are some studies that applied ZFCs for the hydroxylation of phenol.Butthaet al.synthesized Fe2+/Fe3+-HZSM-5 catalyst with different Fe precursors of an equivalent molar concentration of Fe2+and Fe3+to hydroxylate phenol [108].The mixture of highly agglomerated iron-oxo and iron-dioxo species was identified as the main active sites of the catalyst.Iron-oxo species reacted with H2O2to form FeOOH, and then reacted with phenol to form CAT and HQ.As for iron-dioxo species, phenol was adsorbed on Fe sites doped on the zeolite surface and then degraded to CAT.It could achieve a 24% yield of DHB, as well as 71% selectivity to CAT and 29% to HQ.In order to further increase the conversion of phenol, Diaoet al.encapsulated iron oxide nano particles into ordered mesopores [74].It further increased the stability and dispersion of iron species, and thus improved the interaction between iron species and ZSM-5 carrier, resulting in greater Fe(III) reduction as well as•OH generation.For Fe@MZ5, the oxidation of phenol by•OH to CAT and HQ mainly occurred in the ordered mesopores.It could achieve 82.8% of phenol conversion, 94.8% of DHB conversion and 49.2% of HQ conversion.In addition, there are studies using the traditional ZFCs to convert pollutants to other useful chemicals.For example, Shenet al.selectively converted chlorophenol into high-value formic acid [112].The system worked with an ironcontaining ZSM-5 catalyst (with an 80 Si/Al ratio and 1.4% Fe) and H2O2as the oxidant, achieving 50.7% HCOOH yield.
Based on the studies to date, iron-based heterogeneous catalysis can promote green catalytic degradation or conversion of various organic contaminants in water treatment.However, the easy precipitation of Fe(III) under the neutral conditions may limit its further application.One method to solve this problem is to use copper-based zeolite Fenton-like catalysis (Cu(II) is more soluble in the neutral conditions than Fe(III)).
3.3.Copper-based heterogeneous catalysis
In addition to using iron-containing solid matrices as catalysts,copper-containing zeolites have become a hotspot in Fenton-like chemistry owing to their good active and stable properties recently[113,114].Compared to Fe(III), Cu(II) organic complex compounds are more easily decomposed by•OH, which is conducive to the Cu2+/Cu+cycle and has better efficiency [115].Furthermore, the Cu(II) complex can be dissolved at circumneutral pH, while Cu(I)can be rapidly oxidized to Cu(II) in a few minutes at circumneutral pH conditions [116].This makes the Cu2+/H2O2Fenton-like system useable in a wide pH range (2–7).As shown in Eqs.5 and 6, the reaction is mainly carried out by electron transfer, specifically from the reduced form of copper to the oxidizing agent, and then from the oxidant to the oxidized form of copper, simultaneously generating•OH and HO2•[60,117].


Singhet al.used several Cu/Y zeolite catalysts to degrade Congo redviaheterogeneous Fenton-like reaction [60].When the initial pH=7, the maximum decolorization, degradation and mineralization were 95.34%, 93.58% and 79.52%, respectively.The adsorption and Fenton-like reaction (active copper species and H2O2) played a major role in the overall degradation of Congo Red.First, the dye molecules enter the pores of the zeolite and were mineralized through a Fenton-like reaction shown in Fig.S3a (Supporting information).The ROS generated by Cu/Y zeolite and H2O2destructed the azo bond and naphthalene rings to produce low molecular weight intermediates to achieve further mineralization of dye molecules.In addition, several Cu/zeolite Y samples were synthesized to oxidize quinoline [118].The reaction between H2O2and the copper species fixed over the catalyst surface was crucial in the oxidation of quinoline, because the degradation of quinoline by•OH was at the solid-liquid interface (Fig.S3b in Supporting information), classifying this as heterogeneous catalysis.Cu-ZSM-5 zeolite membrane was synthesized for catalytic wet peroxide of phenol in the presence of H2O2(Fig.S3c in Supporting information) [68].At first, H2O2was in touch with the ZSM-5 membrane catalyst, and the•OH radicals were generated over the Cu sites in the ion-exchange zeolite channels.The effective contact between Cu+species and the ZSM-5 membrane could increase the generation of•OH, further increasing the reaction effi-ciency.It could achieve total organic carbon (TOC) conversion of 76.6% at pH 6.Liet al.synthesized Cu decorated zeolite A@Void@ethyl bridged periodic mesoporous organosilicon nanocomposites(Cu/YS-A@Et-PMO) to degrade methylene blue (MB) without any pH adjustment [119].The removal achieved 95% of 60 mg/L MB within 10 min.The specific mechanism was that MB molecules were adsorbed on the catalyst shell and internal zeolite channels through electrostatic interactions.Then Cu/Cu2O served as the active sites to catalyze H2O2to generate•OH which further degraded the mineralized MB molecules.The porous framework of Cu/YSA@Et-PMO could expose a large number of active sites as well as provide void space, improving electron transfer between nanoparticles as well as catalytic performance.
Based on the studies of copper-based heterogeneous Fenton catalysis, it can be thought that it is a promising technology for the wastewater treatment because of the high catalytic oxidation ability and relatively wide pH range.Compared to the iron-based Fenton-like catalysis, there is not enough research on the application of copper-based zeolite Fenton-like catalysis at present.Thus,the mechanisms and applications of copper-based zeolite Fentonlike catalysis need to be further studied in future.
4.Methodological strategies to improve zeolite-based Fenton-like catalysis
In order to further improve the pollutants degradation effi-ciency of traditional ZFCs, specific methodological strategies have been introduced in this section.At present, there are many studies combining photocatalysis or electrocatalysis with traditional ZFCs.The presence of UV/visible light or electricity can accelerate Fe(II) regeneration in order to facilitate the generation of ROS[120].Introducing various transition metals into ZFCs can also be an excellent alternative to facilitate the generation of ROS.In this section, the application of methodological strategies in promoting the degradation efficiency of organic pollutants as well as related mechanisms are introduced.The specific parameters assessment and removal effectiveness are summarized in Table S4.
4.1.External synergetic strategies via light/electric field
4.1.1.Photo-Fenton
At present, the combination of heterogeneous Fenton oxidation and photocatalysis,i.e., heterogeneous photo-Fenton oxidation, is highly attractive [121].This is due to the synergetic effects of light and Fenton reaction that can make charge transfer more efficient,thereby promoting ≡Fe(II) regeneration and H2O2activation.In solution, photochemical regeneration of Fe(II) occurs through photoreduction of Fe(III) under light irradiation, and continues through the generation of the second•OH and Fe(III) by newly produced Fe(II) and H2O2, as shown in Eqs.7 and 8.Thus, the hydroxyl radicals are released with the enhanced Fe(II) regeneration.For instance, the reduction of Fe(III) to Fe(II) can be accelerated and the direct H2O2photolysis under light irradiation can occur [98].The use of zeolite-based Fenton-like catalysts can increase the reaction frequencies between Fe species and H2O2due to the high specific surface areas of zeolites, thereby improving reaction efficiency.
Studies have shown that irradiating ultraviolet light on the ZFCs can enhance the catalytic degradation rate.Velichkova found that UV/vis irradiation can significantly promote the production of radicals [105].At near neutral pH, photo-Fenton oxidation of paracetamol over Fe-containing zeolite could achieve complete conversion after 5 h, as well as TOC removal of as high as 60%.Ghaffariet al.used Fe-ZSM-5 to degrade phenol by photo-Fenton [91].Compared to the phenol degradation efficiency in separate photocatalysis and Fenton processes, the efficiency observed in the combined photo-Fenton process was significantly enhanced, because of the synergetic effect of light and H2O2on the generation of•OH (Fig.S4a in Supporting information) [122].The Fe(III) species in the zeolitebased catalyst can be reduced to Fe(II) by light irradiating.However, only by generating more ROS can the organic pollutants be better degraded.
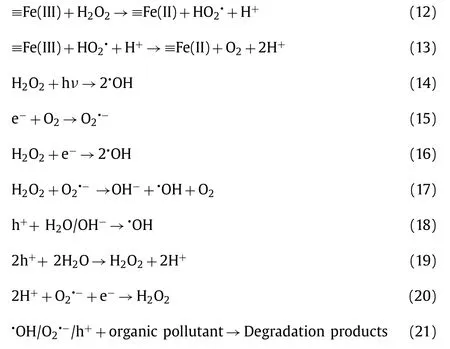
At present, the semiconductors are the most widely used photocatalyst [123–126].However, the low specific surface area, poor affinity for hydrophobic organic compounds, difficulty to separate and recover, and agglomeration issues limit their actual application [51].Many attempts have been made to enhance the removal efficiency by immobilizing the semiconductors onto/into suitable supports, which need to have a high specific surface area, relatively good stability, affinity for hydrophobic organic compounds and prevent semiconductors from forming clusters [51,62,127,128].Zeolite can be considered as a promising support in the application of heterogeneous photo-Fenton because of its enhanced adsorption performance and the ability to reduce electron-hole pairs recombination [51].There have been studies combining zeolite with semiconductors to develop prospective heterogeneous photo-Fenton/photo-Fenton like process.As shown in Eqs.9–21, the H2O2can not only generate•OH radicals under ultraviolet irradiation, but also effectively prevent e−/h+recombination [129,130].This is due to the reaction of H2O2with conduction band electrons and the superoxide radical anion to generate•OH radicals [131].The mechanism of the iron-containing zeolite-based catalysts includes the cycling of ≡Fe(III)/Fe(II) (Eqs.10–13).In contrast, the mechanism of zeolite-based catalysts containing no or little iron but combined with semiconductors includes the production of electron-hole pairs over the catalyst and the production of ROS.
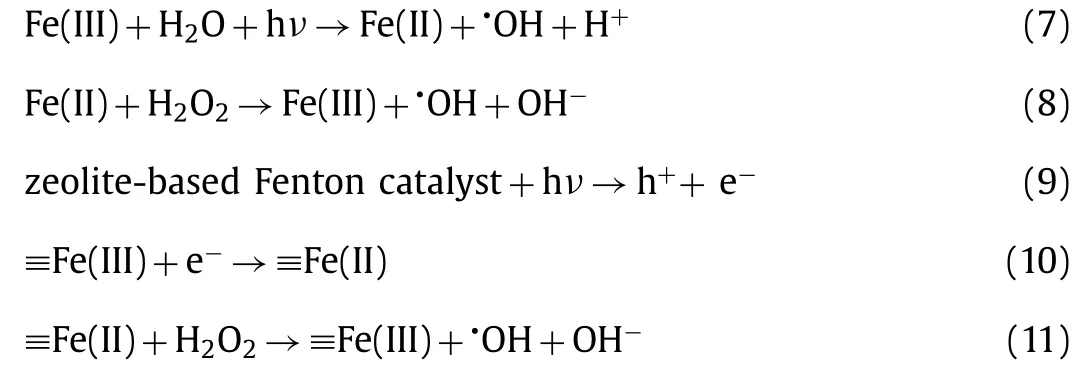
Nezamzadeh-Ejhiehet al.investigated the effectiveness of ZnO/nano-clinoptilolite zeolite in the removal of 4-nitrophenol under UV irradiation [131].The adsorption capacity of this nano zeolite could make more•OH radicals, resulting in faster degradation of the adsorbed pollutants.Also, zeolite could enhance the photocatalytic efficiency by preventing e−/h+recombination.The mechanism of ibuprofen removal by the "Zeolite-titanium-H2O2-UV-ultrasonic" system was studied by Farhadiet al.(Fig.S4b in Supporting information) [76].At the optimum pH of 5, various elements of the zeolite reacted ibuprofen, while the reaction between photocatalyst and ultrasound occurred, leading to•OH generation.The generation of electron-hole pairs occurred when the energy of TiO2in the suspension was equal to or higher than the UV bandgap.Electrons that existed on the surface of TiO2or in the reaction suspension could be accepted by the excited optical cavities.Then, the organic compounds were oxidized by•OH,h+and O2•−.This caused the oxidation of organic compounds by the•OH, the photovoltaic, and O2•−.The combination of ultraviolet radiation and H2O2can significantly improve the efficiency of the photolysis process through the generation of•OH by ultrasonic waves.This integrated system produced•OH radicals in large quantities to degrade ibuprofen, and also decreased the recombination of electron-hole in the photocatalyst through the role of H2O2as an electron scavenger accepting a photo-generated electron from the conduction band, thereby increasing the purification efficiency.Thus, the excited electrons by ions in this photocatalyst could prevent the recombination of electron and hole, as well as continue the ROS generation.Phanet al.used the impregnation calcination method for the first time to dope LaFeO3(LFO) into a natural zeolite modified by acid [132], and the resulting effectiveness to decolorize Rhodamine B by photo-Fenton catalysis was evaluated.Compared with the pure LFO and the acid-modified zeolite, the 1HNZ-30LFO sample could better degrade pollutants through heterogeneous photo-Fenton reaction, with 98.3% of RhB removal.This was caused by the synergistic effect between abundant adsorption sites on the modified zeolite as well as active sites for photocatalysis on LFO.The conduction electron scavenging (Eqs.9 and 16) and the Fenton-like reaction over the catalyst surface (Eqs.11–13) work together on the RhB removal through heterogeneous photo-Fenton.
The combination of photocatalysis and ZFCs can effectively boost the ≡Fe(II) regeneration and H2O2activation by driving efficient charge transfer due to the photo-assisted synergistic effects.In order to generate more active oxygen to effectively degrade organic pollutants, it is necessary to combine semiconductors that can generate electron-hole pairs with the ZFCs.For instance, graphene-based inorganic materials can be used in heterogeneous photo-Fenton, with advantages of excellent chemical stability and mechanical properties, as well as high mobility of charge carriers.
4.1.2.Electro-Fenton
The so-called electro-Fenton process is the combined process of electrochemistry and traditional Fenton reaction.The electrochemical generation of H2O2is reduced by O2at the cathode, which can avoid the hazards of H2O2in transportation, storage, and operation, as shown in Eq.22 [133,134].Also, Fe2+can be obtained by the reduction of Fe3+at the cathode, thereby regenerating Fe2+more rapidly and reducing Fe-containing sludge, as shown in Eq.23 [135].It still has shortcomings such as a narrow pH range, using soluble iron ions as well as incapability in reusing the catalysts [136].Thus, the heterogeneous Fenton-like process can be combined with the electro-Fenton process.This combination of two processes is termed the heterogeneous electro-Fenton process,which is suitable for neutral pH conditions and no requirement for additional iron salt.The heterogeneous electro-Fenton has the advantages of both heterogeneous Fenton-like process and classical electro-Fenton process, and can maximize the effects of degradation [137,138].The high adsorption ability and catalytic properties of the zeolite makes it suitable for use as an electro-heterogeneous Fenton catalyst in water treatment [75].Zeolite-based catalysts combined with transition metals, such as Fe, Cu, can be applied in the electro-Fenton to treat organic contaminants in water.In addition, they can enable continuous circulation.

Based on the function of heterogeneous Fenton catalysts, the heterogeneous electro-Fenton process is divided into two types.In Type 1, the catalyst serves to catalyze the decomposition of H2O2.In Type 2, the catalyst functions as both catalyst and cathode [139].
According to the above-mentioned principle of electro-Fenton,the electrochemical regeneration of H2O2at the cathode, which is very essential in the Fenton electrical process, is affected by the performance of the cathode.Thus, the key to electro-Fenton is the choice of cathode [140].Since zeolites are almost nonconductive, when the zeolite-based catalysts are applied to electro-Fenton, they are categorized as Type 1.Sruthiet al.investigated the application of heterogeneous electro-Fenton process with ironmanganese dioxide supported zeolite (IMZ) for the treatment of landfill leachate [141].Both anode and cathode were made of graphite, and some IMZ was added.At the optimal conditions,the COD removal was 87.5% and the biodegradability of landfill leachate was significantly enhanced from 0.03 to 0.52.The demand for low catalyst usage in the heterogeneous electro-Fenton justifies its better feasibility for stable leachate treatment relative to heterogeneous Fenton.Fe-ZSM-5 nano catalyst and an innovative reactor with surrounding graphite electrodes were applied for removal of Acid Blue 25 [57].The pH dependence markedly reduced when a heterogeneous catalyst was used.The nano Fe-ZSM-5 was used consecutively in 5 cycles without a remarkable decrease in decolorization, and approximately 90% of Acid Blue 25 was removed under optimal operating conditions.
However, Leet al.used a composite material of zeolite and carbon felt (CF) as the cathode, classifying this as Type 2 [75].Carbon felt (CF) has been known as a commonly used cathode for electro-Fenton process because of its advantages of cost, and excellent conductivity and stability.Besides, it has a relatively high specific surface area, which can provide a large number of active redox sites [142,143].Fe-MFI@CF was not only used as a catalyst but also a cathode in electro-Fenton reaction in the degradation of acid orange 7 [75].Results indicate the heterogeneous electro-Fenton system exhibited more effectiveness compared to the homogeneous electro-Fenton system.200 mL of acid orange 7 (0.1 mmol/L) was completely degraded over the Fe-MFI@CF within 40 min, and the cathode showed good recovery after five circulations.
The efficiency of degrading pollutants in the heterogeneous electro-Fenton is mainly related to the functionalized cathode.There are few studies on using modified zeolites to functionalize cathode [75].Materials such as carbon nanotube, graphite and graphite felt, which has excellent conductivity, can be combined with modified zeolites to be used as the cathode.
4.2.Internal synergetic strategies with the aid of multi-metals catalyst
In addition to using external physical energies to increase the yield of ROS, introducing other transition metals, like Cu, Mn, Cr as well as Co on the iron-based catalysts, can also be effective.Multi-metals co-supported systems can exhibit synergistic properties, and regulate the physical and chemical performances of catalysts, thereby increasing catalytic activity.Different multi-metal co-supported systems have been studied.In recent years, Fe-Cu bimetallic catalyst systems have attracted increasing attention, as Cu has similar redox properties to iron.The introduction of Cu in Fe-containing catalyst can improve the catalytic ability and extend the pH range in the Fenton process [144].Meanwhile, due to thermodynamic advantages, Cu(I) can make Fe(III) convert to Fe(II)[145].
Because of the synergy of the metals, Fe-Cu bimetallic systems usually show new functions [146].For instance, according to Xuet al., a zeolite-loaded Fe-Cu bimetallic system (Fe/Cu@zeolite) was used to degrade bisphenol A (BPA) by enhancing the formation of O2•−[147], resulting in a degradation efficiency of 87% under optimal conditions.It was thought that the synergetic effect of iron and copper played a major role in BPA degradation.The Cu(I) regeneration over the surface of zeolite contributed to the production of O2•−.Subsequently, O2•−oxidized ≡Fe(II) and ≡Cu(II) into≡Fe(III) and ≡Cu(II), respectively, and was converted into H2O2.Then the decomposition of H2O2induced by ≡Fe(II) occurred in order to produce more•OH through Fenton (Fig.4a).Finally, macromolecular bisphenol A was completely mineralized into CO2and H2O in the presence of•OH.

Fig.4 .(a) The mechanism of BPA degradation by bimetallic Cu/Fe@zeolite.Reprinted with permission [147], Copyright 2020, Elsevier.(b) The mechanism of the phenol degradation by the Fe-based catalysts.Reprinted with permission [149].Copyright 2017, Elsevier.(c) The mechanism of phenol degradation by core-shell hierarchical 4A zeolite/Fe@Cu.Reprinted with permission [150].Copyright 2020, Elsevier.(d) The mechanism of MO degradation by Fe-Mn/SAPO-18 at the presence of UV light.Reprinted with permission [151].Copyright 2019, Elsevier.(e) The mechanism of participation of transition metals in iron oxidation states redox cycle and H2O2 degradation in heterogeneous electro Fenton.Reprinted with permission [152].Copyright 2020, Elsevier.
For multi-metals co-supported systems, there are still challenges on how to make metallic components disperse more uniformly, as well as how to improve stability and catalytic performance of catalysts.Using mesoporous zeolites or hierarchical and porous nanoarchitectures as multi-metal supports has become a highly effective strategy to meet these challenges.Luoet al.treated the mesoporous ZSM-5 zeolite with alkali as a superior carrier for bimetallic FeCu [148].The mesoporous ZSM-5 carrier obtained by alkali treatment resulted in rough surfaces, increasing the metal migration barrier, and thereby enhancing the dispersion and prohibiting metal migration.Using the alkali-modified mesoporous zeolite as a carrier could prevent the aggregation of nanoparticles as well as enhance the multi-metallic interaction.This strategy could effectively promote catalytic oxidation of phenol and is a dependable way for the preparation of multi-metallic catalysts with good dispersion of active components.However, these open-pore systems also enable the active components to suffer a loss during the reaction, which leads to high Fe and Cu concentrations in the product solution of greater than 1 ppm.
Encapsulating iron and copper bimetal oxides in hollow zeolite could make the active components highly dispersed in pores and channels of zeolite (Fig.4b), which may become a more effective measure to resolve the above limitations.Highly dispersed Fe-Cu bimetallic oxide nanoparticles in hollow Silicalite1 zeolite single crystals (Fe2O3CuO@Hol S-1) were successfully encapsulated[149], which achieved almost 100% phenol conversion due to the significantly improved transmission of reactants/products in microporous channels of 20 nm as well as the well-dispersed active components with small encapsulated Fe2O3–CuO particle sizes of about 3.7 nm.The much larger size than the 0.53 nm of zeolite micropores could enable the active components to decrease leaching.
Huanget al.prepared core-shell hierarchical 4A zeolite/Fe@Cu to degrade phenol in heterogeneous Fenton-like process [150],which could achieve 95.3% of phenol removal at pH 5 in the presence of H2O2, which was better than the single metal Fe or Cu catalysts under the same condition.This was attributed to the synergy of the 4A Fe/zeolite (core) and two-dimensional copper hydroxide nanosheets (shell).The Fe(III) and Cu(II) could be reduced by H2O2to Fe(II), Cu(I), respectively, and HO2•/O2•−was generated(Fig.4c).Subsequently, Fe(II) and Cu(I) could react rapidly with H2O2to generate hydroxyl radicals.Simultaneously, Cu(I) promoted reduction of Fe(III) to Fe(II).The copper hydroxide nanosheets had an open network structure on this catalyst surface, which could improve the Fe(III) stability under acidic pH significantly and prevent the decreased Fe(III) catalytic activity due to the embedding effect.
It has also been reported that light can be introduced into multi-metal co-supported Fenton catalysis to improve the production of ROS and the catalytic degradation of organic pollutants.This was mainly because light could facilitate the conversion of the metal ions valence.Liet al.used Fe, Mn co-supported SAPO-18 zeolite to remove methyl orange under light [151], and the resulting Fenton reaction over Fe-Mn/SAPO-18 achieved almost complete degradation of 50 mg/L methyl orange in 20 min.Under the same conditions, its methyl orange degradation efficiency was higher than that of single metal Fe or Mn catalysts.The probable mechanism of methyl orange degradation over Fe-Mn/SAPO-18 is shown in Fig.4d.The UV light could not only speed up H2O2decomposition to produce•OH, but also facilitate the different valence of metal ions to convert.Moreover, the conversion between different valences of Mn could release ROS (HO2•), and it could also have a synergetic effect with Fe ions, thereby further improving the reaction efficiency.Hence, the photo-assisted method and the synergy of multi-metal promoted the conversion of metal ions valence and generated more ROS,i.e.,•OH, which can promote the increase of MO degradation efficiency.
There is also research to combine the multi-metal co-supported heterogeneous Fenton catalysis and electro-Fenton.This can facilitate metal ion valences to convert, thereby improving the production of ROS and the catalytic degradation effectiveness of organic pollutants.Zahraniet al.improved the Fe-ZSM-5 catalysts by using three transition metals (Cr Cu, and Co) to improve the removal of Acid Blue 25 [152].The existence of transition metals could enhance Acid Blue 25 removal efficiency (approximately 8%) as well as the speed of reaction (approximately 64%), because transition metal ions can boost the redox cycle of Fe3+to Fe2+and decomposition of H2O2, as shown in Fig.4e [153].Also, the presence of transition metals increased the specific surface area of the nano catalyst, which could increase active sites on the nano catalyst surface.Under optimum operating conditions, Fe/Cu-ZSM-5 was capable of removing 97% of Acid Blue 25, 79% of COD as well as 65% of TOC.Even after 5 cycles of using the nano catalysts, the Acid Blue 25 removal efficiency was well-maintained.Various metal oxide catalyst particles with zeolites (Cu, Cu-Zn, Cu-Zn-Pr, Cu-Zn-Pr-La)were applied in the treatment of actual dyeing wastewater through a Fenton process with the aid of electricity [154].After 50 min,the actual dyeing wastewater was decolorized by 83.2% by Cu-Zn-Pr-La-zeolite, which was17.85%, 13.1%, and 7.0% higher than that of Cu-zeolite, Cu-Zn-zeolite, and Cu-Zn-Pr-zeolite, respectively.The COD removal in actual dyeing wastewater by Cu-Zn-Pr-La-zeolite reached 52.1% after 60 min, which was 14.4%, 11.52%, and 5.37%higher than values for the Cu-zeolite, Cu-Zn-zeolite, and Cu-Zn-Pr-zeolite, respectively.The superior performance of Cu-Zn-Pr-Lazeolite was attributed to the increased loading of metal species increasing the efficiency of decolorization of actual dyeing wastewater and removal of COD.
Thus, the multi-metals co-supported zeolite Fenton catalysis can exhibit high catalytic activity in the degradation of organic pollutants.However, if the metals leached from the multi-metals cosupported catalyst, it may cause the unsustainable use of catalysts and environmental pollution.Future research is supposed to pay more attention to the control of metal leaching of this catalysis system.
5.Limitations of zeolite-based Fenton-like catalysis for practical water treatment
According to the current studies, it can be thought that the ZFCs can be effectively applied to the removal and reclamation of various organic contaminants in water treatment.And there are also a few studies on treating real wastewater.Arimiet al.used impregnated natural zeolite to remove colored recalcitrant in molasses distillery wastewater (MDW), increasing the biodegradability of MDW by 4% and decreasing the color of the effluent[46].Sruthiet al.investigated the application of the heterogeneous electro-Fenton process with iron-manganese dioxide supported zeolite (IMZ) for the treatment of landfill leachate [141].Liuet al.used various metal oxide catalyst particles with zeolites (Cu, Cu-Zn, Cu-Zn-Pr, Cu-Zn-Pr-La) by impregnation method in the treatment of actual dyeing wastewater through an electro-Fenton process [154].Athitayaet al.used Fe-impregnated natural zeolite as the Fenton-like catalyst to degrade antibiotics (including amoxicillin, tetracycline, and tiamulin) in real treated swine wastewater [155].The degradation efficiencies were above 75%.It could be found that the zeolite-based Fenton catalysts were not extensively used in the treatment of real wastewater.One possible reason was that despite the micropores of zeolites could prevent the entrance of most background organic molecules, there may be small molecules similar in size to the target organic contaminants in the real wastewater, which might compete with the adsorption and oxidation of the target organic contaminants [50,88].Thus, it is necessary to further investigate the modification of zeolite proprieties, such as structural properties (e.g., pore opening and pore space) and surface hydrophobicity in order to improve the selectivity for target contaminants.Another possible reason was that most of them were natural zeolites modified by impregnation or ion-exchange method.Because the cost of natural zeolites was relatively lower than synthetic zeolites.However, using impregnation or ion-exchange method to modify them may cause the inevitable metal species agglomerated, leaching, and even reexchanged from zeolite by other cations in industrial wastewater [61].This decreases the sustainable utilization of the catalysts.Designing more efficient, stable, and economic zeolite-based Fenton catalysts should be concerned.And the zeolite-based catalysts prepared by hydrothermal methods can be considered as one approach.It could make the catalysts have higher dispersion of metal species and stability [61,71].Although the cost of this method may be higher than the impregnation and ion-exchange method, it can be further reduced by recycling the catalyst with high stability.Another approach is to use industrial wastes as a source of silicon and aluminum and metal species to prepare more environmentally and economic zeolite-based Fenton catalysts.Studies on degrading trace organics, such as personal care products and steroids estrogens, by ZFCs are scarce.Thus, the ZFCs should be developed for these emerging pollutants and implemented in the real fields to validate its efficiency and economic feasibility.
In addition to the above existing problems, how to improve the utilization of H2O2in ZFCs also needs to be focused on.There are some methodological strategies (e.g., ultraviolet light, electricity)applied to ZFCs, which can favor the utilization of H2O2.As for photo-Fenton, the synergetic effects of ultraviolet light and Fenton reaction can make charge transfer more efficient, thereby promoting H2O2activation [98].As for electro-Fenton, the electrochemical generation of H2O2is reduced by O2at the cathode, which can avoid the hazards of H2O2in transportation, storage, and operation [133,134].However, these technologies may come at the cost of consuming energy and resources.How to reduce energy consumption and utilize the low-cost source should be paid more attention to.For example, sunlight is a low-cost and environmentally source, so the synthesis of zeolite-based catalysts with high quantum yields needs to be studied in the future.Easy-operated equipment involving in rationally collecting and utilizing light energy is recommended.In order to improve current efficiency and save costs in electro-Fenton, zeolite-based materials as electrodes with high catalytic activity, long working life, and low costs can be developed.Wanget al.reported a Fe3O4-zeolite-cyclodextrin (F-Z-C)heterogeneous Fenton-like catalyst [156], which can extend the duration of the reaction and increase the utilization rate of H2O2.The contaminants were absorbed to the surface of F-Z-C by the zeolite and Fe3O4cyclodextrin nanostructure, thereby increasing the local contaminant concentration on the composite surface.The H2O2dispersed in water could be temporarily captured and stored byβ-cyclodextrin, reducing the ineffective consumption of free H2O2,after which the F-Z-C catalyst could release H2O2slowly and continuously.This is termed the “storage-release” effect of the catalyst on H2O2.Thus, further studies can also be concentrated on improving the utilization efficiency of H2O2in ZFCs through a green and low-cost way, which can make ZFCs have more potential for practical applications.
For the further application of zeolite in practical engineering,the regeneration of zeolite-based catalysts was also an important problem that needs to be concentrated on.Moraleset al.applied•OH driven from Fenton chemistry to reactivate a fouled completely microporous ZSM-5 catalyst [157].Reactivation by Fenton chemistry was effective in removing organics and recovering its original catalytic activity.Moreover, the properties of the reactivated zeolites were retained.For instance, they had similar structure and texture characteristics to the initial zeolites, and the acidity was also improved.Fenton activation was used to recover the catalytic activity of the fouled catalyst.This was proven to be very effective in terms of using Fenton process to activate fouled ZSM-5.Exploring more promising approaches for zeolite-based catalysts regeneration are research topics worth investigating in the future.
Despite considerable developments in recent years, the practical applications of zeolite-based Fenton catalysis are still at the initial stage and require more effort in further research.Future studies should not only focus on efficient technologies, but also on environmental sustainability and economic assessments (including resources, energy, chemical, reactor investment and operating costs,etc.) and do so to establish a universal and scientific index system to evaluate the practicability of ZFCs.
6.Conclusion and perspectives
This article presented an overview of the latest developments in various ZFCs, summarized their synthesis and methodological strategies to improve reaction efficiency, as well as discuss the environmental application of ZFCs.The impregnation method and the wet ion exchange method could perfectly combine the metal components with the zeolite as well as preserve the original framework structure of the zeolite.But the limitations, such as loss of active centers and the risk of metal leaching, need to be resolved.The hydrothermal method could effectively address these problems, thereby making the zeolite-based catalyst more stable.Most mechanisms of ZFCs are heterogeneous catalysis.Methodological strategies in improving ZFCs include the aid of photocatalysis or electrochemistry in zeolite-based heterogeneous Fenton-like catalysis, boosting ≡Fe(II) regeneration and H2O2activation effectively.Introducing multi-metals could also improve the catalytic activity through the synergistic effect between the various metals.The combination of external driving force and the multi-metals can further improve the generation of active species.Moreover, the ZFCs could not only effectively degrade organic pollutants in water, but also convert harmful organic pollutants into useful chemicals, such as the hydroxylation of phenol to DHB, as well as the conversion of chlorophenol to high-value formic acid.
However, there are still some aspects that need to be considered in the synthesis and implementation of zeolite-based-like catalysis.
(1) More stable and efficient synthesis of zeolite-based Fentonlike catalysts can be developed.The sol-gel method is an attractive method to synthesize zeolite-based Fenton-like catalysts.It has the ability to prepare stable carriers with a high surface area and porosity, which can be considered as a rational and controlled method to promote Fenton-like oxidation of organic pollutants.
(2) Apart from iron-based catalysis, other transition metals such as copper- and manganese-based catalysis have also shown excellent efficiency in heterogenous Fenton.For instance,copper-based zeolites have become a hotspot in the field of Fenton chemistry owing to their high activity and stability in recent years, but studies remain scarce.More research is needed to delve into the combination of these transition metals with ZFCs and their reaction mechanisms.
(3) Developing the combination of ZFCs with other materials with excellent performance.For instance, the combination of zeolite-based heterogeneous Fenton catalysis and semiconductors (e.g., g-C3N4, TiO2, MoS2, CoS2) can generate more active oxygen to degrade organic pollutants.Besides, the combination of zeolite-based heterogeneous Fenton catalysis and good conductive materials can play dual roles of catalyst and cathode in heterogeneous electro-Fenton catalysis.There are few studies in these areas, and future studies can be developed in these directions.
(4) Considering economic feasibility and environmental friendliness, more coupled Fenton optimization processes can be explored to improve the degradation efficiency of organic contaminants.The economic costs of these coupled Fenton processes should also be comprehensively evaluated in further studies, which can provide useful support information for large-scale engineering applications.
(5) The environmental applications of ZFCs to degrade organic pollutants and extract useful chemicals from wastewater have been studied extensively, whereas other environmental applications such as using Fenton chemistry to reactivate a fouled zeolite, and improving the utilization efficiency of H2O2have been less studied.ZFCs can be further comprehensively studied in more environmental applications.
Declaration of competing interest
The authors declare that they have no competing interest.
Acknowledgments
The authors gratefully acknowledge the financial support provided by National Natural Science Foundation of China (Nos.72088101, 51739004, 21776066) and the Fundamental Research Funds for the Central Universities (No.531118010675).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.001.
杂志排行
Chinese Chemical Letters的其它文章
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
- Heterostructures of NiFe LDH hierarchically assembled on MoS2 nanosheets as high-efficiency electrocatalysts for overall water splitting
