Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
2022-12-07ZeHuYqunXuHoWngGoChoFnXilingLuo
Ze Hu, Yqun Xu, Ho Wng, Go-Cho Fn,b,∗, Xiling Luo,∗
a Shandong Key Laboratory of Biochemical Analysis, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
b State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
Keywords:Photoelectrochemistry Immunoassay Zwitterionic peptide Photocathode Anti-interference
ABSTRACT Accurate detection of important biomarkers with ultra-low levels in complex biological matrix is one of the frontier scientific issues because of possible signal interference of potential reductive agents and protein molecules.Herein, a self-powered anti-interference photoelectrochemical (PEC) immunosensor was explored for sensitive and specific detection of model target of cardiac troponin I (cTnI).Specifically, a novel ternary heterojunction served as the photocathode to offer a remarkable current output and a zwitterionic peptide was introduced to build a robust antifouling biointerface.CuInS2 (CIS) film with porous network nanostructure was first prepared and then modified in order with ZnIn2S4 (ZIS) nanocrystals and Au nanoparticles to fabricate the Au/ZIS/CIS heterojunction photocathode.After capture cTnI antibody (Ab) was immobilized, the zwitterionic peptide KAEAKAEAPPPPC was then anchored to compete the immunosensor.The elaborated PEC immunosensor exhibited high sensitivity for target cTnI antigen (Ag)detection, with good anti-interference against reductive agents and nonspecific proteins.This integration strategy of heterojunction photocathode with zwitterionic peptide provides a new sight to develop advanced PEC immunosensors applying in practical biosamples.
Sensitive and precise probing of disease-related biomarkers with low abundances have shown great prospect especially for early diagnosis and timely treatment of serious illnesses or cancers[1–3].As a result, constant efforts are now still being made to develop advanced biosensing technics.Among the reported biosensing community, photoelectrochemical (PEC) biosensing has shown its obvious merits such as simple device, easy operation, low price,high sensibility, and weak background signal [4–7], which bestows real-time, fast and high-throughput test upon this sensing technic.Since its inception, PEC immunosensing as a potential evolving direction has been actively pursued by the researchers [8–13].The explored PEC immunosensors alternatively can be divided into photoanode- and photocathode-based types in terms of the nature of the photoelectrodes [14,15].Photocathode-based immunosensors have been verified to have better anti-interference abilities to incidental reductive agents in biological samples [16–19], showing its bright future in real applications.Yet, the current outputs of the photocathodes are generally much lower than that of the photoanodes, bringing about limited sensitivity of the photocathode-based immunosensors.
Actually, except for reductive species, many potential protein molecules also co-exist in the biological matrixes (such as blood,plasma and serum), which easily latch onto the sensor surfaces through nonspecific adsorption [20,21], causing limited accuracy of detection.In order to minimize unwished nonspecific adsorption or biofouling, some special antifouling materials for biosensors have been explored [21,22].Thereinto, zwitterionic peptides have been considered as promising antifouling materials with distinct advantages of easy synthesis, good biocompatibility, low cost,tunable structure, and structural stability [23,24].The zwitterionic peptides have two critical features of electric neutrality and hydrophilic property, which can availably prevent the accessing of biofouling molecules whetherviaopposite charge attraction orviahydrophobic interaction.As a result, anchoring of zwitterionic peptides to the sensor surfaces would form strong hydration layers at biointerface against nonspecific protein adsorption [24–26].However, to the best of our knowledge, the pioneer works on this excellent antifouling strategy have not been reported in any PEC immunosensors.
We herein introduced a superior heterojunction photocathode with zwitterionic peptide anchoring to develop a powerful PEC immunosensor, as illustrated in Scheme 1.Cardiac troponin I (cTnI,Ag), which is a typical indicator of myocardial injury and necrosis[27,28], was used as a model target to appraise the immunosensor.CuInS2(CIS) film with a porous network nanostructure was firstly prepared on a fluorine-doped tin oxide (FTO) electrode through a hydrothermal method.Subsequently, the guests of ZnIn2S4(ZIS)nanocrystals and Au nanoparticles were modified on the CIS film to fabricate the expected Au/ZIS/CIS ternary heterojunction photocathode.After capture cTnI antibody (Ab) was immobilized on the PEC matrix of the Au/ZIS/CIS photocathodeviaNH2−Au bond, the zwitterionic peptide KAEAKAEAPPPPC was then anchored with the bonding of S–Au, forming an antifouling biointerface on the sensing electrode.The target Ag of cTnI was finally probed by an obvious decline of the current signal deriving from striking steric hindrance of the immunocomplex formed between Ab and Ag.This effective integration of heterojunction photocathode with zwitterionic peptide granted the PEC immunosensor with good performances of high sensitivity and anti-interference ability for target biomarker detection in the biological media.The experimental details are presented in Supporting information.
The electron transfer mechanism of the PEC immunosensor was depicted in Scheme 2.In the sensing system, a novel Au/ZIS/CIS heterojunction photocathode acted just as the PEC matrix to generate an evident current output without the assistance of bias potential (that is, it was self-powered).As a typical p-type semiconductor, CuInS2(CIS) possesses a large absorption coefficient and a narrow band gap of 1.5 eV that allows it to work as a promising light absorber [29,30].However, the intrinsic fast charge (electronhole pair) recombination in CIS causes a weak current output [31],which has limited further practical application of CIS.ZnIn2S4(ZIS)is also a narrower band-gap semiconductor (∼2.4 eV) and popular in photoelectric and photocatalytic fields for its high visible light utilization, good photostability, and environmentally friend [32,33].More importantly, the valence band (VB) and conduction band (CB)of CIS match well with ZIS after effective contact [34], and thus the ZIS/CIS coupling structure could evidently promote the current output for the synergistic action of sufficient light absorption (Fig.S1 in Supporting information) and efficient charge separation.In addition, metallic Au nanoparticle can produce many hot electrons thanks to its surface plasmon resonance (SPR).The effective combination of semiconductor with Au nanoparticle would not only increase the density but also enhance the lifetime of excited photoelectrons due to the exciton-plasmon coupling effect [35–37].Hence, the decoration of Au nanoparticles on the ZIS/CIS hybrid could further enhance the current output.
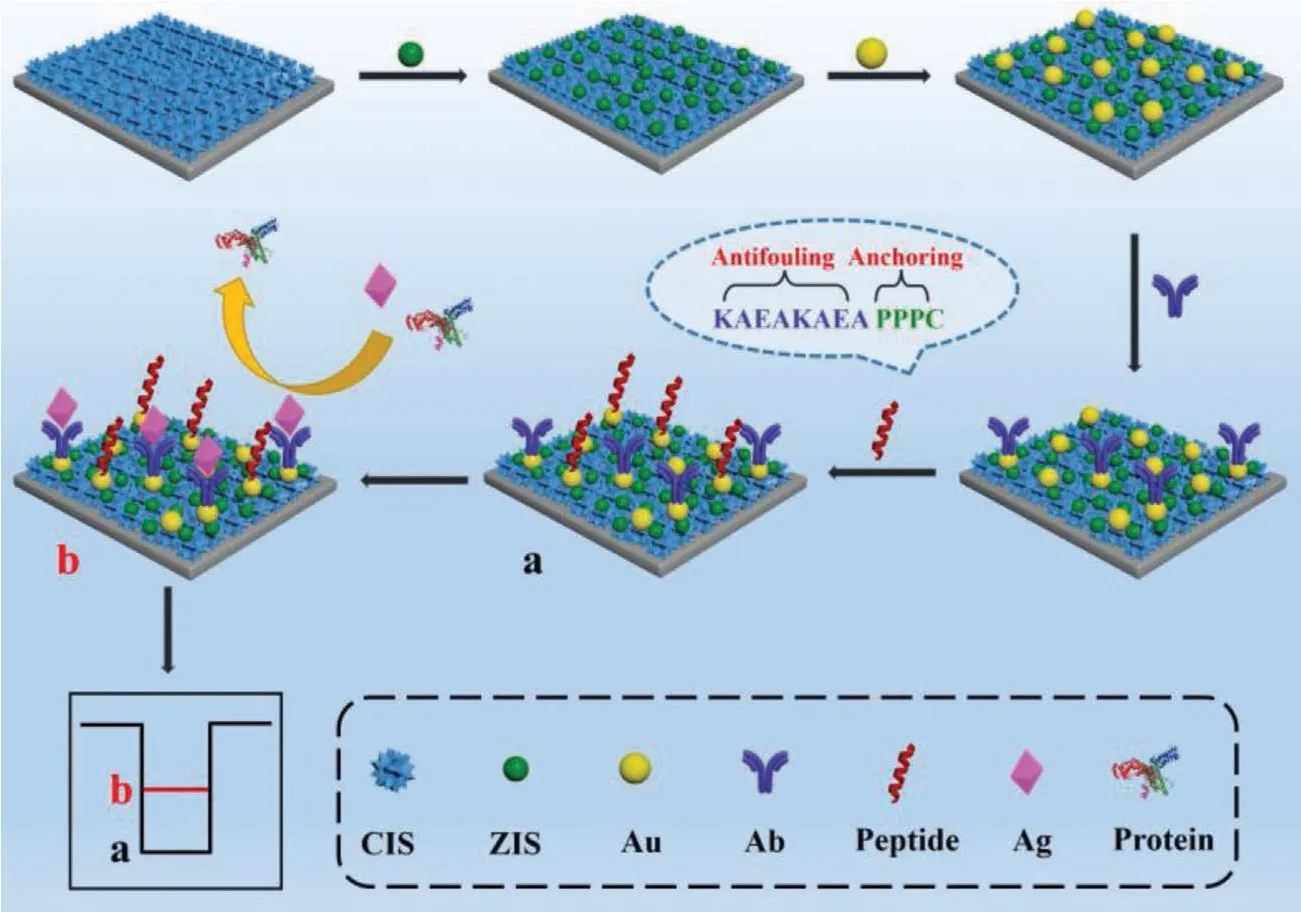
Scheme 1 .Development of the PEC immunosensor combining heterojunction photocathode with zwitterionic peptide.
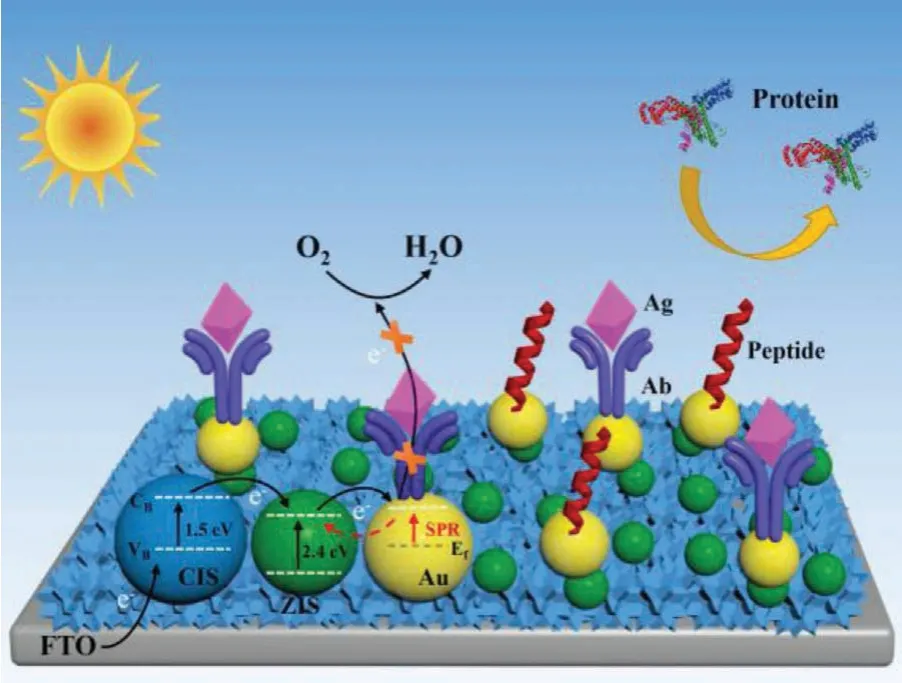
Scheme 2 .Photogenerated electron transfer process of the designed PEC immunosensor for target Ag detection.
In the sensing system, the zwitterionic peptide with sequence KAEAKAEAPPPPC was applied to build a robust antifouling biointerface, and its molecule structure is presented in Fig.S2 (Supporting information).The zwitterionic peptide was composed of a KAEAKAEA antifouling segment and a PPPPC anchoring segment.The antifouling segment played the main role in resisting nonspecific protein absorption, while the anchoring segment assisted the antifouling segment to generate a dense antifouling layer[38,39].In contrast to conventional blocking agent such as the protein of bovine serum albumin (BSA), the zwitterionic peptide exhibits not only the reinforced antifouling capability for surface blocking but the smaller steric hindrance for reduced signal loss[40,41].
Toward target Ag detection, the specific immunoreaction of Ag with Ab brought about large steric hindrance to the PEC immunosensor, causing a significant decline in the current signal because of serious impediment to electron transfer.Accordingly, profiting from evident current output of the heterojunction photocathode and excellent antifouling ability of the zwitterionic peptide,sensitive and precise probing of target cTnI in a biological specimen was realized.
The obtained Au/ZIS/CIS heterojunction photocathode was firstly characterized by scanning electron microscopy (SEM).As can be seen in Fig.1A, the CIS film appeared a porous network nanostructure with densely interlinked smooth nanoflakes.After ZIS deposition, as depicted in Fig.1B, abundant nanoparticles with the sizes of 8–12 nm were closely adhered on the CIS nanoflakes, and the smooth surfaces of the previous nanoflakes got obvious roughness and thickness.After Au nanoparticles decoration, as presented in Fig.1C, many nanoparticles with average sizes of about 20 nm were scattered evenly on the surfaces of the latter nanoflakes.Incidentally, the elemental mappings in the insets of Figs.1B and C also suggested successive modification of ZIS and Au.Moreover, as shown in Fig.1D, X-ray diffraction (XRD) analysis was utilized to characterize the Au/ZIS/CIS photocathode.The XRD pattern of CIS on a FTO substrate was shown as curve a, in which the characteristic diffraction peaks at 2θ= 28.24°, 32.68°, 55.36° and 57.98° can be well signed to the crystal planes of (112), (200), (215) and (224)of pure CuInS2phase (PDF No.38–0777).After ZIS deposition on CIS, as shown in curve b, the newly appeared diffraction peaks at 21.58°, 30.44°, 39.77°, 47.17° and 52.21° were indexed to the crystal planes (006), (104), (108), (110) and (1012) of pure ZnIn2S4phase(PDF No.72–0773).After Au nanoparticles decoration, the XRD pattern has no obvious difference with ZIS/CIS, which might be attributed to low content of loaded Au on the electrode.Alternatively, the pure Au nanoparticles were characterized, as presented in Fig.S3 (Supporting information).
The stepwise building of the PEC immunosensor was first monitored by current outputs of the electrodes, as revealed in Fig.1E.The CIS electrode showed a minor cathodic current output (curve a), owning to the intrinsic property of fast charge recombination for CIS.After ZIS deposition, the current output enhanced markedly(curve b), which was 1.7 times as high as the initial CIS electrode.This positive phenomenon can be explained that the coupling of ZIS adequately increased the light absorption and meanwhile effectively facilitated the charge separation.After Au nanoparticles decoration, a further increase in the current output was obtained(curve c), which was because the SPR effect of the Au nanoparticles increased the density and prolonged the lifetime of the excited photoelectrons.It could be estimated that the current output of the desired Au/ZIS/CIS heterojunction photocathode was about 2.5 times as high as the single CIS electrode, showing the excellent PEC property.Besides, time-varying current output was employed to inspect the stability of the Au/ZIS/CIS heterojunction photocathode,and a positive result of high stability was obtained, as presented in Fig.S4 (Supporting information).After the heterojunction photocathode underwent Ab modification and then zwitterionic peptide anchoring, the current output reduced continuously (curves d and e), due to the obvious steric hindrance of the protein and peptide molecules.After the sensing electrode underwent target Ag incubation, an evident decrease in the current output was obtained(curve f), illustrating the specific immunoreaction between Ag and Ab happened.The PEC test thus demonstrated successful building of the immunosensor.
The stepwise building of the PEC immunosensor was further inspected by electrochemical impedance spectroscopy (EIS), as revealed in Fig.1F.The CIS electrode presented a relatively small charge-transfer resistance (Rct) (curve a).After ZIS deposition, theRctenlarged (curve b) resulting from weak conductivity of the semiconductor ZIS.After Au decoration, theRctobviously reduced(curve c) owning to good electron conductivity of the metallic Au.After Ab immobilization and zwitterionic peptide anchoring, gradual increase in theRctcould be found (curves d and e) due to insulating effects of the protein and peptide molecules.After target Ag incubation for the sensing electrode, the further increase in theRctwas clearly observed (curve f), indicating the occurrence of the specific immune recognition of Ab with Ag.The expected variation tendency of EIS thus also suggested successful construction of the immunosensor.
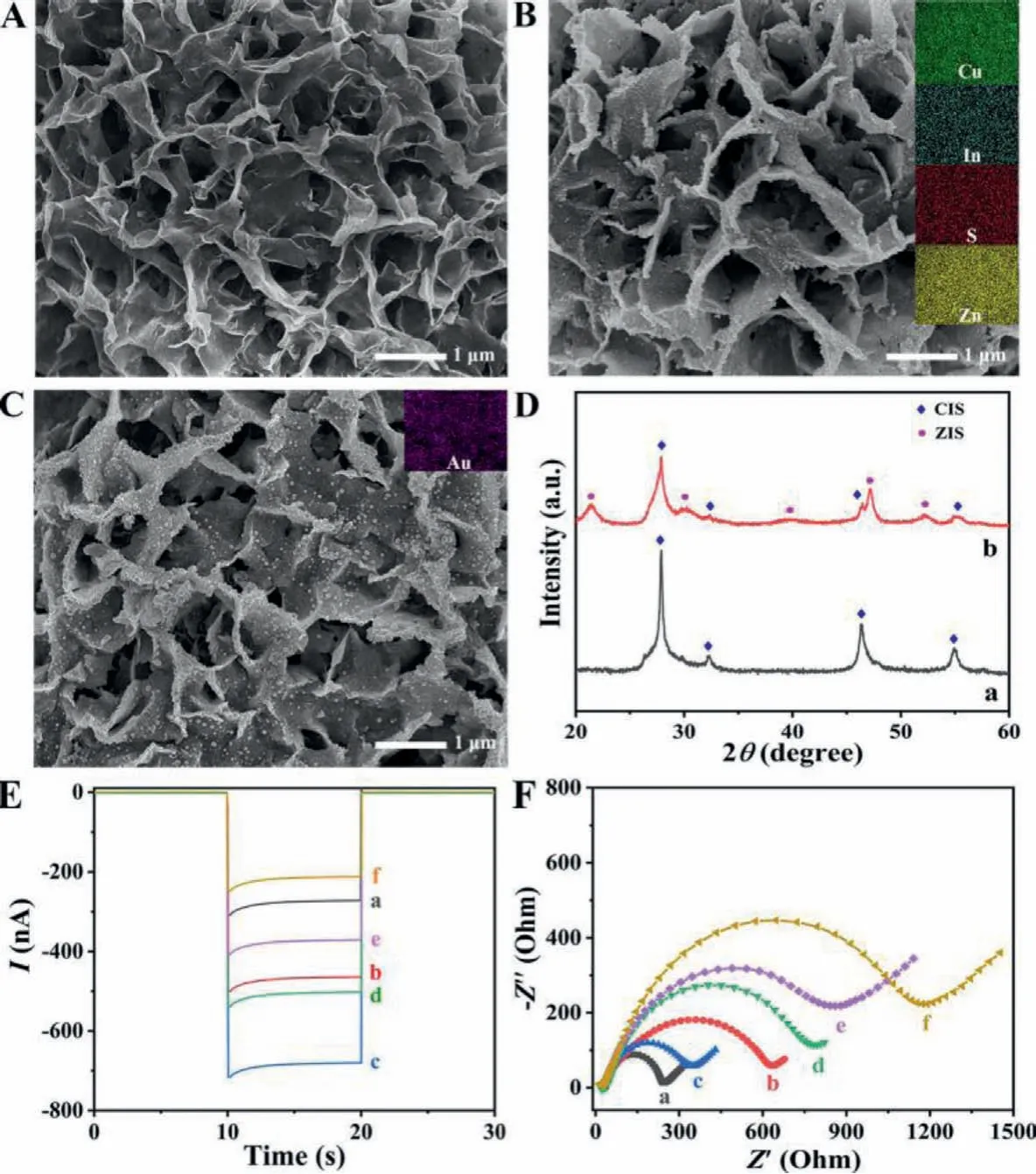
Fig.1 .SEM images of the (A) CIS electrode, after with (B) ZIS nanocrystals deposition, and (C) Au nanoparticles decoration; (D) XRD patterns of CIS and ZIS/CIS.(E) Current outputs and (F) impedance spectra of (a) the CIS electrode, after with (b) ZIS deposition, (c) Au decoration, (d) Ab immobilization, (e) peptide anchoring, and (f) finally target Ag incubation.Insets in B and C: elemental mappings of (Cu, In, S, and Zn) and (Au).
Nonspecific adsorption or biofouling is a grand challenge for an immunosensor to apply in biological matrixes.Zwitterionic peptides with high hydrophilicity and electric neutrality have been testified to own good capacity of repelling nonspecific protein adsorption [24–26].To assess antifouling capacity of the zwitterionic peptide, the characterizations of zeta potential and water contact angle were conducted.Fig.S5 (Supporting information) presents zeta potential of the zwitterionic peptide, and the obtained value of approximate 0.0 mV indicated its electric neutrality.Water contact angle analysis was used to investigate hydrophilicity of the zwitterionic peptide, as exhibited in Fig.S6 (Supporting information).The fabricated Au/ZIS/CIS photocathode had a contact angle of 75.19°, and the contact angle reduced to 49.35° after Ab immobilization.After zwitterionic peptide further anchoring, the contact angle reduced evidently to 20.56°, showing excellent hydrophilicity of the zwitterionic peptide.The result reflected the PEC immunosensor might have a good potential in antifouling property.
The antifouling property of the designed PEC immunosensor was further inspected by fluorescence microscopy, and the labeled protein of FITC-BSA was used as the fluorescent indicator.It was observed in Fig.2A that the Ab-modified electrode presented an evident green FITC fluorescence, reflecting many labeled proteins were absorbed nonspecifically on the electrode surface.In contrast,the immunosensor with zwitterionic peptide anchoring showed nearly no green FITC fluorescence, as revealed in Fig.2B, illustrating a relatively clean biointerface without obvious nonspecific adsorption of labeled proteins.The optical monitoring verified that the PEC immunosensor had a favorable antifouling ability.
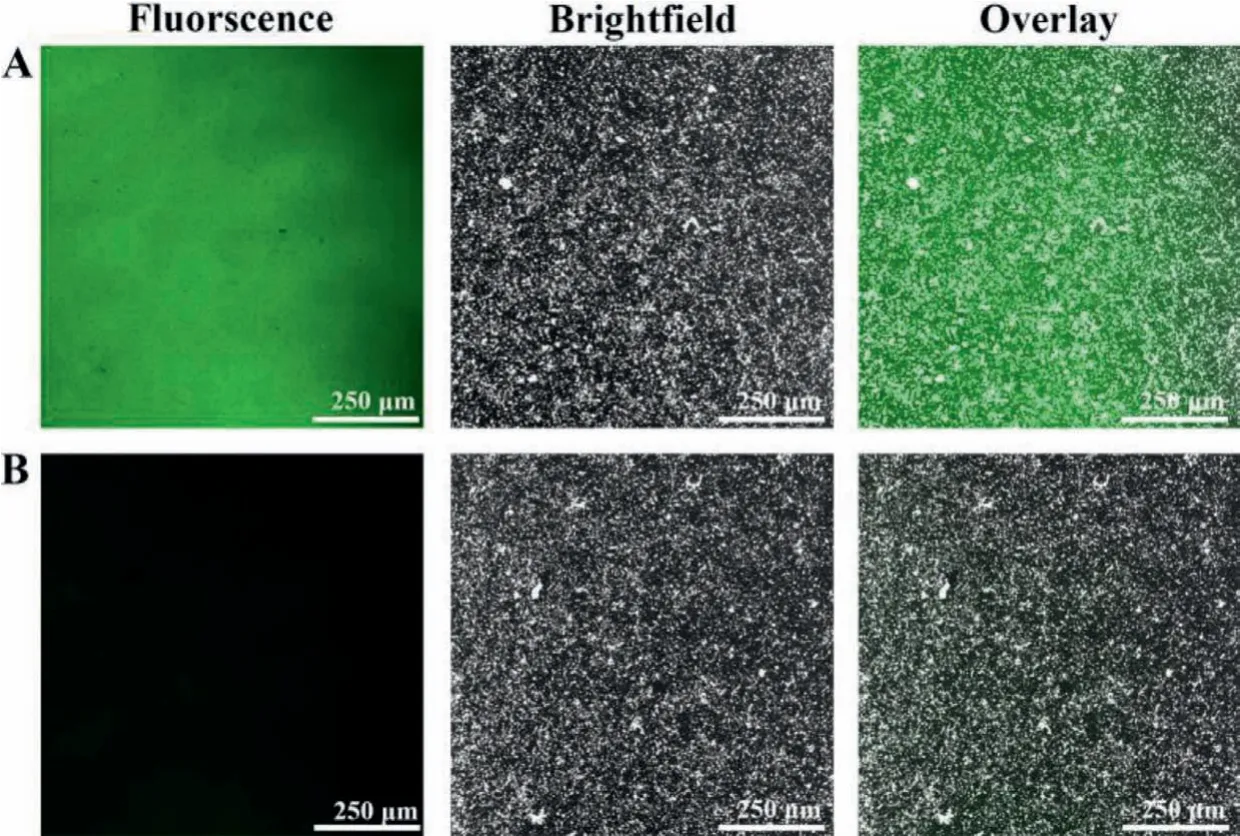
Fig.2 .Fluorescence images of the (A) Ab/Au/ZIS/CIS, and (B) ZP/Ab/Au/ZIS/CIS electrodes after with incubation of 2.0 mg/mL FITC-BSA.ZP denotes zwitterionic peptide.
The PEC detection of target cTnI depended on the change of the current signal produced by obvious steric hindrance of the formed immunocomplexes after the specific recognition of Ab with Ag.Fig.3A depicts current signal of the optimized PEC immunosensor against varied concentrations of target cTnI (See optimum conditions in Fig.S7 in Supporting information).As expected, the current signal weakened accordingly with increased concentration of cTnI, illustrating more captured target molecules on the immunosensor.Fig.3B exhibits calibration curve of the PEC immunosensor against target cTnI concentration range between 0.5 pg/mL and 10 ng/mL.The linear regression equation was experimentally obtained asI= –259.58 + 28.60logC(ng/mL), with 0.998 as the correlation coefficient.The limit of detection (LOD, S/N = 3)was estimated to be 0.20 pg/mL target cTnI, which was lower than or comparable to many sensitive cTnI biosensors reported previously [42–48].
To estimate antifouling performance of the immunosensor, the current outputs of the immunosensors with and without zwitterionic peptide anchoring were tested while they underwent incubation of the biological matrix of human serum sample.It was apparently observed in Fig.3C that the peptide-based immunosensor showed a minor current change rate than that without peptide anchoring, and meanwhile it also exhibited an excellent antifouling ability even to 10% diluted human serum (with the current change rate of 4.6%).The result hereby illustrated the potential utility of the PEC immunosensor to real biological specimens.
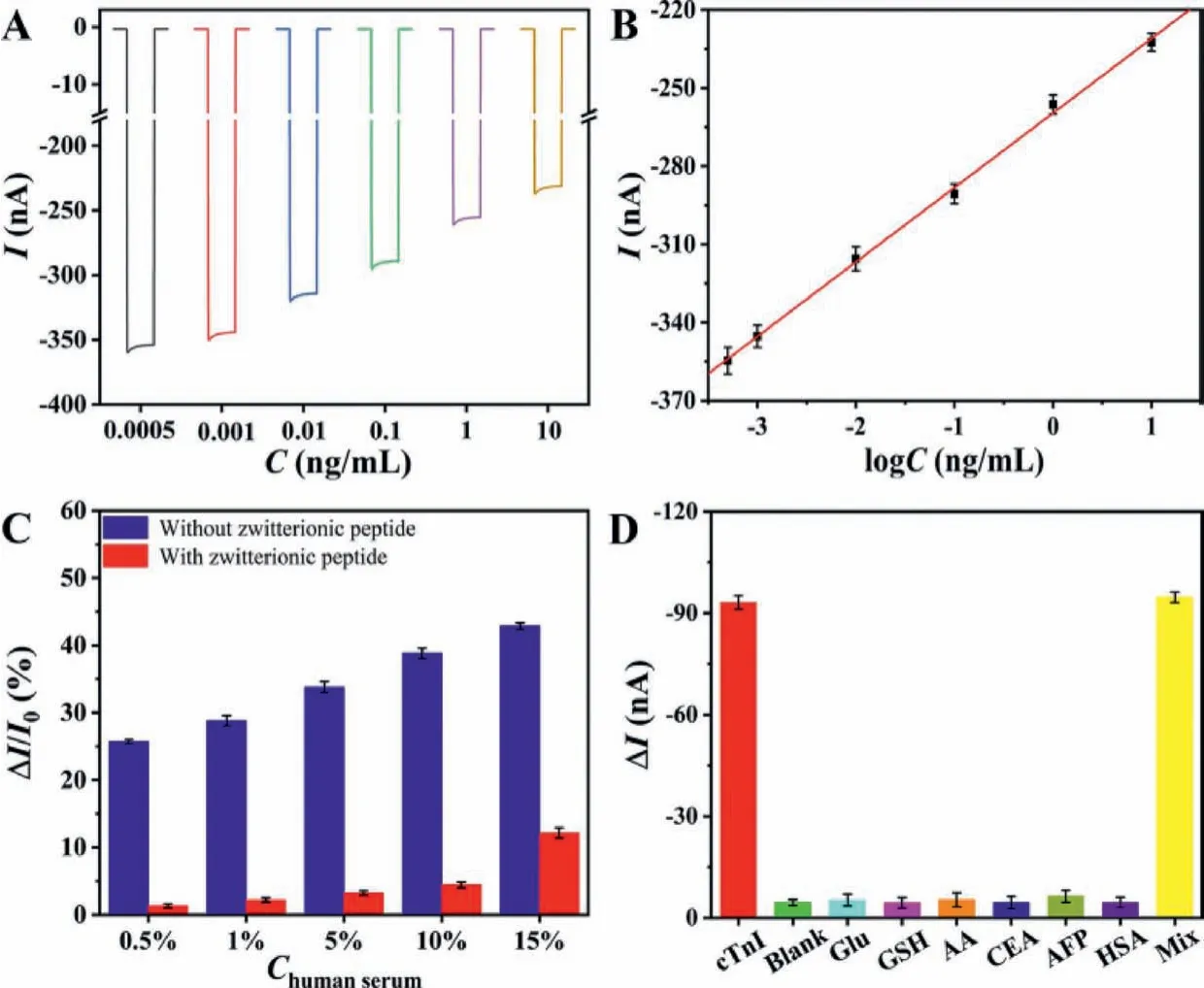
Fig.3 .(A) Current signal and (B) calibration curve of the PEC immunosensor to different concentrations of target cTnI; (C) current change rate (ΔI/I0) of the immunosensor with and without zwitterionic peptide after underwent incubation in a diluted human serum; (D) current change (ΔI) of the immunosensor against 0.1 ng/mL of cTnI,5 mmol/L of Glu, GSH, AA, 0.5 ng/mL of CEA, AFP, HSA, and the mixture of all (Mix). ΔI = I0 −I, I and I0 stand for current signal of the immunosensor after (I) and before(I0) incubation.
High specificity is of great importance for immunosensors to achieve accurate detection in complex biological matrixes.Since the common species like protein molecules and reductive agents usually co-exist in biological specimens, for interference test, some typical substances including Glu, GSH, AA, CEA, AFP and HSA were chosen to be added into 2% diluted human serum sample.As depicted in Fig.3D, the negligible changes in the current signals against both reductive agents and interfering proteins were clearly observed comparing with target cTnI detection.The result testified that the PEC immunosensor had a good specificity and was not affected by these possible interfering substances.
The reproducibility of the immunosensor was evaluatedviarelative standard deviation (RSD) of the current signals.Through testing five immunosensing electrodes that prepared separately, the current signals gave out RSD values of 3.3% and 3.8% against the detection of 100 pg/mL and 10 pg/mL target cTnI, illustrating acceptable reproducibility.The stability of the immunosensor was inspected by the change of the current signal.After ten days of storage in a refrigerator, the current signal of the immunosensor still kept above 95% of the initial response, indicating good stability.
The feasibility of the PEC immunosensor was assessedviaa recovery test on probing target cTnI in 5% diluted human serum.The result showed that the recovery rates of target cTnI with spiked concentrations of 20, 50, and 100 pg/mL were 103.8%, 95.4%, and 97.1%.Based on the acceptable outcome, potential practicability of the PEC immunosensor was verified.
In summary, a novel ternary heterojunction as photocathode and a zwitterionic peptide as antifouling biomaterial were integrated to construct an advanced PEC immunosensor.The Au/ZIS/CIS ternary heterojunction with porous network nanostructure projected a distinct current response without the assistance of bias potential, being qualified as the expected photocathode to anchor guest biomolecules.The zwitterionic peptide KAEAKAEAPPPPC presented electric neutrality and good hydrophilicity, which served an interfacial brush of the immunosensor to defense nonspecific protein adsorption.Integrating excellent PEC property of the heterojunction photocathode with robust antifouling ability of the zwitterionic peptide, the elaborated PEC immunosensor exhibited high sensitivity and good anti-interference for target biomarker of cTnI detection.Such a self-powered anti-interference PEC immunosensor offers a new thought for developing other powerful PEC biosensors capable of detecting in complex environments.
Ethical agreement
The blood samples from healthy adult donors were provided from the Eighth People’s Hospital of Qingdao (Qingdao, China), as well as the informed consent for use of the human blood was obtained.All sample preparations were approved by the Institutional Review Committee of relevant hospital and carried out in accordance with institutional guidelines and conformed to the relevant regulatory standards Ethical
Declaration of competing interest
We herein declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.We also declare that the manuscript has been read and approved by all authors named out (Ze Hu, Yaqun Xu, Hao Wang, Gao-Chao Fan, Xiliang Luo) and there are no other persons who satisfy the criteria for authorship but are not listed out.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Nos.22074073, 21275087), the Natural Science Foundation of Shandong Province of China (No.ZR2021YQ11), and the Taishan Scholar Program of Shandong Province of China (No.ts20110829).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.088.
杂志排行
Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
- Heterostructures of NiFe LDH hierarchically assembled on MoS2 nanosheets as high-efficiency electrocatalysts for overall water splitting
