Dual modulation of morphology and electronic structures of VN@C electrocatalyst by W doping for boosting hydrogen evolution reaction
2022-12-07DnyngHeLiyunCoLinglingFengShuinnLiYongqingFengGuodongLiYifeiZhngJinhnLiJinfengHung
Dnyng He, Liyun Co,∗, Lingling Feng,∗, Shuinn Li, Yongqing Feng,Guodong Li, Yifei Zhng, Jinhn Li, Jinfeng Hung,∗
a School of Materials Science & Engineering, International S&T Cooperation Foundation of Shaanxi Province, Xi’an Key Laboratory of Green Manufacture of Ceramic Materials, Shaanxi Key Laboratory of Green Preparation and Functionalization for Inorganic Materials, Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Ministry of Education, Shaanxi University of Science & Technology, Shaanxi 710021, China
b State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China
Keywords:Tungsten doping Vanadium nitride Graphitic carbon Electronic structure Hydrogen evolution
ABSTRACT Developing high-efficiency and robust durability electrocatalyst for hydrogen evolution reaction (HER) in water electrolysis functions as a crucial role for the construction of green hydrogen economy, herein,ultrafine W-doped vanadium nitride nanoparticles anchored on N-doped graphitic carbon framework(WVN@NGC) are synthesized through a one-step simple pyrolysis protocol.Owing to the enlarged catalytically active sites, enhanced electrical conductivity and optimized electronic structure, the resultant VN/WN@NGC delivered the prominent HER performance with overpotentials of 143 mV and 158 mV at 10 mA/cm2 in acid and alkaline media, respectively, accompanied by the long-term stability for at least 50 h.This work highlights a novel strategy for a metal-triggered modulation of nitride-based HER electrocatalyst for sustainable energy conversion device.
With the over-consumption of fossil fuels and ever-rising CO2emissions, bettering the energy structures to fulfill the carbon neutrality before 2060 has become an irresistible trendency and a global challenge [1–3].Renewable energy (e.g., solar, wind, hydropower), regarded as an alternative energy source, is severely hindered by its inherent intermittency, volatility and randomness, while hydrogen (H2) as an ecofriendly secondary energy carrier can be conveniently transferred into electricity and heat with high-efficiency and multiple pathways [4–6].To date, electrocatalytic water splitting can be functioned as a far more effi-cient and sustainable strategy to obtain the desired H2.Obviously,platinum (Pt)-based materials present brilliant in catalyzing hydrogen evolution reaction (HER), whereas soaring cost, scarce reserves, environment-hagardous and unsatisfactory stability significantly hamper the widespread commercialization [7,8].Accordingly, a great deal of endeavor has been devoted to seeking the Pt-free electrocatalysts with highly efficiency and robust durability for HER.
Nowadays, transition metal nitrides (TMNs) have an overwhelming superiority in candidates for electrocatalytic materials encompassing of Pt-like properties, favorable electrochemical stability, high corrosion resistance, melting point and conductivity[9–12].Notably, TMNs are regarded as interstitial compounds, in which nitrogen atoms are intercalated into the transition metal lattice to modulate the d-band state density and redistribute the charge density, thus forming a particular structure to boost electrochemical performance [13,14].Among them, vanadium nitride(VN) is known as an excellent catalyst owing to unique physical and chemical properties whereas its insufficient d electron density still renders it somewhat difficult to production of adsorbed hydrogen (Hads), resulting in the undesirable electrocatalytic HER performance [15].To further enhance the electrochemical properties of catalyst, the incorporation of heteroatoms with highly dband density of state was demonstrated to an effective approach to promote its intrinsic activity, which could optimize the electronic configuration to facilitate the water dissociation and adsorption processes [16–19].Tungsten (W), featuring with relatively substantial resources, flexible valence state and sufficient d electron density, can be served as fascinating dopant to tailor the morphology and electronic state of original compound, thereby favoring the electrochemical reactivity and stability of catalyst [20–22].In response to this, incorporating W atom into VN material is undoubtedly a promising tactic to modulate the electronic structure for improving the electrocatalytic HER performance, but the correlative research and mechanism involved in hydrogen production remained ambiguous and challenging.Moreover, nitrogen (N)-doped graphitic carbon materials are sharing the spotlight as electrocatalysts for HER due to their well-defined structure with large specific surface area, where the graphitic carbon plays an essential role in regulating the electronic and geometric characteristics of carbon substrate [23,24].Meaningfully, confining TMNs within N-doped graphitic carbon materials enable to fully expose catalytic active sites, smoothen electron transport, and strengthen structural stability, leading to the enhancement of HER properties of catalysts.
In this work, we hereby design a novel W-doped vanadium nitride anchored on N-doped graphitic carbon framework(WVN@NGC) electrocatalystviaa facile one-step calcination protocol.Impressively, the WVN required a quite low overpotential of 143 mV and 158 mV at a current density of 10 mA/cm2in 0.5 mol/L H2SO4and 1.0 mol/L KOH media respectively, and sustained long-term durability for over 50 h in continuous operation.This intriguing work represented a strategy for constructing groups of outstanding noble-metal-free nitrides electrocatalysts by incorporating metal atom in water electrolysis for hydrogen production.
As schematically depicted in Fig.1a, a novel W doped VN nanoparticles anchored on graphitic carbon framework was prepared by simple pyrolysis in Ar atmosphere (details in experimental section in Supporting information).The structural information of as-synthesized samples was estimated by X-ray diffraction(XRD) analysis.Fig.1b presented the diffraction patterns of VN@NC and WVN@NGC.All the characteristic peaks at 37.6°, 43.7°, 63.5°and 76.2° that assigned to (111), (200), (220) and (311) planes of cubic VN (JCPDS No.35–0768) were observed in as-prepared catalysts, affirming the purity and successful preparation of the desired VN phase.Note that, the patterns of WVN@NGC-900 underwent a slightly positive deviation in comparison with VN@NC-900, which indicated W atom was incorporated into the lattice of VN material.Besides, the phase compositions of contrast samples with different W contents and pyrolysis temperatures were depicted in Figs.S1 and S2 (Supporting information).Notably, when melamine is used as nitrogen source, V2O3(PDF #34–0178) impurity still exists in the prepared sample, making it impossible to produce pure VN.Therefore, pure VN was synthesized successfully and employed CO(NH2)2as nitrogen source for comparison, which demonstrated that W-doping was beneficial to the formation of VN in the WVN@NGC-900 synthesis process (Fig.S3 in Supporting information).Furthermore, Raman spectra of as-synthesized catalysts were employed to evaluate the carbon structure feature (Fig.1c).The WVN@NGC-900 contained two visible peaks positioned at 1349 cm−1and 1596 cm−1, which attributed to D band and G band[25], representing the structural disorder and the degree of sp2hybridization respectively, accompanying with the intensity ratio value (ID/IG) of 0.86.Evidently, the high graphitization endowed WVN@NGC-900 with enhanced conductivity, thereby accelerating the electron transfer rate and leading to the boost of HER kinetics during the electrocatalytic process.
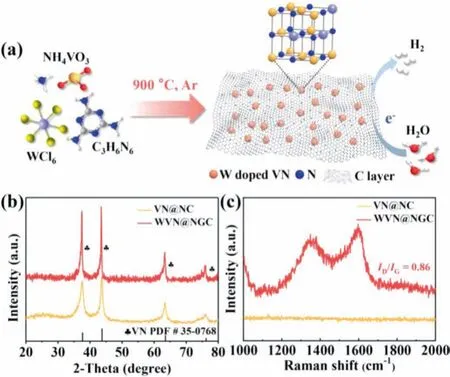
Fig.1 .(a) Schematic representation of WVN@NGC-900 synthesis.(b) XRD patterns.(c) Raman spectra of VN@NC-900 and WVN@NGC-900 electrocatalysts.
In this work, the doped tungsten served a vital role in the formation of W-doped vanadium nitride anchored on N-doped graphitic carbon framework (WVN@NGC) during the pyrolysis process.It is worth noting that W can not react with N, as confirmed in Fig.1b, and the W-doping acts as the blocking agent to inhibit the aggregation of VN particles, downsizing and dispersing them in the carbon layer.Meanwhile, the W-doping facilitates the crystallization of carbon as the support (see Raman spectra of VN@NC-900 and WVN@NGC-900 in Fig.1c), which in turns quickly wrap VN nanoparticle, resulting in ultrafine and highly dispersed VN nanoparticles confined into the carbon layer.
To investigate the technological conditions influences on modulating the final morphology and structure of as-fabricated samples,the scanning electron microscope (SEM) and the transmission electron microscope (TEM) observations were shown in Fig.2.As revealed in Fig.S4 (Supporting information), the WVN@NGC-900 was composed of uniformly rich ultrathin N doped carbon layer with high dispersivity.In contrast, the FESEM distinctly indicated that VN@NC-900 was comprised of aggregated lumpy particles (Figs.S5 and S6 in Supporting information).Evidently, Fig.2a demonstrated that a large number of nanoparticles in WVN@NGC-900 were located homogeneously in wrinkled N doped carbon nanosheets.The high-resolution TEM (HRTEM) image of WVN@NGC-900 exhibited interplanar spacings with 0.21 nm and 0.24 nm (Figs.2b and c),belonging to the (200) and (111) crystallographic planes respectively of cubic VN, which was in well accordance with the XRD result.It was noteworthy that in the resultant WVN@NGC-900 with crystalline ultrafine nanoparticles in a size of approximately 2 nm, confining evenly in amorphous N-doped graphitic carbon.For comparison, the morphologies of WVN@NGC with different W amounts and calcination temperature were also studied, as displayed in Figs.S7-S10 (Supporting information).It was found that the VN nanoparticles in WVN@NGC-900 had the similar size of 2–3 nm, which is smaller than that of WVN@NGC-800 (3–5 nm) and WVN@NGC-1000 (4–5 nm), respectively.Moreover, the X-ray energy dispersive spectroscopy (EDS) mappings elucidated the uniform distribution of C, V, N and W elements in the WVN@NGC-900 catalyst (Figs.2d-h).
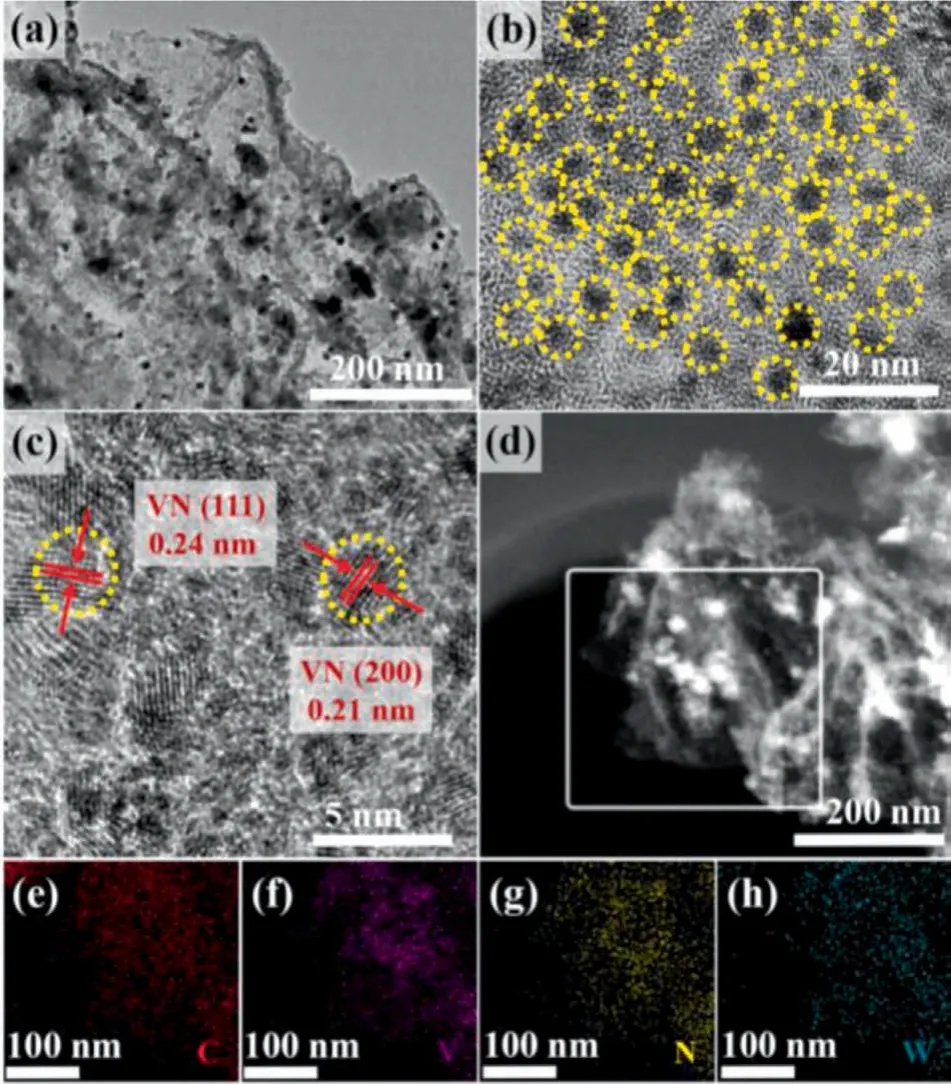
Fig.2 .Morphology characterization of WVN@NGC-900.(a) TEM image, (b, c)HRTEM image, (d) scanning transmission electron microscopy (STEM) image of WVN@NGC-900.(e-h) Energy dispersive spectrometry (EDS) mappings of N, V, C and W elements for WVN@NGC-900.
To get a deeper understanding of electronic interplay derived from chemical components and bonding configuration, X-ray photoelectron spectroscopy (XPS) of as-prepared catalysts was performed in Fig.3.The XPS spectra of WVN@NGC samples elucidated the presence of C, N, V and W elements, in line with the EDS analysis results.The high-resolution XPS spectra of C 1s in WVN@NGC samples can be resolved into four obvious peaks of C−W (283.0 eV), C−C (284.6 eV), C−N (285.2 eV) and C−O (287.6 eV) bonds(Fig.3a), which revealed the successful doping of W atom in carbon support [26].Fig.3b displayed that the four well-fitted peaks of N element focusing on 396.3 eV, 397.0 eV, 398.7 eV and 400.7 eV assigned to metal-N, pyridinic-N, pyrrolic-N and graphitic-N in WVN@NGC-900, respectively [27,28].In terms of N 1s, the corresponding peaks in WVN@NGC-900 exhibited negative shift in comparison with VN@NC-900, indicating N atom functioned as electron acceptor influenced by W doping.In addition, different types of nitrogen content were demonstrated in Table S1 (Supporting information), in which the atomic proportion of graphitic N in WVN@NGC-900 (46.68%) was much higher than VN@NC-900(6.45%), suggesting the W incorporation helped speed up electrons transfer, further boosting kinetics of WVN@NGC-900 in electrochemical process.In the V 2p spectra of WVN@NGC-900 (Fig.3c),the peaks centered at 513.8 eV, 516.0 eV and 517.3 eV were allocated to V−N, V−O−N and V−O respectively, along with three shakeup satellite peaks at 521.40 eV, 523.96 eV and 525.28 eV,which shifted towards lower binding energy relative to VN@NC-900 as a whole [29,30].As depicted in Fig.3d, the three pair of deconvoluted peaks of W 4f can be ascribed to W −C (32.3 eV, 34.3 eV), W−N (33.0 eV, 35.5 eV) and W−O (37.7 eV, 40.1 eV) in WVN@NGC-900 respectively [31,32].In view of the abovementioned results, W served as an electron donator to transfer electrons to electron acceptors of N and V sites, effectively modulating the electronic structure in WVN@NGC-900.In order to elaborate electronic interaction of WVN@NGC, the atomic orbitals of W−N−V model were adopted to analyze in Fig.3e.Admittedly, the electron configuration of W and V atoms are 3d34s2and 5d46s2respectively, which suggests the higher d electron density of W atom than V atom.Nevertheless, VN sample possessing of insufficient d electron density extremely impeded the formation of adsorbed hydrogen (Hads) on the surface of VN electrode.Accordingly, the incorporation of W atom featuring with sufficient d electron density can provide more electrons to VN and compensate the intrinsic deficiency of the VN, thus modulating the electronic configuration of VN, boosting the Hadsproduction and H2release, and further optimizing the hydrogen binding energy to conform the Sabatier principles [33,34].Given above this, the incorporation of W atom could efficiently regulate the charge/electron redistribution and optimize the electronic structure in WVN@NGC catalyst, thus enhancing their electrocatalytic HER performance.
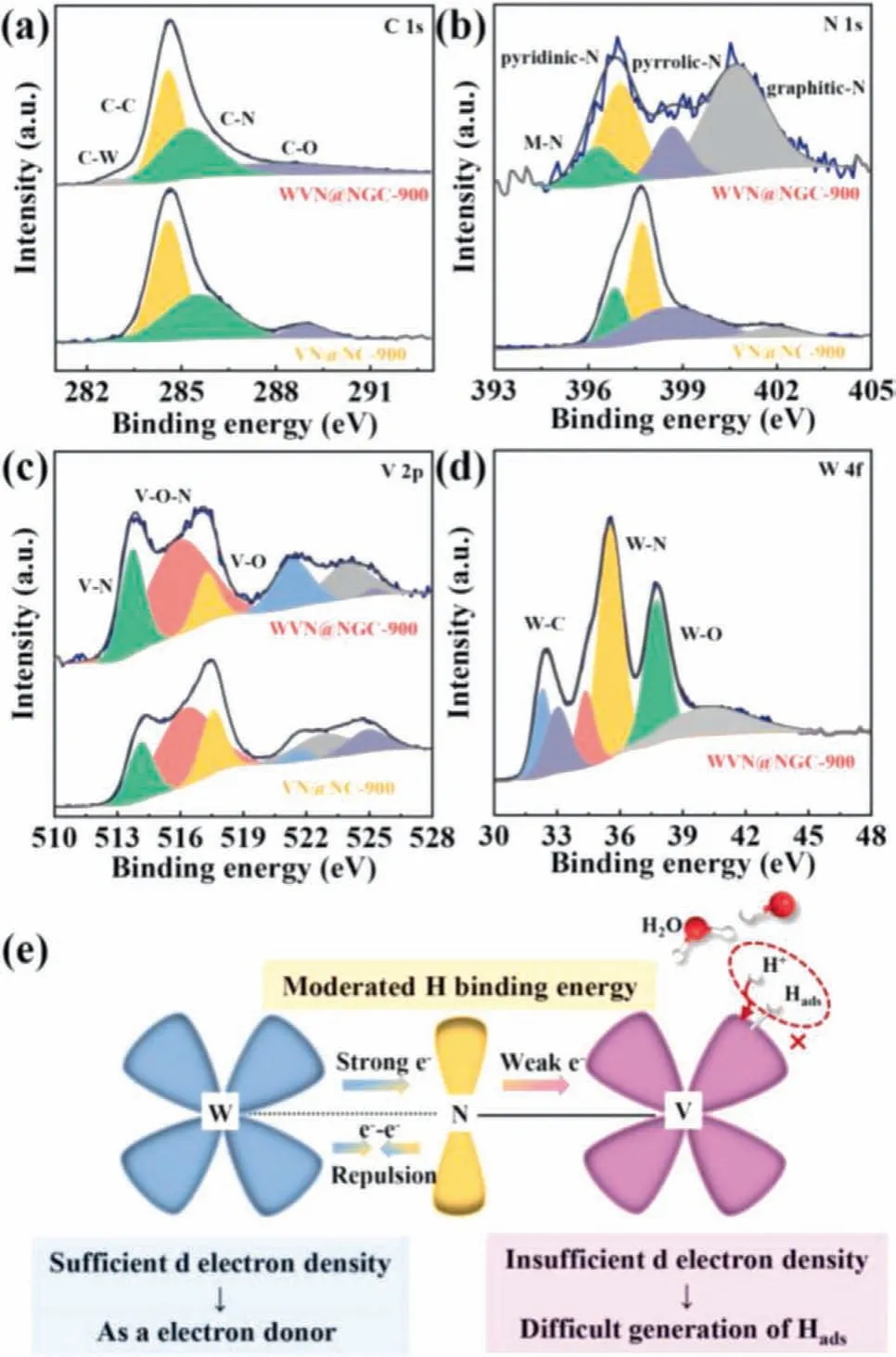
Fig.3 .XPS spectra of VN@NC-900 and WVN@NGC-900.(a) C 1s; (b) N 1s; (c) V 2p; (d) W 4f.(e) Schematic representation of the electronic interaction among W,N and V of WVN@NGC-900.
The electrocatalytic HER performance of as-fabricated VN@NC,WVN@NGC, bare GCE and 25% Pt/C samples was explored in acid and alkaline electrolytes employing conventional three-electrode configuration.To evaluate the influences of various related factors on electrochemical properties, the amounts of tungsten sources and the pyrolysis temperatures as key indicators were to analyze the catalytic activity in 0.5 mol/L H2SO4and 1.0 mol/L KOH media respectively (Figs.S11 and S12 in Supporting information).Figs.4a and e displayed the linear sweep voltammetry (LSV) curves of all the samples.Notably, WVN@NGC-900 required only the overpotentials of 143 mV and 158 mV to achieve a current density of 10 mA/cm2in 0.5 mol/L H2SO4and 1.0 mol/L KOH media, respectively, outperforming the corresponding VN@NC-900 (361 mV)and VN@NC-900 (460 mV).The experimental results demonstrated that the incorporation of W into VN@NC could greatly enhance the electrocatalytic HER activity.In addition, the electrocatalytic HER performance over the resultant WVN@NGC-900 was better than that of the most of VN-based materials, as shown in Table S2(Supporting information).Furthermore, the linear portion of Tafel slope derived from LSV curves was carried out to assess the behavior of reaction kinetics for as-prepared catalysts, as demonstrated in Figs.4b and f.The WVN@NGC-900 displayed smaller numerical value (acid medium: 84 mV/dec, alkaline medium: 50 mV/dec) of Tafel slope compared with VN@NC-900 (acid medium:410 mV/dec, alkaline medium: 188 mV/dec), revealing the Volmer-Heyrovsky mechanism was regarded as the predominant step of WVN@NGC-900 sample [35].To further analyze HER kinetics of synthetic samples during electrochemical process, the electrochemical impedance spectroscopy (EIS) test was performed in Figs.4c and g.The charge transfer resistance (Rct) for WVN@NGC-900 was lower than that of VN@NC-900 in both acid and alkaline solutions, which implied W dopant was quite advantageous to expedite electron transmission and favor HER kinetics.Furthermore, the equivalent circuit diagram and corresponding resistance were illustrated in the inset of Figs.4c and g and Tables S3, S4 (Supporting information).By ascertaining the prominent HER electrocatalytic activity of WVN@NGC-900 ultrafine nanostructure, the doublelayer capacitance (Cdl) of as-fabricated catalysts was to calculated to estimate the electrochemically active area (ECSA).As we all know, the ECSA was proportionate toCdlvalue, namely the half of slope for as-synthesized catalysts, acquired from cyclic voltammetry (CV) at different scanning rates (Figs.S13 and S14 in Supporting information).As expected, theCdlvalues of WVN@NGC-900 (pH 0:31 mF/cm2, pH 14: 143 mF/cm2) was larger than that of VN@NC-900 (pH 0: 16 mF/cm2, pH 14: 63 mF/cm2), which indicated the largest catalytically active sites of WVN@NGC-900 that boosting the electrocatalytic activity toward HER.To evaluate the durability of WVN@NGC-900, the time-dependent curves were measured, as shown in Figs.4d and h.Evidently, the WVN@NGC-900 could sustain robust electrocatalytic hydrogen evolution with negligible decay for 50 h consecutive operation in both acid and alkaline electrolytes, confirming the superior durability in HER electrochemical process.After 50 h long-termi-tmeasurement in both media,the microstructure and components of WVN@NGC-900 electrocatalyst maintained basically intact, certifying the excellent structural robustness throughout the electrochemical test in both 0.5 mol/L H2SO4and 1.0 mol/L KOH conditions (Figs.S15-S18 in Supporting information).
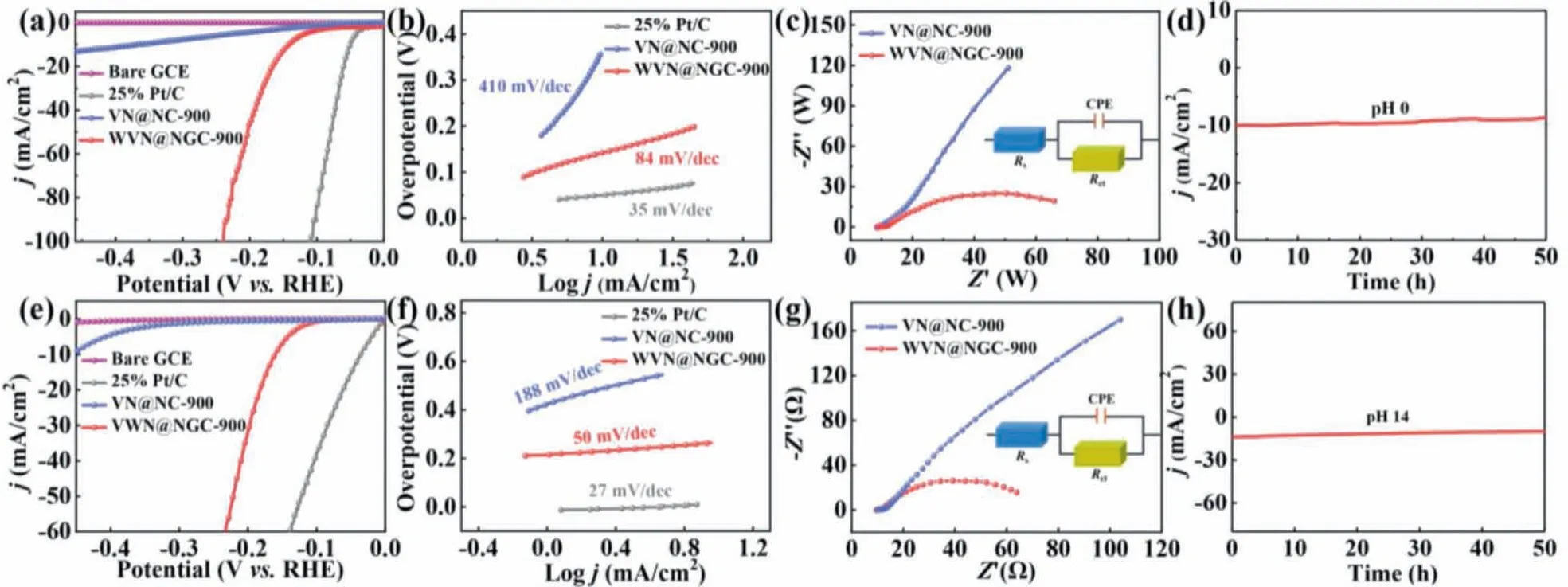
Fig.4 .LSV polarization curves of WVN@NGC-900 and the control samples in (a) 0.5 mol/L H2SO4 and (e) 1.0 mol/L KOH solutions; the corresponding Tafel plots in (b) 0.5 mol/L H2SO4 and (f) 1.0 mol/L KOH solutions.Nyquist plots of VN@NC-900 and WVN@NGC-900 in (c) 0.5 mol/L H2SO4 and (g) 1.0 mol/L KOH solutions.Chronoamperometry(i-t) curve of WVN@NGC-900 in (d) 0.5 mol/L H2SO4 and (h) 1.0 mol/L KOH solutions.
In summary, we successfully constructed the W-doped vanadium nitride anchored on graphitic carbon framework(WVN@NGC)viafacile one-step pyrolysis approach.Notably, the WVN@NGC catalyst showed fantastic electrocatalytic HER performance in 0.5 mol/L H2SO4and 1.0 mol/L KOH media.Such results were primarily ascribed to the following three aspects:i) Highly dispersed ultrafine nanostructures exposed abundant catalytic active sites for accelerating the penetration of electrolyte and releasing H2produced on the electrocatalyst surface;ii) the upgraded electrical conductivity of graphitic carbon in WVN@NGC facilitated the electron transport and charge transfer during HER process; iii) the incorporation of W atom modulating the electronic configuration of WVN@NGC, facilitating the rapid adsorption/desorption of HER intermediates.This work affords a rational strategy to design massive highly-effective and robust carbon-based electrocatalysts by metal doping toward HER in future energy conversion devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos.22179074, 52073166, 52072226), Xi′an Key Laboratory of Green Manufacture of Ceramic Materials Foundation (No.2019220214SYS017CG039), Key Program for International S&T Cooperation Projects of Shaanxi Province (Nos.2020KW-038, 2020GHJD-04), Science and Technology Program of Xi’an,China (No.2020KJRC0009), Scientific Research Program Funded by Shaanxi Provincial Education Department (No.20JY001), Science and Technology Resource Sharing Platform of Shaanxi Province(No.2020PT-022), Science and Technology Plan of Weiyang District, Xi’an (No.202009), Fund of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (No.2021–14) and Open Project of Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Ministry of Education (No.KFKT2020-06).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.006.
杂志排行
Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
