Glossopterids survived end-Permian mass extinction in North Hemisphere
2022-12-07ZHANGYiZHENGShaolinKamalSinghWANGYongdong
ZHANG Yi, ZHENG Shaolin, Kamal J. Singh, WANG Yongdong,
ZHANG Shanzhen6 and Anju Saxena4
1. College of Paleontology, Shenyang Normal University, Shenyang 110034, China;
2. Key Laboratory for Evolution of Past Life in Northeast Asia, Ministry of Natural Resources, Shenyang 110034,China;
3. Shenyang Center of Geological Survey, China Geological Survey, Shenyang 110034, China;
4. Birbal Sahni Institute of Palaeobotany, Lucknow 226007, India;
5. State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China;
6. Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China
Abstract: Recently, more attention has been paid to Glossopteris, the most significant representative fossil of the Gondwanan Supercontinent in the Paleozoic. It has been regarded as an important clade of Angiophytes on the basis of its reproductive organ related to Angiosperms. Since Brongniart erected Glossopteris in 1928,reliable Glossopterids attached by fertile organs were only collected from the Permian Gondwanaland. Here,the authors found a new element of Glossoptetids, Sinoglossa sunii gen et sp. nov., with attached female organs from the Middle Triassic Linjia Formation in Benxi, Northeast China. This demonstrates that Glossoptetids not only distributed in South Hemisphere, but also in North Hemisphere, and successfully survived the end-Permian mass extinction in North Hemisphere. The distinguished environment in Northeast China influenced by both warm and cold currents, probably resulted in the Paleozoic relic elements, such as Glossopterids associated with Lobatannularia successfully survived the end-Permian mass extinction.
Keywords: Glossopterids; Sinoglossa sunii; Northeast China; Pangea; Middle Triassic; end-Permian mass extinction
Introduction
The origin of angiosperms as an abominable mystery addressed by Charles Darwin has been focused by paleobotanists for a long time (Sunet al.,1998; Herendeenet al., 2017; Liet al., 2019; Shiet al., 2021). Recently, Shiet al. (2021) suggested that all the Corystosperms,Glossopteris,Caytoniaand Angiosperms form a clade of Angiophytes based on the cladistic analysis (Shiet al., 2021). Due to the new understanding of the implication of Glossopterids,increasing attention has been paid toGlossopteris(Shiet al., 2021; Tayloret al., 2009).
As a typical element of Gondwanan flora,Glossopterisis one of the key evidences for the Gondwana Supercontinent (Tayloret al., 2009). Since Brongniart erectedGlossopterisin 1928, reliable Glossopterids with female and male organs were only collected from the Permian deposits in Gondwanaland (Tayloret al.,2009; Retallack & Dilcher, 1981; McLoughlin, 2011;Cariglino, 2015), although some fossil specimens were described without the fertile organs from the Triassic of both North and South Hemisphere (Tayloret al.,2009; McLoughlin, 2011). The specimens of Glossopterids from North Hemisphere have not been regarded as reliable evidence due to the lack of attached fertile organs and associated important genus,Vertebraria(McLoughlin, 2011).
Pangea was a global supercontinent from Permian to Triassic. The distribution of Glossopterids was only limited in South Hemisphere. Their distribution in North Hemisphere is still poorly known and debated.In order to settle the problem whether Glossopterids can be distributed in North Hemisphere or not, we select the Middle Triassic Linjia Formation as our reinvestigated stratum. This is because Zhanget al.(1983) reported that the plant fossils from the Middle Triassic Linjia Formation in Linjiawaizi of Benxi,East Liaoning, Northeast China (Fig.1) are very complicated in time and space distribution.

Fig.1 Location of the study area occuring Sinoglossa sunii Zhang et al. gen. et sp. nov.
2 Materials and method
The Linjia Formation is only distributed in the Linjiawaizi and surrounding regions of Benxi, eastern Liaoning Province of the North China (Fig.1a-d).The formation is about 158.4 m thick. The lower part predominately consists of yellow-green, gray-white conglomerates and cross-bedded sandstones, while the upper part is composed of yellow-green feldspathic quartzose sandstones and purple or black shales. The Linjia Formation unconformably overlies the Lower Triassic Zhengjia Formation (=Hongla Formation)(Fig.1e). The overlying strata of the Linjia Formation are unexposed in this locality.
The age of the Linjia Formation is assigned to the Middle Triassic based on the comprehensive evidence from zircon dating age (Zhanget al., 2019) combined with stratigraphic correlation, lithologic characters, as well as parallel unconformity, typical Triassic fossil plants includingPleuromeia, and the fossil insect ofSogdoblatta(Zhanget al., 2019, 2020).The dating results of 30 grains of zircon crystal, collected from the grey-white metamorphic tuffaceous sandstone in the base of the Linjia Formation (Fig.1f), show that the average206Pb/238U age of 4 newest zircons was 239.5±4.1 Ma. All specimens were collected from Layer 4 in the Linjia Formation of Qiandianzi,about 750 meters southwest of Linjiaweizi village(123°41′3.24″E, 41°16′24.18″N). The specimens are housed in PMOL (Paleontology Museum of Liaoning)and were studied and photographed with the digital camera Canon A700 and the VEX-5000 microscope.
3 Systematic paleobotany
Glossopteridales
Lidgettoniaceae
GenusSinoglossagen. nov.
Type species:Sinoglossa suniiZhanget al.gen. et sp. nov.(Figs.2-30)
Diagnosis: Fertile ultimate branch slender, attached by scale leaves and young branches with a whorllike arrangement; Both ultimate and young branches showing theVertebrariastructure in both cross and longitudinal sections; Scale leaf simple, linear, petiolate, with an entire margin, cuneate base, and probably an obtuse apex; Midrib strong, persisting to the apex; Lateral veins bifurcating and anastomosing to form narrow, elongate reticulate meshes; Several cupules each with a strong and short pedicel attached to the abaxial midrib; Each cupule with six linear lobes;Seed broadly ovate, platyspermic, with wide furrows in the nucule.
Sinoglossa suniiZhanget algen. et sp. nov.(Figs.2-30)
Etymology: The prefix of the genus name,“Sino-”,represents China, while the suffix, “glossa” represents glossopterids. The specific epithet name is given in honor of Prof. Sun Ge, Dean of College of Paleontology, Shenyang Normal University, who supports this research work.
Holotype: PMOL–B01320A/B;Paratype: PMOL–B01321A/B, B01322, B01323A/B, B01325
Occurrence: Qiandianzi of Benxi, East Liaoning,Northeast China; Linjia Formation (Middle Triassic).Diagnosis: same as the genus.
Description: Specimens include a fertile ultimate branch attached by a scale leaf developing three cupules (Fig.2a-f, 2k; Figs.3-16, 18), a suggested fertile ultimate branch (Figs.19-27) and an apex of leaf(Fig.2i-j, Fig.17), a detached cupule (Fig.2g, Fig.28),and a detached seed (Fig.2h, Fig.29).
The specimen No. PMOL–B01320A represents a fertile slender ultimate branch, attaining a width of 3.5–4 mm, attached by a scale leave, several petioles of leaves and one young branch. The young branch is more slender, up to 1.5–2 mm thick, attached to the ultimate branch with a narrow angle. Both petioles of the scale leaf and young branch are arranged in whorls on the ultimate branch (Fig.2a-d, Figs.3-8).Petioles of the young leaf are attached to the young branch and also show a whorl-like arrangement (Figs.5-8). The cross section shows theVertebraria-type structure developing in the present specimen (Figs.5-8). Six rigid septa are clearly developed within both the ultimate and young branches. A central vascular bundle is enclosed by a sheath and is preserved in the centers of the ultimate and young branches, attaining about 1 mm and 0.3–0.4 mm in diameter, respectively(Figs.5-8). Longitudinal and transverse grooves(Figs.5-6, GL and GT) develop in the surface of both ultimate and young branches. Ridge septa (Figs.5-6,RSP) inside the ultimate and young branch, correspond to longitudinal grooves on the surface of the ultimate and young branch respectively (Figs 5-6).
Scale leaf in the specimen No. PMOL–B01320A is simple, linear, up to 8.5 cm long and 1.4 cm wide,with an entire margin, a cuneate base and a marked petiole (Fig.2a-d). Transverse grooves are clearly shown in the petiole, while longitudinal grooves are unclear (Figs.5-6). Midrib is distinct, broad, about 2 mm in width, persisting to the apical part, with a strong vascular bundle with 0.8–1 mm in thickness (Fig.2a-d,Figs.9-16). Lateral veins are very fine in preservation and numerous in number, emerging from the midrib at acute angle of 10º–15º and passing laterally to the margin at an angle of 30º–40º. They repeatedly bifurcate and anastomose, forming oblique, narrow,and elongate meshes, with an average concentration of meshes about 70–80 per cm2(Fig.2k, Fig.18). Three ovoid cupules are attached to the abaxial midrib by their short pedicels (Fig.2a-d, Figs.3-16). Pedicels are strong, 6–8 mm long and 2 mm wide. The distance between the point of the lowest cupule attached to the scale leaf and the point of the scale leaf attached to the branch is about 17.5 mm (Fig.3).

Fig.18 Middle part of a Sinoglossa scale leaf (PMOL–B01320A, enlargement of Fig.2k, secondary veins bifurcating and anastomosing, arrows)
The specimen No. PMOL–B01322 shows a detached cupule probably developing six linear lobes(Fig.2g, Fig.28), while the specimen No. PMOL–B01323A, B shows a detached seed, broadly ovate,platyspermic, with a length of 5 mm and a width of 4.5 mm; lateral wings with almost uniform breadth extending from the micropyle end to the chalaza end;nucule broadly ovate, with a wide furrow from the cordate base to the apical part (Fig.2h, Fig.29).
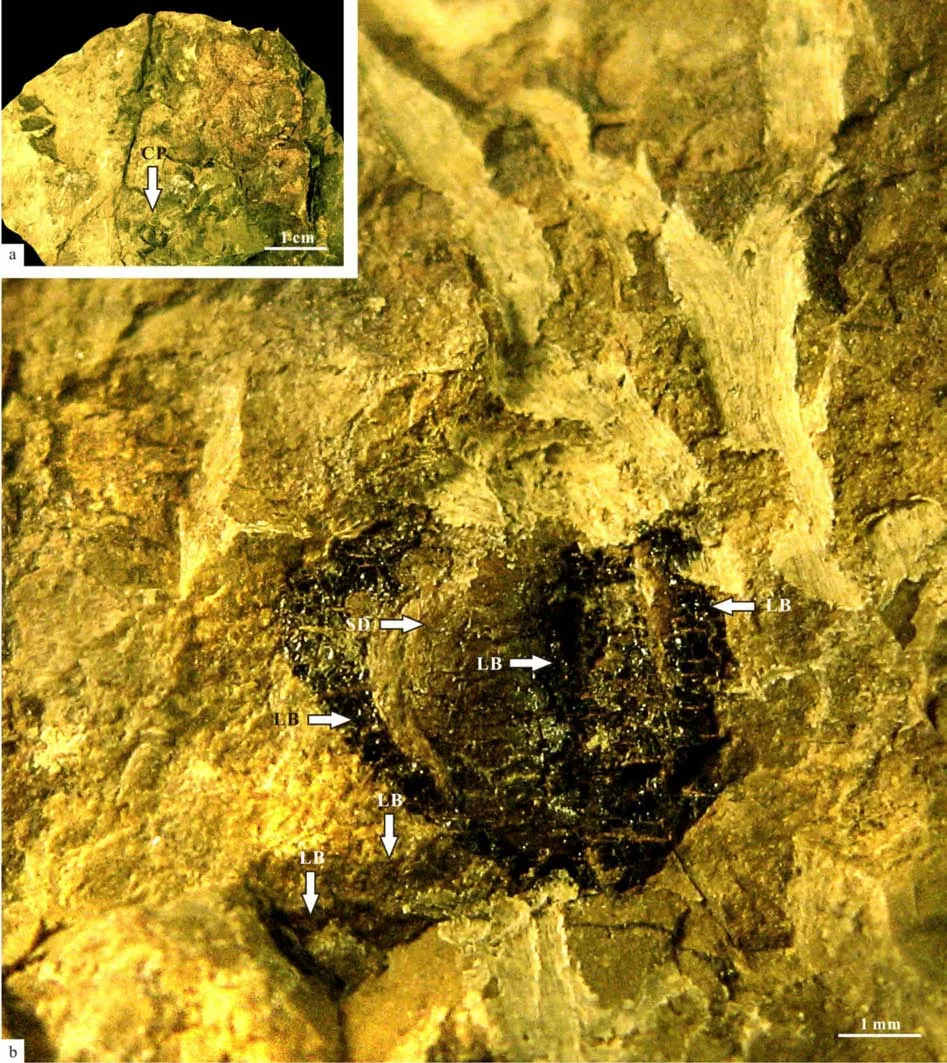
Fig.28 A detached cupule of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01322)

Fig.29 A detached seed of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01323A, B)
The specimen No. PMOL–B01325 probably represents the apex of the scale leaf, with an obtuse apex, 1 cm wide and 4.5 cm long (Fig.2i,j, Fig.17),similar to the commonGlossopterisleaf. Midrib is distinct, broad, up to 2 mm in width, persisting to apex (Fig.2i,j, Fig.17). Lateral veins are very fine and numerous in number, emerging from midrib at an acute angle of 10º–15º and passing laterally to margin at an angle of 30º–40º, repeatedly bifurcating and anastomosing, to form oblique, narrow, elongate meshes. The average concentration of meshes is also about 70-80 per cm2(Fig.2j, Fig.17).
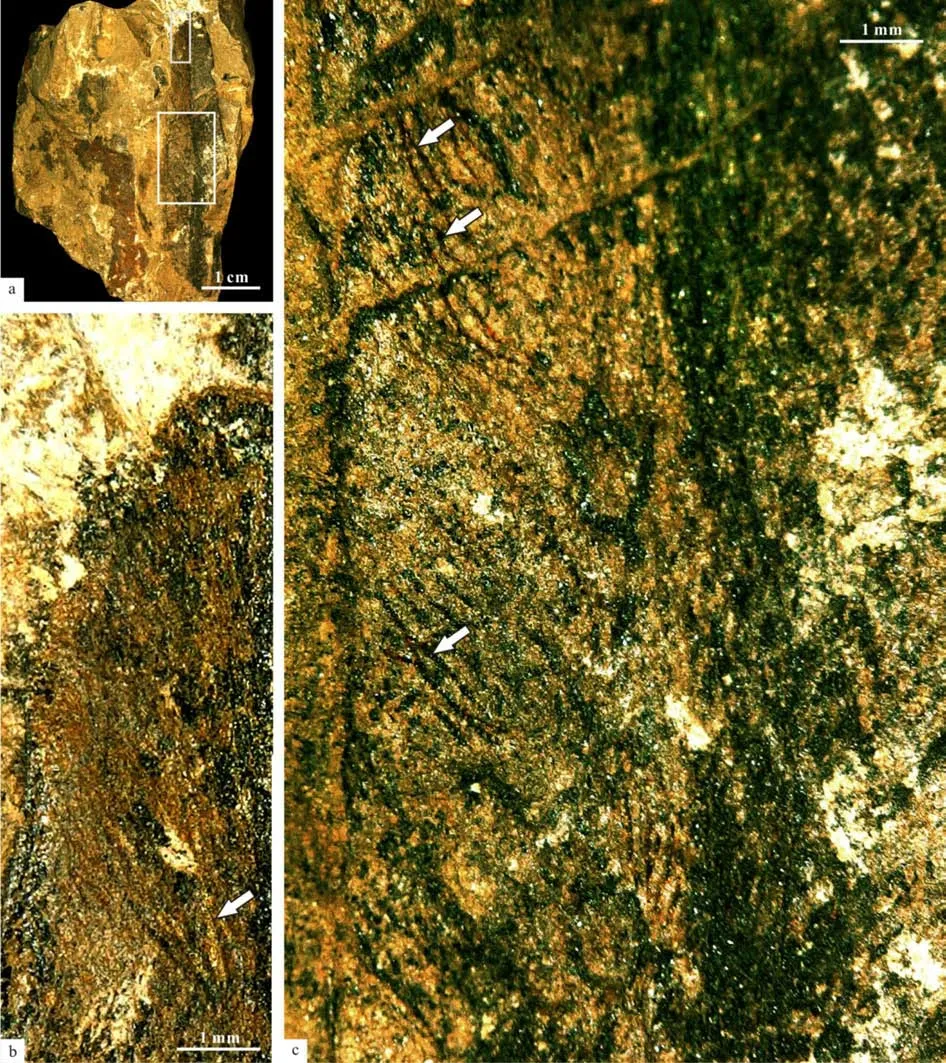
Fig.17 Apex of a Glossopteris leaf, with bifurcating and anastomosing veins, similar to those of scale leaf (No. PMOL–B01325)
The specimens No. PMOL–B01321A, B show two leaves attached to an ultimate branch with aVertebrariastructure, and are 3–3.5 mm wide, associated with a cupule abaxially attached to the scale leaf and its detached seed (Figs.19-27). The distance between the point of the cupule attached to the scale leaf and the point of the scale leaf attached to the branch is also about 17.5 mm (Fig.20), almost the same as the specimen No. PMOL–B01320A in Fig.3.

Fig.2 Sinoglossa sunii Zhang et al. gen et sp. nov.

Fig.3 Specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, whole branch with a scale leaf attached by 3 cupules. One of cupules is unclear)

Fig.4 Counterpart specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320B, whole branch with a scale leaf attached by 3 seeds. One of seeds is unclear)

Fig.7 Specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, whole branch with a scale leaf attached by a cupule)

Fig.8 Reconstruction of whole branch with a scale leaf attached by a cupule of Sinoglossa sunii Zhang et al. sp. nov. (No.PMOL–B01320A, whole branch with a scale leaf attached by a cupule)

Fig.9 Specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, three cupules in detail)

Fig.10 Specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, suggested complete structure of the scale leaf with 3 attached cupules by dot lines)

Fig.11 Part specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, central vascular bundle through the centers of midrib and pedicel)

Fig.12 Reconstruction of scale leaf with three cupules of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A)

Fig.13 Counterpart specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320B, three cupules in detail)

Fig.15 Counterpart specimen of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320B, central vascular bundle through the centers of midrib and pedicel)

Fig.16 Reconstruction of scale leaf with three cupules of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320B)
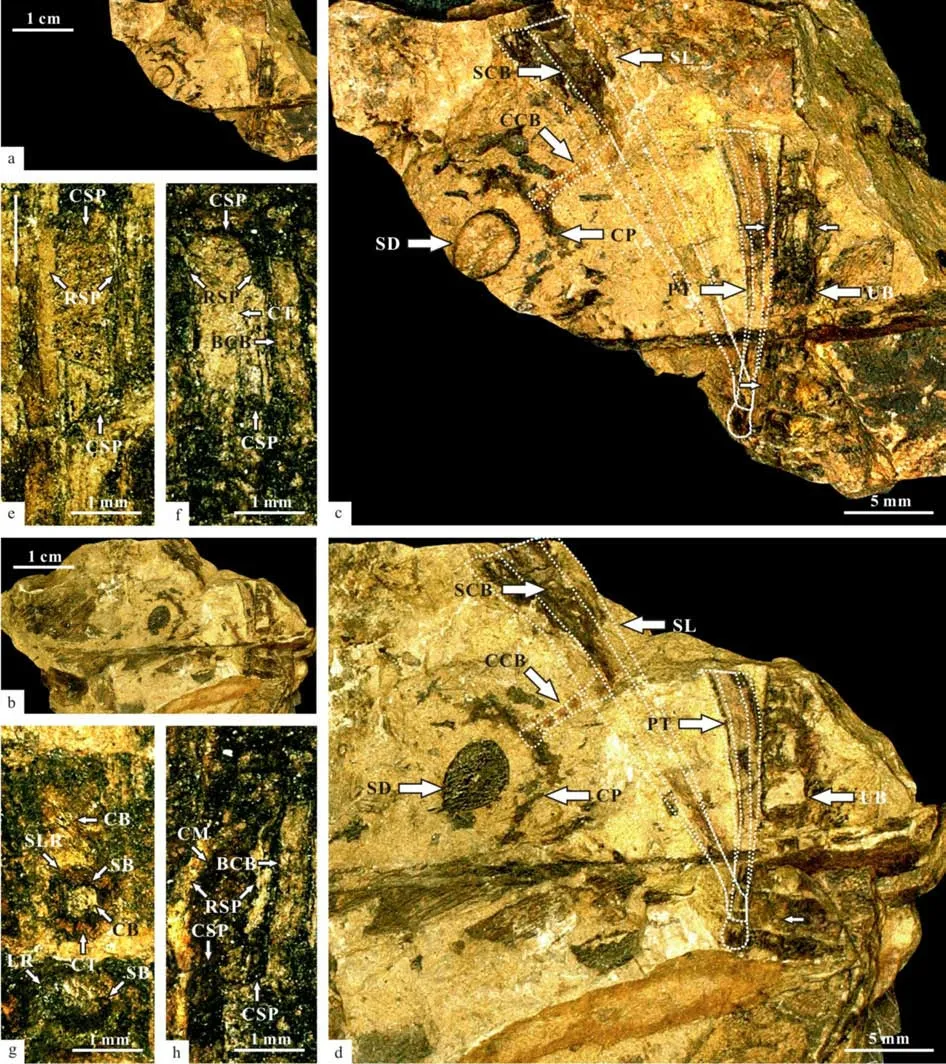
Fig.19 Suggested scale leaves attached to a branch of Sinoglossa sunii Zhang et al. gen et sp. nov.

Fig.20 A leaf attached to a branch showing the Vertebraria structure, associated with a cupule attached to a scale leaf and a detached seed (No. PMOL–B01321A, enlargement of the specimen in Fig.19a, the Vertebraria structure inside the branch, associated with a cupule attached to a scale leaf and a detached seed in detail)

Fig.21 A leaf attached to a branch showing the Vertebraria structure, associated with a cupule attached to a scale leaf and a detached seed (No. PMOL–B01321B, enlargement of the specimen in Fig.19b, showing the Vertebraria structure inside the branch, associated with a cupule attached to a scale leaf and a detached seed in detail)

Fig.23 A leaf attached to a ultimate branch showing the Vertebraria structure (No. PMOL–B01321B, enlargement of the upper frame in Fig.21)
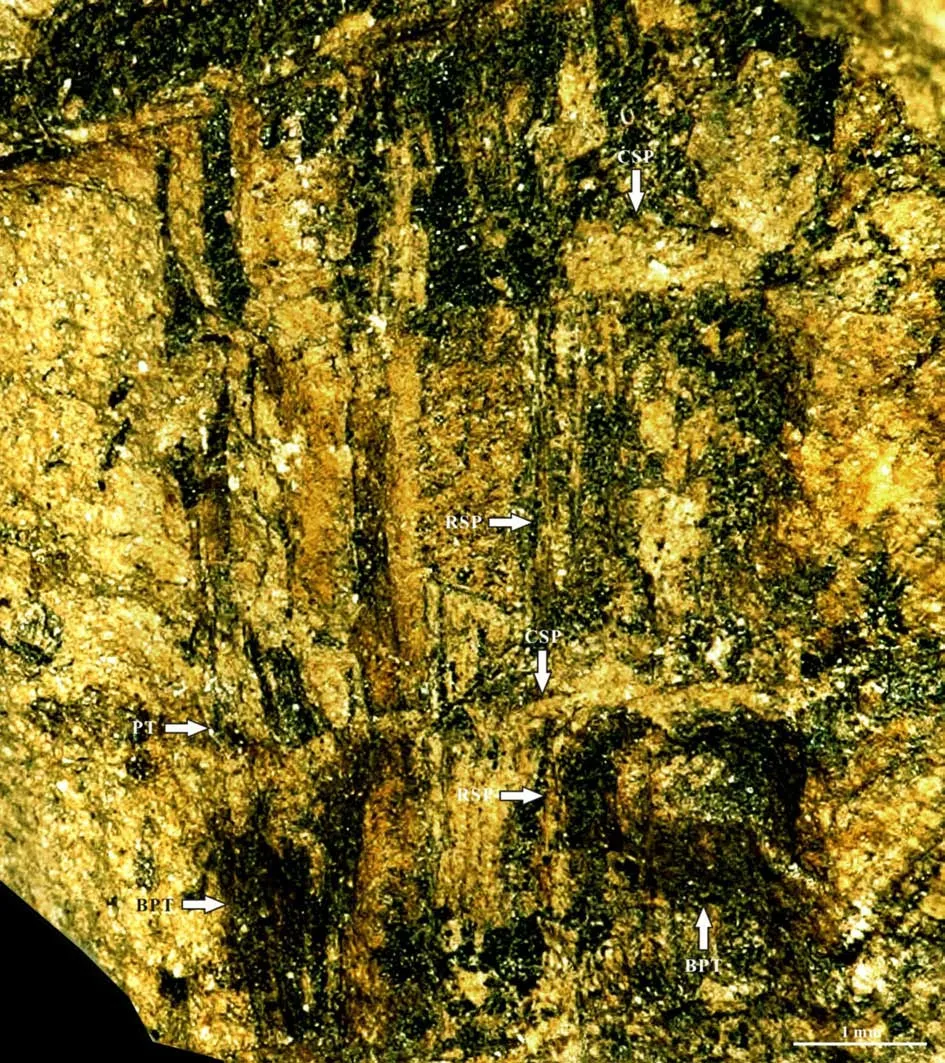
Fig.24 A leaf attached to an ultimate branch showing the Vertebraria structure (No. PMOL–B01321A, enlargement of the lower frame in Fig.20, showing the vascular-bundle scars of leaves and the base of petiole).

Fig.25 A leaf attached to an ultimate branch showing the Vertebraria structure (No. PMOL–B01321B, enlargement of the lower frame in Fig.21)
The surface of the ultimate branch in the specimens No. PMOL–B01321A, B (Figs.19-27)shows small protuberlance and unclear leaf scars(Fig.26a, b). Under the surface, six chambers are observed around the center vascular bundle of the branch. The central vascular bundle of the branch is enclosed by its sheath (Fig.22). Each chamber is covered by cortex outside, and enclosed by the lower and upper cross septa and lateral ridge septa. Two leaf traces are present in each chamber. Each leave trace includes the outside cortex, the sheath of the central vascular bundle and the central vascular bundle. Eight ridges cross over the cortex with a radial arrangement(Fig.27a).

Fig.22 A leaf attached to an ultimate branch showing the Vertebraria structure (No.PMOL–B01321A, enlargement of the upper frame in Fig.20)

Fig.26 Details of Vertebraria structure (PMOL–B01321A)
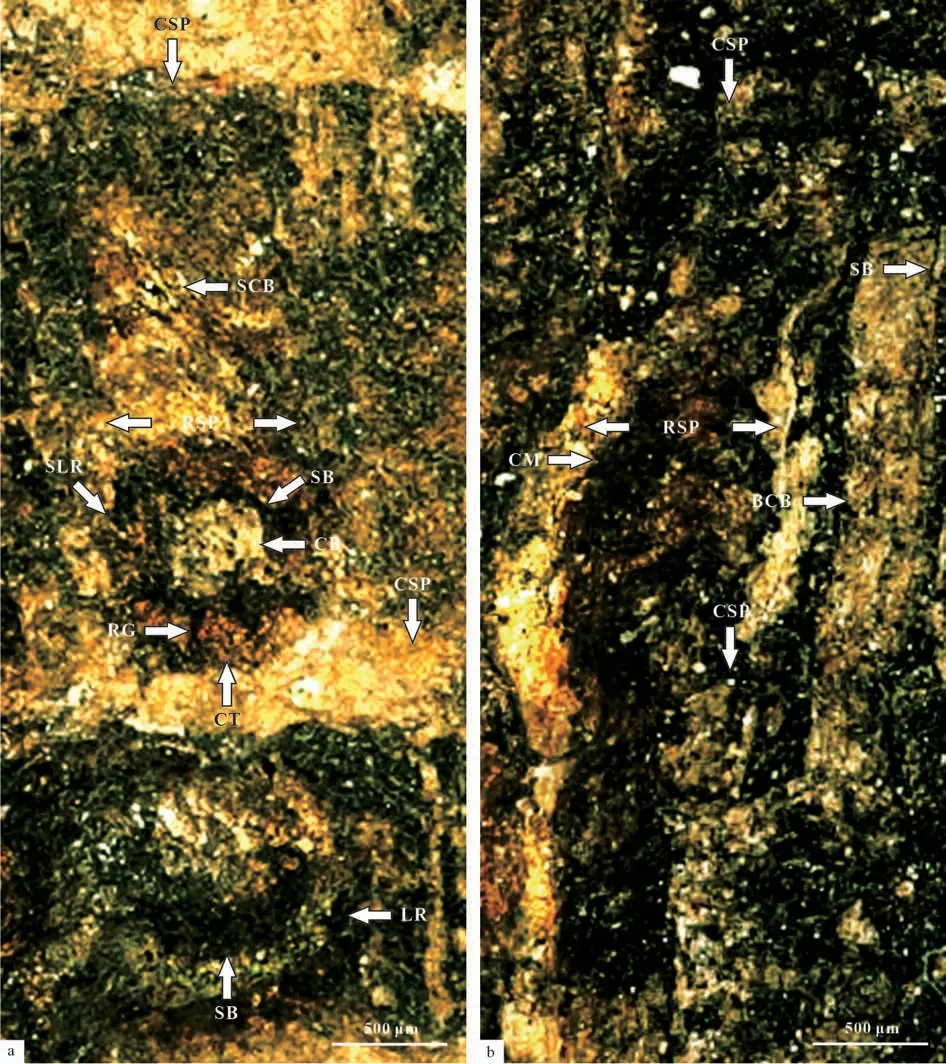
Fig.27 Details of Vertebraria structure (PMOL–B01321A, B)
4 Discussion
4.1 Reconstruction of the new species
The lobes of the cupule are six in number because two right cupules in the specimen PMOL–B01320A from the front view show two lobes located between the furrow, and two lobes located in each lateral side of the cupule, indicating that the arrangements of the lobes are same from the back view (Fig.2a-f, Figs.9-16). The detached cupule No. PMOL–B01322 (Fig.2g;Fig.28) shows a more direct evidence of the number and morphology of lobes in the cupule.
The specimen No. PMOL–B01321A, B was collected in the same layer from the same fossil site of the holotype of the new species. It also shows theVertebrariastructure of 3-3.5 mm in width. The specimen is also associated with the very similar seed and cupule as the holotype. Furthermore, the distance between the point of the lowest cupule attached to the scale leaf and the point of the scale leaf attached to the branch is about 17.5 mm (Fig.20), while that of holotype is also about 17.5 mm (Fig.3). All of these evidences indicate that the specimen could be assigned to the new speciesSinoglossa sunii.
The surface of the branch showing longitudinal and transverse grooves (Fig.5, 6), is very similar to Fig.1 shown by Sen in 1955 (Sen, 1955), and Fig.6 in Page. 322 of the book“Palaeoflora of Southern Africa. Prodromus of Southern African Megafloras:Devonian to lower Cretaceous” shown by Anderson and Anderson (1985), but the present specimens show one wider longitudinal groove in the middle of the branch, and two thinner grooves in the two lateral sides of the branch; while the above two specimens show two longitudinal grooves in the branch. Walton and Wilson (1932) addressed that this situation is resulted from different positions of “the axis in relation to the direction of compression would determine the form of the resulting fossil”.
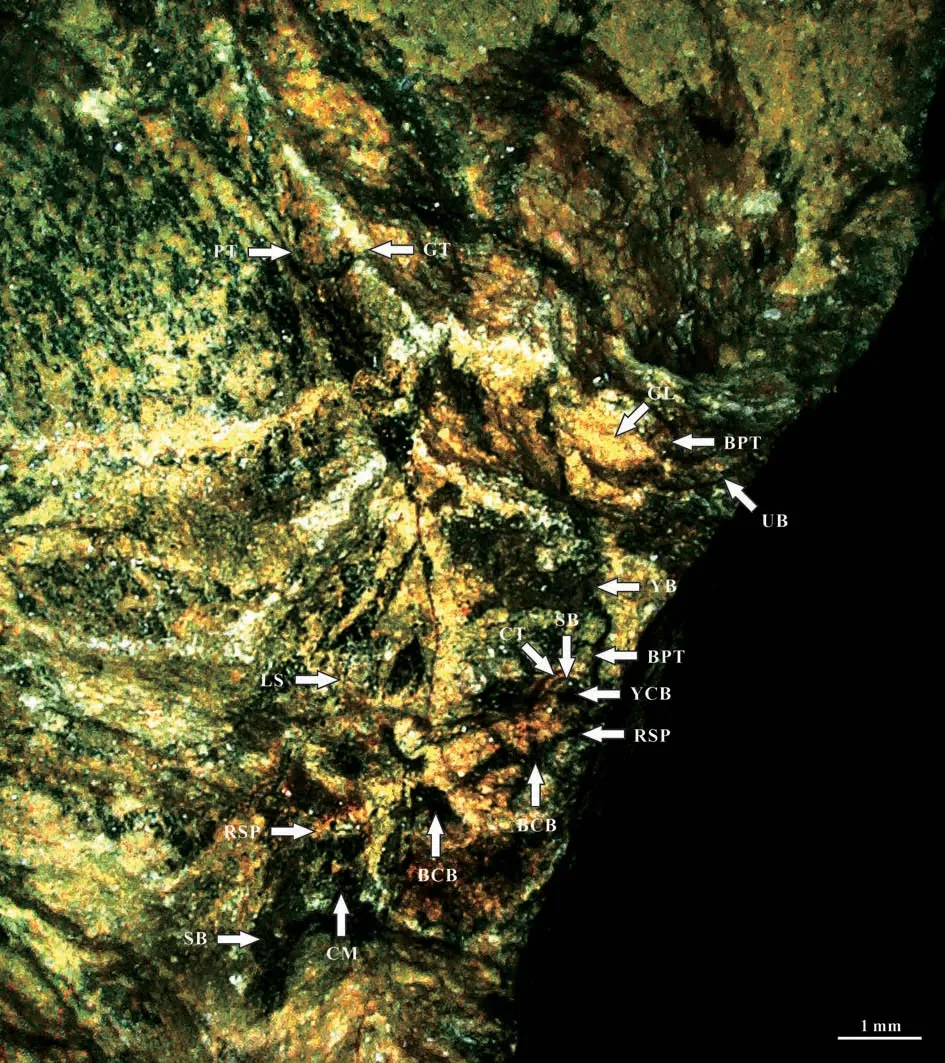
Fig.5 Branch structure of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320A, branch structure in detail)
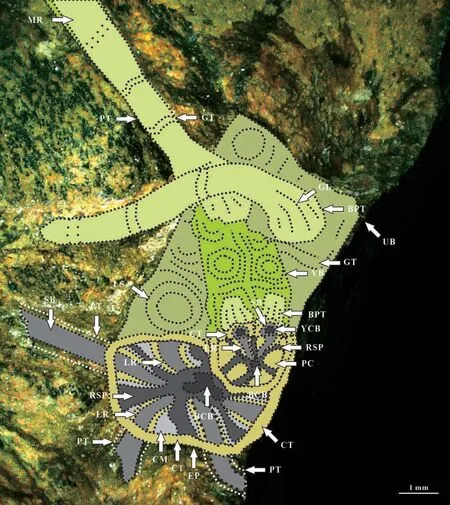
Fig.6 Reconstruction of branch of Sinoglossa sunii Zhang et al. sp. nov. (No. PMOL–B01320, branch structure in detail)
Another difference of the branch surface between the present specimens and the specimens shown by Sen (1955) as well as Anderson and Anderson (1985)is the arrangement of transverse grooves. Those of the two later specimens are irregular in arrangement along the direction of the axis, but those of the present specimens are around a plane.
The specimen No. PMOL–B01320A (Fig.2a)preserved the middle and lower part of the scale leaf,while the specimen No. PMOL–B01325 (Fig.2i)preserved the upper part of the scale leaf. Both of them share the entire margin and venation pattern, including“lateral veins very fine, numerous, emerging from midrib at acute angle of 10º-15º and passing laterally to margin at an angle of 30º-40º, repeatedly bifurcating and anastomosing, to form oblique, narrow, elongate meshes, with an average concentration of meshes about 70-80 per cm2”, and typical characteristics of the Glossopterids leaf. It is thus indicated that the specimen No. PMOL–B01325 is actually representing the apex of the new species. However, the maximum width of the specimen No. PMOL–B01325 is 1 cm,smaller than the that of the specimen No. PMOL–B01320A. This difference probably is resulted from their different locations attached on the ultimate branch. This phenomenon is popular in the branch.
The length of the scale leaf can be calculated by the two specimens. The distance between the petiole base to and the maximum width of the leaf in the specimen No. PMOL–B01320A is about 7.5 cm, while the distance between the apex and the maximum width of the leaf in the specimen No. PMOL–B01325 is about 2.5 cm, so the whole length of the scale leaf is at least 10 cm.
The scale leaf represented by specimen PMOL–B01320A, B is very flattening with a strong midrib(Fig.2a.b), indicating that the pedicel of cupules is attached to the abaxial surface of the midrib. This is because the midrib of extant Angiosperm leaves is generally marked on the abaxial surface, comparing with the adaxial surface. For example, almost all Fagales show the marked midrib on the abaxial surface. Their midribs on the adaxial surface are generally slender (Kuang & Li, 1979). The specimen PMOL–B01321A, B also shows that the cupule is attached on the abaxial surface of the midrib.
The young branch is an immature organ of the new species. It is more or less like the buds of Angiosperm, but shows some differences. The bud of Angiosperm is in dormant state, enclosed by bud scales, while the young branch of the new speciesSinoglossa suniiare not enclosed by bud scales.
Based on the above analysis, the suggested reconstruction ofSinoglossa suniigen. et sp. nov. is shown in Fig.30, including an ultimate branch with scales leaves attached by cupules.

Fig.30 Reconstruction of Sinoglossa sunii Zhang et al. gen et sp. nov.
4.2 Comparison with similar taxa
Sinoglossa suniigen. et sp. nov. is very similar toDenkania indica(Surange & Chandra, 1971) in its cupules attached to the scale leaf, but the scale leaf of the former develops a very marked and strong midrib,and cupules abaxially attached to the midrib, as well as theVertebrariastructure developed in the branch;whereas the later one lacks strong midrib, and cupules attached to which side of the midrib, as well as the scale leaf attached to which kind of the branch is unclear(Surange & Chandra, 1971).
The morphology of the scale leaf inSinoglossasuniigen. et sp. nov. (No. PMOL-B01320A) is very similar toGlossopteris recurva(Maithy 1965) Pant et Singh 1974, but the scale leaf ofS. suniiis a petiolate leaf attached by fertile organs, whileG. recurvais a petiolate leaf without fertile organs attached.
Generally, the genus nameVertebrariais used to the Glossopterids root (Tayloret al., 2009). But based on the cross and longitudinal sections of the present specimens, it can also represent the branch of Glossopterids (Fig.2a-d; Figs 3-16; Figs.19-27).Therefore, there are two types of branches or stems of Glossopterids. One isAraucarioxylon-type (Tayloret al., 2009),and another one isVertebraria-type.Retallack and Dilcher (1981) show two reconstructions of Glossopterids. The left reconstructionin Fig.2B by by Retallack and Dilcher(1981) developsAraucariaxylon-type branch / stem, while the right in Fig.2DbyRetallack and Dilcher(1981) probably shows theVertebraria-type branch / stem because the scars on the branch / stem in Fig.2Bby Retallack and Dilcherwith a whorl-like arrangement, are very similar to the present specimen.
4.3 Implication of paleogeography, paleoclimate and paleoecology
Scutumwas the first ovuliferous fructification in organic attachment to the prolific Gondwanan taxonGlossopteris.Since then, over 30 genera of glossopterid fertile organs have been documented(Prevec, 2011), but there is no glossopterid fertile organs attached to the glossopteris leaf found in the Triassic and North Hemisphere.Sinoglossa suniigen.et sp. nov. from the Middle Triassic Linjia Formation in Benxi, Northeast China, is the first record of reliable Glossopterids in the Middle Triassic of North Hemisphere.
In Middle Triassic, fossil localities of plant are very limited. Only about 20 important fossil localities with relatively complete records are documented(Fig.31). In China, the Middle Triassic floras were reported from the Linjia Formation in Northeast China (Zhanget al.,1983; Zhanget al.,2019, 2020);the bottom of the Ermaying Formation in North China (Wang & Wang, 1990); the upper part of the Ermaying and Tongchuang formations in the Ordos Basin in North China (Huang & Zhou, 1980); the Badong Formation in the central Yangtze River Basin(Menget al., 1980); the top of the lower member of the Karamay Formation in the northern margin of the Tarim Basin, Xinjiang (Wu & Zhou, 1996); the Middle Triassic to the upper part of the Late Triassic flora of the Karamay Formation in Xinjiang, Northwest China,including the Junggar Basin in North Xinjiang, Turpan Basin and the northern edge of Tarim Basin in South Xinjiang (Gu & Hu, 1979). Duat al.(2021) assigned the Jiefangcun flora to the Middle Triassic flora in Yangbian, Jilin based on the zircon dating age and the record ofNeocalamites. But the index fossil of the Early-Middle Triassic in the Earth,Pleuromeia, and the typical element of the Triassic Angaran Realm,Scytophyllum, have not been recorded. So, its age needs to be further demonstrated (Dobruskina, 1994;Tayloret al,2009).
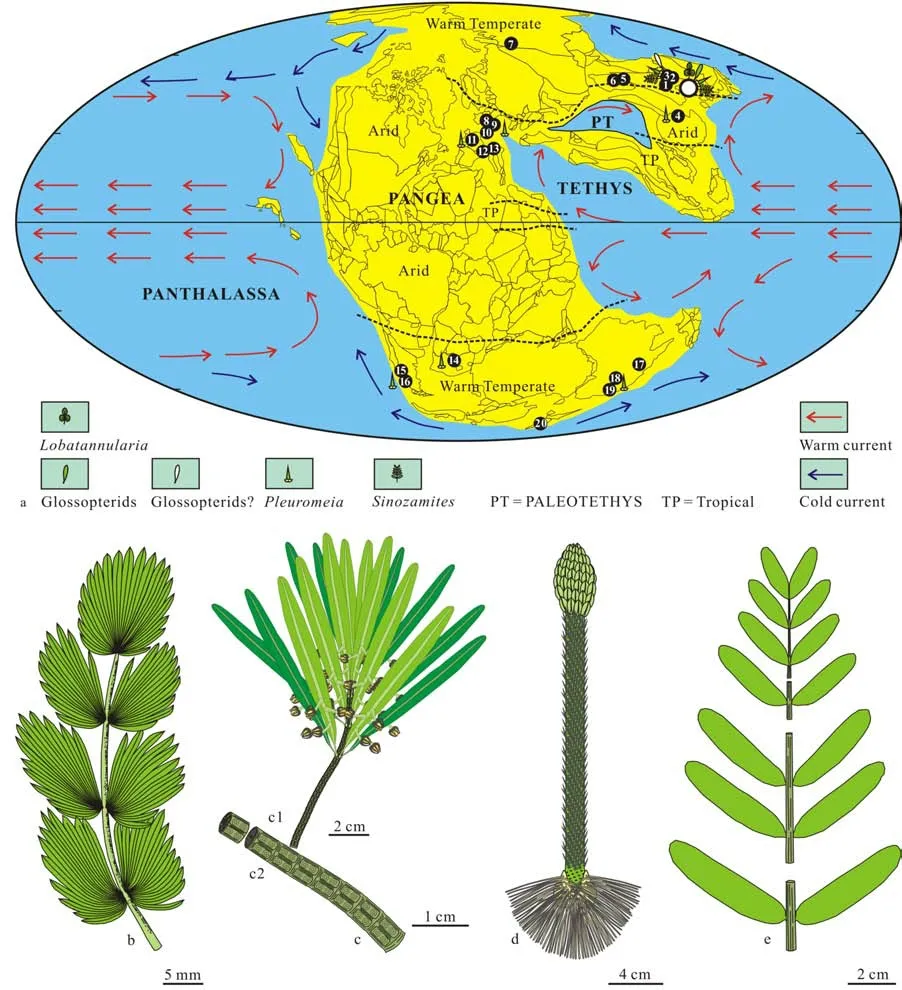
Fig.31 Middle Triassic paleomap, showing the area of Benxi (white circle) developing the Linjia flora, mainly composed of Lobatannularia, Sinoglossa, Pleuromeia and Sinozamites, influenced by and the warm and cold currents in the climatic zone of warm temperate
Only one Middle Triassic flora from Orenburg was reported in Southern Ural, Russia (Vladimirovite,1972).
In Europe, 6 Middle Triassic floras was relatively complete documented, including the Middle Triassic floras from the Upper Buntsandstein and Muschellkalk in Germany (Blancksanfstreins, 1886), the Voltzia sandstone of Vosges in France (Grauvogal-Stamm,1978),Dolomites of Northern Italy in Anisian (Kustatscheret al., 2010; Wachtler, 2011a, b, 2020) and Ladinian (Kustatscher & van Konijnenburg-van Cittert,2005) as well as the Cassina beds of Switzerland(Stockar & Kustatscher, 2010).
In Gondwanaland, the Middle Triassic floras were shown in the Moolayember Formation from the Bowen Basin, Queensland, Australia (Palyfordet al.,1982), Benolong of New South Wales, Australia(Holmes, 1982), the Long gully Formation in Otago,South Island, New Zealand (Retallack, 1981), the Cacheuta Formation from the Mendoza oil deposit in West Argentina (Jain & Delevoryas, 1967), the Quebrada Formation in Mendoza, West Argentina(Cariglinoet al,2018). The Sydney Basin in Australia developed a Smithian (Early Triassic) to Anisian flora (Retallack, 1975, 1977, 1981), The Burgersdorp Formation in the Karoo Basin of South Africa yields a flora from Early to Middle Triassic flora (Anderson &Anderson, 1985).
In these floras,Pleuromeiaas a widelydistributed element in the Early-Middle Triassic strata, has only been documented in the Middle Triassic floras from the Linjia Formation in Northeast China, the upper part of the Ermaying and Tongchuan formations in North China, the Badong Formation in South China, the Upper Buntsandstein in Germany the Voltzia sandstone of Vosges in France, and the Quebrada Formation in Mendoza of West Argentina.P.longicauliswas found in the Sydney Basin (Retallack,1975). Retallack changed its name toCyclostrobus sydneyensisin 1997.Gregicaulis dubiusis supposed by Anderson and Anderson in 1985. Retallack pointed out thatGregicauliswas a junior synonym ofPleuromeiain 1995. So,G. dubiusshould change its name toP.dubia.
Glossopterids, as a typical Paleozoic relic element,were only reported from the Linjia Formation in Northeast China, as well as the upper part of Ermaying and Tongchuan formations in North China, but specimens from the upper part of Ermaying and Tongchuan formations lack fertile organs attached, resulting in these specimens in question of identification (Huang& Zhou, 1980).Sinozamites, as a typical endemic Mesozoic element was only found in the Linjia Formation in Northeast China, and the upper part of the Ermaying Formation, but specimens from the latter is questionable (Huang & Zhou, 1980).Lobatannularia,as a typical Paleozoic relic element of Cathaysian flora was found in the Linjia Formation in Northeast China(Xuet al.,2020).
The Middle Triassic Linjia flora is a very different flora in that time because the flora not only developedPleuromeia,the typical element of the Early-Middle Triassic element (Tayloret al., 2009), andSinozamites,a typical endemic Mesozoic element (Hanet al., 2018),but also developed Glossopterids, a typical Paleozoic relic element from Gandwanaland (Tayloret al.,2009), andLobatannularia,a typical Paleozoic relic element of Cathaysian flora (Xuet al., 2020). In Middle Triassic, only the Linjia flora was under the control of the warm temperate climate, and markedly influenced by both warm and cold currents, probably resulting in a flora under the control of an environment with more participants, similar to the Late Paleozoic environment. In this situation, both Late Paleozoic and Early-Middle Triassic elements can be developed in the Linjia flora. Other floras generally influenced by one kind current, warm or cold, such as the flora from the Upper Buntsandstein in Germany by warm flora,the flora from the Quebrada formation in Mendoza,West Argentina by cold flora (Fig.5).
In the end-Permian, a greatest mass extinction took place(Shenet al.,2011). The new finding ofSinoglossa suniigen. et sp. nov. demonstrates that Glossopterids not only could be distributed in both South and North Hemisphere, but also could survive the end-Permian mass extinction in both South Hemisphere (Gastaldo, 2019) and North Hemisphere.
Generally, the research of Glossopterids in North Hemisphere was insufficient because the fertile organs attached to the sterile organs were absent(McLoughlin, 2011). So, it is difficult to understand why Glossopterids could be distributed in North Hemisphere. There are two suggestions about the presence of Glossopterids in North Hemisphere. One is related to the migration of Glossopterids from South Hemisphere, another is the parallel evolution taken place in both South and North Hemispheres. The first suggestion is more reasonable because Pangea was present from Permian to Triassic, making it possible for the South Glossopterids to migrate the North Hemisphere (Fig.31); another reason is that no evidence can be demonstrated that the vicariance condition was present in Pangea. The vicariance condition is the key factor resulting in the parallel evolution took place for marsupial mammal species in Australia and placental mammal species in other continents.
4.4 Implication of evolution in Angiophytes
The new element of Glossopterids discovered from North Hemisphere, demonstrates that the environment of North Hemisphere was probably better than South Hemisphere after the mass extinction,resulting in the origin of Angiosperms more likely from North Hemisphere.
Angiosperms are widely distributed in both North and South Hemispheres in Late Cretaceous and Cenozoic (Tayloret al.,2009). The reliable earliest Angiosperms found in the Early Cretaceous Yixian Formation in West Liaoning, Northeast China (Sunet al.,1998). The new finding of the reliable Glossopterids in North Hemisphere combined with early reports(Tayloret al.,2009; Gastaldo, 2019) demonstrated that it is possible for Glossopterids to be distributed in both North and South Hemispheres at least from Permian to Middle Triassic.
Recently, Shiet al.(2021) suggested that all Corystosperms,Glossopteris,Caytoniaand Angiosperms form a clade of Angiophytes based on the cladistic analysis. Liet al.(2019) addressed that the crown Angiosperms can been dated back to the Late Triassic. The new finding of the reliable Glossopterids in the Middle Triassic of North Hemisphere supports the above two hypotheses in the views of the age and space distribution.
Glossopterids has been regarded closely related to Angiosperms in the clade of Angiophytes (Shiet al.,2021). In the view of space distribution, if no evidence shows that the Glossopterids could be distributed in North Hemisphere, it is difficult to understandwhy the reliable Angiosperms was discovered from North Hemisphere.
The crown Angiosperms has been dated back to the Late Triassic (Liet al., 2019), and Glossopterids has been regarded as taxon closely related to Angiosperms in the clade of Angiophytes (Shiet al., 2021).In the view of age distribution, if the evidence shows that the Glossopterids could be distributed in Middle Triassic, the crown Angiosperms dated back to the Late Triassic is more understandable.
5 Conclusions
Since Brongniart erectedGlossopterisin 1928,reliable Glossopterids attached by fertile organs were only collected from the Permian Gondwanaland.Recently, a new element of Glossoptetids,Sinoglossa suniigen et sp. nov., has been found with attached female organs from the Middle Triassic Linjia Formation in Benxi, Northeast China, characterized by fertile ultimate branch slender, attached by scale leaves and young branches with a whorl-like arrangement,showing theVertebrariastructure in its cross and longitudinal sections, and several cupules each with a strong and short pedicel attached to the abaxial midrib of scale leaves.Sinoglossa suniigen. et sp.nov. is very similar toDenkania indicain its cupules attached to the scale leaf, but the scale leaf of the former develops a very marked and strong midrib, and cupules abaxially attached to the midrib, as well as theVertebrariastructure developed in the branch.
The reconstruction of the branch structure ofSinoglossa suniigen et sp. nov. is based on different specimens showing the same structure from transverse and longitudinal sections. The reconstruction of the whole leaf is based on different specimens sharing the same structure of their entire margins and venation patterns.The reconstruction of the cupules and containing seeds is based on different specimens showing the same structure with attached and detached situations.
In Middle Triassic, only the Linjia flora was under the control of the warm temperate climate, and markedly influenced by both warm and cold currents,probably resulting in a flora under the control of an environment with more participants, similar to the Late Paleozoic environment. In this situation, both Late Paleozoic and Early-Middle Triassic elements can be developed in the Linjia flora. Glossopterids in North Hemisphere probably migrated from South Hemisphere, and successfully survived the end-Permian mass extinction in North Hemisphere.
Glossopterids is one of key evidences of the presence of Gondawanan continent in Late Paleozoic. The new finding also gives a new sound evidence to demonstrate the presence of Pangea. In addition, the new finding also shows the crown Angiosperms dated back to the Late Triassic is more understandable in light with the age and space distribution of each element of Angiophytes.
Acknowledgments
We are deeply indebted to Dr. R. Prevec, Dr. S.McLoughlin and Dr. B. Cariglinofor their carefully checking the photos of main fossils and giving good advice in the initial stage of the study. We are very grateful to three anonymous reviewers for their valuable comments and suggestions for the final version of the manuscript.
杂志排行
Global Geology的其它文章
- Late Triassic flora of the northern part of the Fore-Caspian depression (Kazakhstan) as exemplified by data from the S. Nurzhanov-509 borehole
- The first occurrence of Scincomorpha lizard from Nenjiang Formation (lower Campanian) of Jilin, Northeast China
- Characteristics of source rocks and paleoenvironment of Lower Silurian Longmaxi Formation in western Hubei
- Characterization of mineral element fingerprints under soilcrop systems in eastern and western rice-producing areas of Jilin Province, China
- Unveiling Ceremony of Academician Li Xingxue’s Former Residence Scientists’ Spiritual Education Base held in Chenzhou of Hunan
- CONTENTS
