Antiviral effects of natural small molecules on aquatic rhabdovirus by interfering with early viral replication
2022-11-29YanZhouTianXiuQiuYangHuLeiLiuJiongChen
Yan Zhou, Tian-Xiu Qiu, Yang Hu, Lei Liu,*, Jiong Chen,*
1 State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-Products, Ningbo University, Ningbo,Zhejiang 315211, China
2 Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Meishan Campus, Ningbo University, Ningbo, Zhejiang 315832, China
3 Key Laboratory of Applied Marine Biotechnology of Ministry of Education, Meishan Campus, Ningbo University, Ningbo, Zhejiang 315832, China
ABSTRACT Spring viremia of carp virus (SVCV) is globally widespread and poses a serious threat to aquatic ecology and aquaculture due to its broad host range.To develop effective agents to control SVCV infection, we selected 16 naturally active small molecules to assess their anti-SVCV activity.Notably, dihydroartemisinin (DHA) (100 μmol/L) and(S, S)-(+)-tetrandrine (TET) (16 μmol/L) exhibited high antiviral effects in epithelioma papulosum cyprinid (EPC) cells, with inhibitory rates of 70.11%and 73.54%, respectively. The possible antiviral mechanisms were determined as follows: 1. Preincubation with DHA and TET decreased viral particle infectivity in fish cells, suggesting that horizontal transmission of SVCV in the aquatic environment was disrupted; 2. Although neither had an effect on viral adhesion, TET (but not DHA)interfered with SVCV entry into host cells (>80%),suggesting that TET may have an antiviral function in early viral replication. For in vivo study, both agents enhanced the survival rate of SVCV-infected zebrafish by 53.3%, significantly decreased viral load, and modulated the expression of antiviralrelated genes, indicating that DHA and TET may stimulate the host innate immune response to prevent viral infection. Overall, our findings indicated that DHA and TET had positive effects on suppressing SVCV infection by affecting early-stage viral replication, thus holding great potential as immunostimulants to reduce the risk of aquatic rhabdovirus disease outbreaks.
Keywords: Spring viremia of carp virus; Natural small molecules; Antiviral effects; Virus internalization; Danio rerio
INTRODUCTION
Due to their biodegradability, low toxicity, and presence of natural small molecules with specific biological functions, there is growing interest in the use of herbal medicinal plants as growth promoters, appetite stimulators, and immunostimulants in aquaculture (Newman & Cragg, 2020; Tadese et al., 2022;Van Hai, 2015), which may help reduce the application of unnecessary antibiotics to prevent and treat diseases (Wu et al., 2022). Compared with plant extracts, plant-derived small molecules are safer and more efficient, with considerable potential as non-toxic and eco-friendly agents for the prevention and treatment of aquatic diseases. Certain small molecules, such as thymol, carvacrol, and curcumin, exhibit specific antibacterial effects against pathogenic bacteria(Kachur & Suntres, 2020; Zheng et al., 2020), while dioscin,polyphyllin Ⅰ, and gracillin exhibit promising anthelmintic activity towardsGyrodactylus kobayashii(a common monogenean ectoparasite in goldfish) (Zhou et al., 2020). At present, however, viruses remain a serious challenge in aquaculture. While several natural compounds display antiviral activity in aquatic species, e.g., hesperetin, geniposidic acid,and genipin show anti-white spot syndrome virus (WSSV)activity in crayfish (Procambarus clarkii) (Huang et al., 2019,2020; Qian & Zhu, 2019), further research is needed to identify natural small molecules with effective antiviral properties.
Spring viremia of carp virus (SVCV) is a member of the genusSprivivirusin the familyRhabdoviridaeand is the causative agent of spring viremia of carp (SVC), an acute contagious and hemorrhagic disease of cyprinids (Walker et al., 2022). Waterborne transmission is thought to be the primary route of infection, with the virus first excreted in the feces and urine of infected fish, then carried by vectors into a new host via the gills and skin (Ahne et al., 2002), causing infections in various carp species, including common carp(Cyprinus carpio) (Adamek et al., 2019), grass carp(Ctenopharyngodon idella) (Shao & Zhao, 2017), zebrafish(Danio rerio) (Wang et al., 2021), and Caspian white fish(Rutilus frisii kutum) (Ghasemi et al., 2014). Given its broad host range and high mortality, SVCV is considered a notifiable pathogen by the World Organisation for Animal Health(formerly the Office International des Epizooties) and the Chinese Ministry of Agriculture. Although vaccines are a traditional preventive strategy for the control of viruses, limited in-depth research on fish immunobiology has resulted in a lack of efficient vaccines against SVCV (Su & Su, 2018). Recently,small interfering RNAs (siRNAs) targeting glycoprotein, matrix protein, RNA-dependent RNA polymerase, phosphoprotein,and nucleoprotein genes have been shown to reduce SVCV replicationin vitro, but the exorbitant cost and lack ofin vivomodels have limited their use (Fouad et al., 2022; Gotesman et al., 2015). Various agents, including natural small molecules, have also been explored for the control and prevention of aquatic diseases. For instance, bavachin can block early events of SVCV replication, while arctigenin and saikosaponin D can inhibit virus-induced autophagy and apoptosis, respectively (Chen et al., 2018; Shen et al., 2018,2019). Therefore, the discovery of medicinal plant-based small molecules with anti-SVCV activity is essential for aquatic studies and viral treatment.
In the current study, to address the need for SVCV prevention and treatment, we tested 16 natural plant-based small molecules. Based on mRNA expression of the SVCV nucleoprotein gene, both dihydroartemisinin (DHA) and (S, S)-(+)-tetrandrine (TET) exhibited anti-SVCV activityin vitro.Their antiviral mechanisms were verified by apoptosis, viral infectivity, and antiviral gene expression analysis.Furthermore, given their well-defined genetic background,zebrafish were chosen as a model species to study the antiviral activities of DHA and TETin vivo. Overall, our results demonstrate the potential of natural small molecules as novel antiviral agents for the control of viral diseases in aquaculture.
MATERIALS AND METHODS
Fish and small molecules
Zebrafish (3.60±0.25 g in weight) were purchased from the Qing-Feng Zebrafish Breeding Center (Shanghai, China), and acclimatized for 2 weeks at 18 ℃ before the experiments. All procedures are performed according to the Experimental Animal Management Law of China and approved by the Animal Ethics Committee of Ningbo University. The natural small molecules were purchased from Aladdin Chemistry(China) and mixed with 100% dimethyl sulfoxide (DMSO;Aladdin, China).
Cell culture and virus infection
Epithelioma papulosum cyprinid (EPC) cells were cultured in medium 199 (M199; Hyclone, USA) supplemented with 10%fetal bovine serum (FBS; Every Green, China), penicillin (100 IU/mL), streptomycin (100 μg/mL), and gentamicin (50 μg/mL)at 25 ℃. SVCV was amplified in EPC cells containing M199 with 5% FBS until approximately 75% of the cells showed cytopathic effect (CPE), which were then stored at -80 ℃ until use.
CCK-8
EPC cell viability was measured using a Cell Counting Kit-8(CCK-8; Beyotime, China) following previous study (Wang et al., 2021). Briefly, monolayer EPC cells were cultured in 96-well plates in M199 supplemented with 5% FBS and incubated with natural small molecules or SVCV at 25 ℃ for 24 or 48 h.After incubation, cell viability was measured using CCK-8 following the manufacturer’s instructions. Optical density at 450 nm (OD450nm) was measured using a PT-3502C microplate spectrophotometer (Potenov, China).
Antiviral assay of natural small molecules
The antiviral activity of natural small molecules was determined according to previous research (Qiu et al., 2020).Monolayer EPC cells were cultured in a 12-well plate at 25 ℃and infected with SVCV (104×TCID50/mL) in the presence or absence of natural small molecules. After 48 h of infection,changes in cell morphology were observed by light microscopy, and the antiviral activity of natural small molecules was assessed by real-time quantitative polymerase chain reaction (qPCR).
Nuclear damage observations
Fluorescence was used to detect nuclear damage, as per previous research (Song et al., 2021). The EPC cells were infected with SVCV (104×TCID50/mL) in the presence or absence of natural small molecules for 48 h, fixed with 4%paraformaldehyde for 10 min, and stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI;Beyotime, China) and 1,1"-dioctadecyl-3,3,3",3"-tetramethylindocarbocyanine perchlorate (Dil; Beyotime,China) for 30 min and 10 min, respectively. The treated cells were then observed by fluorescence microscopy (Nikon,Japan).
Virus incubation and cell pretreatment assay
In the virus incubation assay, SVCV was incubated with DHA or TET for 1, 2, and 4 h before infection. To detect its binding effects, the virus was added to EPC cells for 1 h at 4 ℃ (the virus only binds to the cell surface). For internalization effects,the virus was added to EPC cells for 4 h at 15 ℃ and treated with trypsin for 30 s to remove the bound but not entered virus.
In the cell pretreatment assay, EPC cells were exposed to DHA or TET for 8, 16, and 24 h, then washed with phosphatebuffered saline (PBS) and immediately infected with SVCV. To investigate long-lasting protective effects, the EPC cells were exposed to DHA and TET for 24 h, washed with PBS every 12 h, and replaced with DHA- or TET-free medium.
Antiviral-related gene quantification in vitro
The EPC cells were divided into three groups: controlDMSO,TET, and DHA. All samples were harvested 24 and 48 h after incubation, then extracted using TriQuick Reagent (Solarbio,China). The cDNA was synthesized using HiScript Q Select RT SuperMix (Vazyme, China) and qPCR was performed on an ABI QuantStudio 3 Real-Time PCR System (Applied Biosystems, USA) using AugeGreeen qPCR Master Mix (US Everbright Inc., China). The primers used for qPCR analysis are listed in Supplementary Table S1. The relative expression levels of genes were analyzed using the 2-△△CTmethod, withβ-actinas the reference.
Analysis of protective effects of DHA and TET on SVCVinfected fish
In total, 80 zebrafish were used to examine the survival of SVCV infection under DHA and TET treatment. Fish were divided into six groups (15 fish each) and placed in 500 mL of fresh water at 16 ℃. Three groups were infected with SVCV(103×TCID50/mL). After 12 h of immersion infection, the fish were fed with 50 μL of 100 mol/L DHA or 16 mol/L TET or the same volume of DMSO. The fish were inspected every 24 h for 9 days to record death and moribund state.
In the viral load experiment, a total of 54 healthy zebrafish were selected and randomly divided into three groups:controlDMSO, DHA+SVCV, and TET+SVCV. Each group was treated as above and analyzed for viral load at 1, 3, and 5 days post-infection (dpi).
Analysis of preventative effects of DHA and TET on SVCV infection in vivo
To evaluate the preventive effects of DHA and TET on SVCV infection, 189 healthy zebrafish were randomly divided into three groups: SVCVDMSO, DHA+SVCV, and TET+SVCV. Each group was separately fed 50 μL of 100 μmol/L DHA or 16 μmol/L TET or the same volume of DMSO. For the next 1, 4,and 7 d, three fish per group were harvested for antiviralrelated gene expression quantification. Others were infected with SVCV at a concentration of 103×TCID50/mL and sampled at 1, 3, and 5 dpi to analyze viral load. The relative expression levels of genes were analyzed using the 2-△△CTmethod, with18SrRNA as the reference.
Statistical analyses
A logistic sigmoidal function with a maximal effect level and Hill coefficient indicating sigmoidal transition was used to construct drug response curves with Origin 2021 (Origin Lab,USA). Virus titers were log10 transformed before statistical analysis. Zebrafish survival was determined using the nonparametric Kaplan-Meier test. Statistical analysis was performed using student’st-test with SPSS v13.0 (SPSS Inc,USA). All data are presented as mean±standard deviation(SD). Statistical significance was defined as aP-value of 0.05 or less.
RESULTS
Antiviral activity of natural small molecules
The anti-SVCV activity of 16 natural small molecules was evaluated in EPC cells. Their CAS number, purity,concentration, and viral inhibition rate are presented in Table 1. Small molecules were considered active if the inhibition rate of SVCV N was over 50%. As shown in Table 1,DHA and TET showed anti-SVCV activity, with inhibition of SVCV nucleoprotein gene expression of 70.11% and 73.54%,respectively.

Table 1 CAS number, purity, and SVCV inhibition rate of small molecules used in this study
Anti-SVCV activity of DHA and TET in EPC cells
To determine the antiviral activities of DHA and TET in EPC cells, their cytotoxicity was first detected by CCK-8 assay. As shown in Figure 1A, B, after 48 h, DHA and TET showed no substantial cytotoxicity at concentrations less than 100 μmol/L and 16 μmol/L, respectively (Figure 1A, B). We next tested whether the antiviral activities of DHA and TET were dosedependent. Results showed that SVCV nucleoprotein gene expression gradually decreased with increasing concentrations of DHA and TET after 24 and 48 h (Figure 1C,D). In a parallel trial, both DHA and TET inhibited CPE caused by SVCV infection (Figure 1E). Compared with the DMSOtreated group, the SVCV-infected cells were round and granular, forming clusters of grape-like cells and small cellular plaques, while the number of cytopathic cells was significantly reduced after DHA and TET treatment (Figure 1E).
Inhibition of SVCV-induced apoptosis by TET and DHA
As SVCV can induce apoptosis in host cells (Licata & Harty,2003), we investigated whether DHA and TET reduced SVCVinduced apoptosis in the EPC cells. As shown in Figure 2A, B,SVCV infection caused typical apoptotic features, including cell fragmentation, morphological irregularities, and nuclear division. In contrast, DHA and TET had significant protective effects on SVCV-infected cells, maintaining a normal spindle structure and reducing the number of apoptotic bodies(Figure 2A, B). Taken together, DHA and TET significantly attenuated SVCV-induced apoptosis, thus indicating they may be highly effective against SVCV infection.

Figure 1 Antiviral activity of DHA and TET in vitro
Effects of DHA and TET on viral infectivity
To evaluate the effects of DHA and TET on SVCV infectivity,we performed viral incubation and cell pretreatment assays. In the viral incubation assay, SVCV was first incubated with DHA or TET at 25 ℃ for 1, 2, and 4 h before infection (Figure 3A).Compared with the DMSO-treated group, TET, but not DHA,decreased SVCV nucleoprotein gene expression by 0.58-fold after incubation for 1 h and suppressed SVCV-induced CPE(Figure 3B, C). The effect of TET on reducing viral infectivity increased with incubation time (Figure 3B, C). Furthermore,TET significantly interfered with the internalization process of SVCV in EPC cells but had no effect on virus binding(Figure 3D, E). These results suggest that TET, but not DHA,may negatively impact viral infectivity.
In the pretreatment assay, DHA and TET were first incubated with EPC cells for 8, 16, and 24 h, followed by infection with SVCV (Figure 4A). As expected, both DHA and TET pretreatment enhanced the antiviral activity, with a 0.18- fold and 0.09-fold decrease in SVCV nucleoprotein gene expression after 24 h of treatment, respectively (Figure 4B, C;Supplementary Figure S1B). Pretreatment for 36 and 48 h also enhanced antiviral activity but showed no time dependence (Supplementary Figure S1A). The antiviral effects of DHA and TET pretreatment gradually declined over time(Figure 4E; Supplementary Figure S1C). However, despite the obvious CPE phenomenon, DHA and TET treatment reduced SVCV nucleoprotein gene expression by 0.68-fold and 0.47-fold, respectively, after 48 h of recovery compared to the SVCVDMSOgroup (Figure 4E, F).
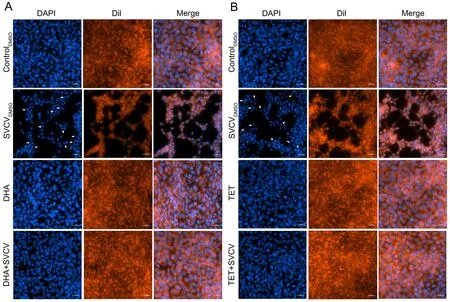
Figure 2 Nuclear damage from SVCV infection in vitro

Figure 3 Effect of DHA and TET on viral infectivity
Protective effects of DHA and TET on SVCV-infected fish
Given their anti-SVCV activities in EPC cells, we next evaluated the antiviral effects of DHA and TETin vivo. As seen in Figure 5, the SVCVDMSOgroup exhibited higher mortality and viral load. Fish without DHA or TET treatment started to die at 2 dpi, reaching 46.7% mortality at 5 dpi and 100% mortality at 9 dpi (Figure 5B). Interestingly, 86.67% of zebrafish were alive in the DHA- and TET-treated groups at 5 dpi, and the survival rate was 53.3% on the last day of the experiment (Figure 5B). To investigate the therapeutic effects of DHA and TET on SVCV-infected zebrafish, we measured viral loads at 1, 3, and 5 days after SVCV infection. As expected, viral load in the SVCV-infected fish under DHA and TET treatment was significantly lower than that in the SVCVDMSOgroup at all time points (Figure 5C). Thus, these results indicate that DHA and TET have therapeutic effects on SVCV-infected zebrafish.
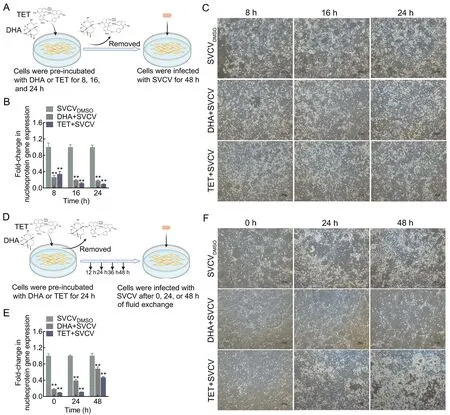
Figure 4 Antiviral effects of DHA and TET pretreatment on EPC cells
Preventative effects of DHA and TET on SVCV infection
Following the positive DHA and TET treatment of SVCVinfected zebrafish, we subsequently investigated the inhibitory properties of DHA and TET pretreatment against virus infectionin vivo. Zebrafish were randomly selected and infected with SVCV on days 1, 4, and 7 after feeding with DHA or TET, followed by detection of SVCV nucleoprotein gene expression using qPCR to determine changes in viral load(Figure 6A). As shown in Figure 6B, DHA or TET treatment one day before infection effectively inhibited SVCV in zebrafish, with inhibition rates greater than 50% at all time points. Additionally, following infection with SVCV on day 4,the viral load was significantly decreased by DHA at all tested time points, while viral load was only inhibited at 3 and 5 dpi under TET treatment (Figure 6C). Neither DHA nor TET had a significant inhibitory effect on SVCV infection at day 7 postfeeding (Figure 6D).
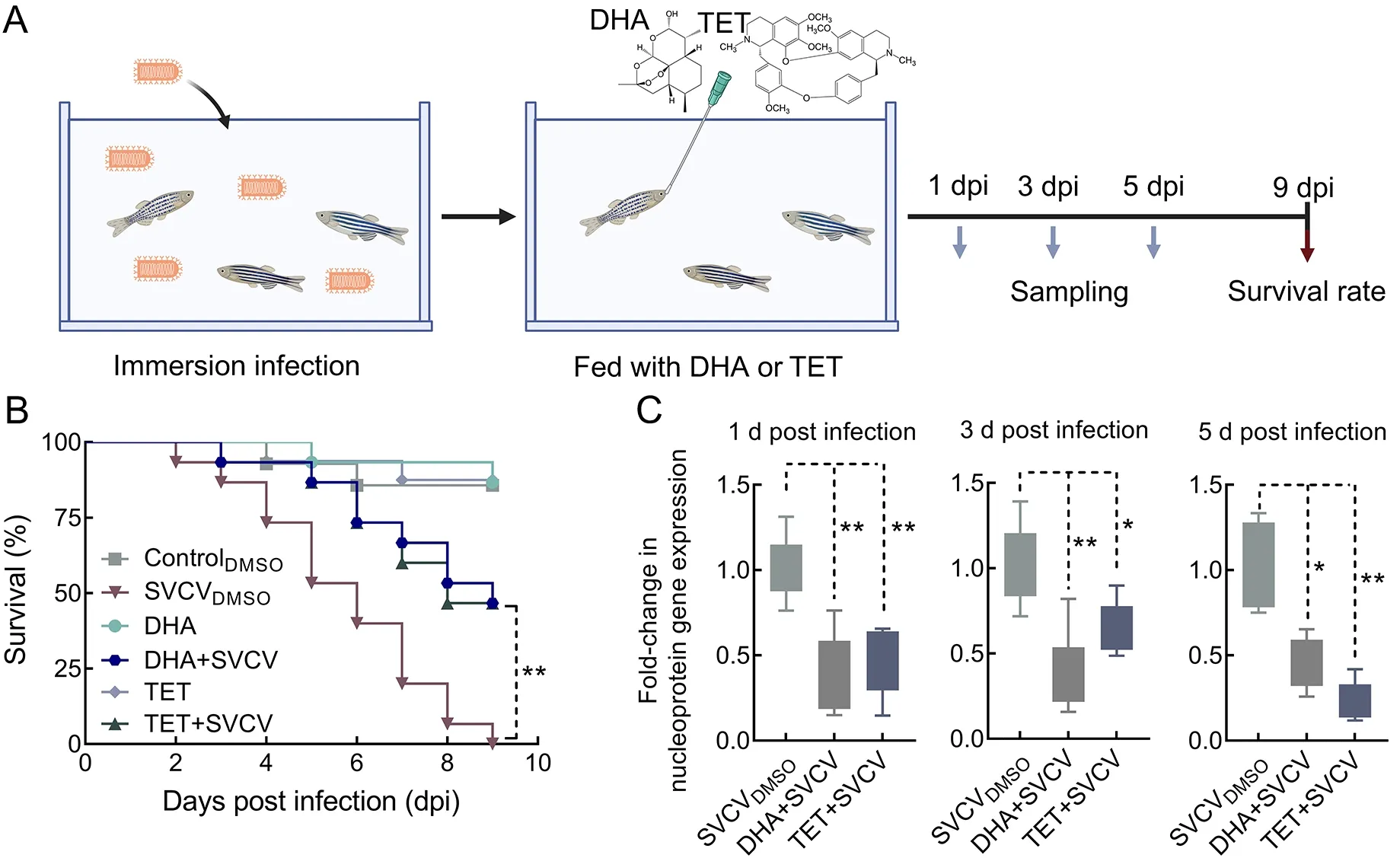
Figure 5 Effects of DHA and TET on survival rate and viral loads of SVCV-infected zebrafish
Effects of DHA and TET on expression of antiviral-related genes
To determine whether the antiviral effects of DHA and TET on zebrafish were obtained through modulation of host immunity,we analyzed the expression of antiviral-related genes. Results indicated that DHA and TET enhanced the expression of target genes, although the time points at which they reached significant up-regulation differed (Figure 7; Supplementary Figure S2). Treatment with DHA rapidly up-regulated interferon-φ (IFNφ) expression, with a significant 3.59-fold increase on day 1 after feeding and a significant 6.71-fold increase on day 4 (Figure 7A). The expression levels of retinoic acid-inducible gene-I (RIG-Ⅰ), myxovirus resistance isoforms A-B (Mxab), and nuclear factor E2-related factor 2(Nrf2) were significantly up-regulated on days 4 and 7 after feeding, withRIG-ⅠandMxabexpression increasing 5.87-fold and 4.65-fold on day 4, respectively. Compared to the controlDMSOgroup,Nrf2and heme oxygenase-1 (HO-1)reached maximum 2.41-fold and 4.46-fold increases on day 7,respectively (Figure 7C, D, F). Interferon-induced proteins with tetratricopeptide repeats 13a (Ifit13a) expression was significantly up-regulated only on day 1 after DHA treatment,while IFN-stimulated gene product 15 (ISG15) was greatly increased on day 4 (Figure 7B, E). Similar results were observed after DHA treatment in the EPC cells, with the expression levels of theIFN1,ISG15,viperin,RIG-Ⅰ,mitochondrial antiviral signaling (MAVS), and tumor necrosis factor-α(TNF-α) genes peaking at 48 h (Supplementary Figure S2). Results also showed that TET treatment had a positive effect onIfit13a,Mxab, andNrf2, withIfit13aandMxabexpression levels increasing 1.97-fold and 1.62-fold,respectively, andNrf2expression markedly increasing on days 4 and 7 (Figure 7B, D).In vitro, TET also enhanced the expression ofIFN1and IFN-induced genes (Mx1,ISG15, andviperin), as well as pro-inflammatory factorsTNF-αand interleukin-1β (IL-1β) (Supplementary Figure S2). Thus, DHA and TET appear to enhance the expression of antiviral genes and show great potential to strengthen the antiviral immune response of zebrafish.

Figure 6 Effect of DHA and TET pretreatment on viral load of zebrafish
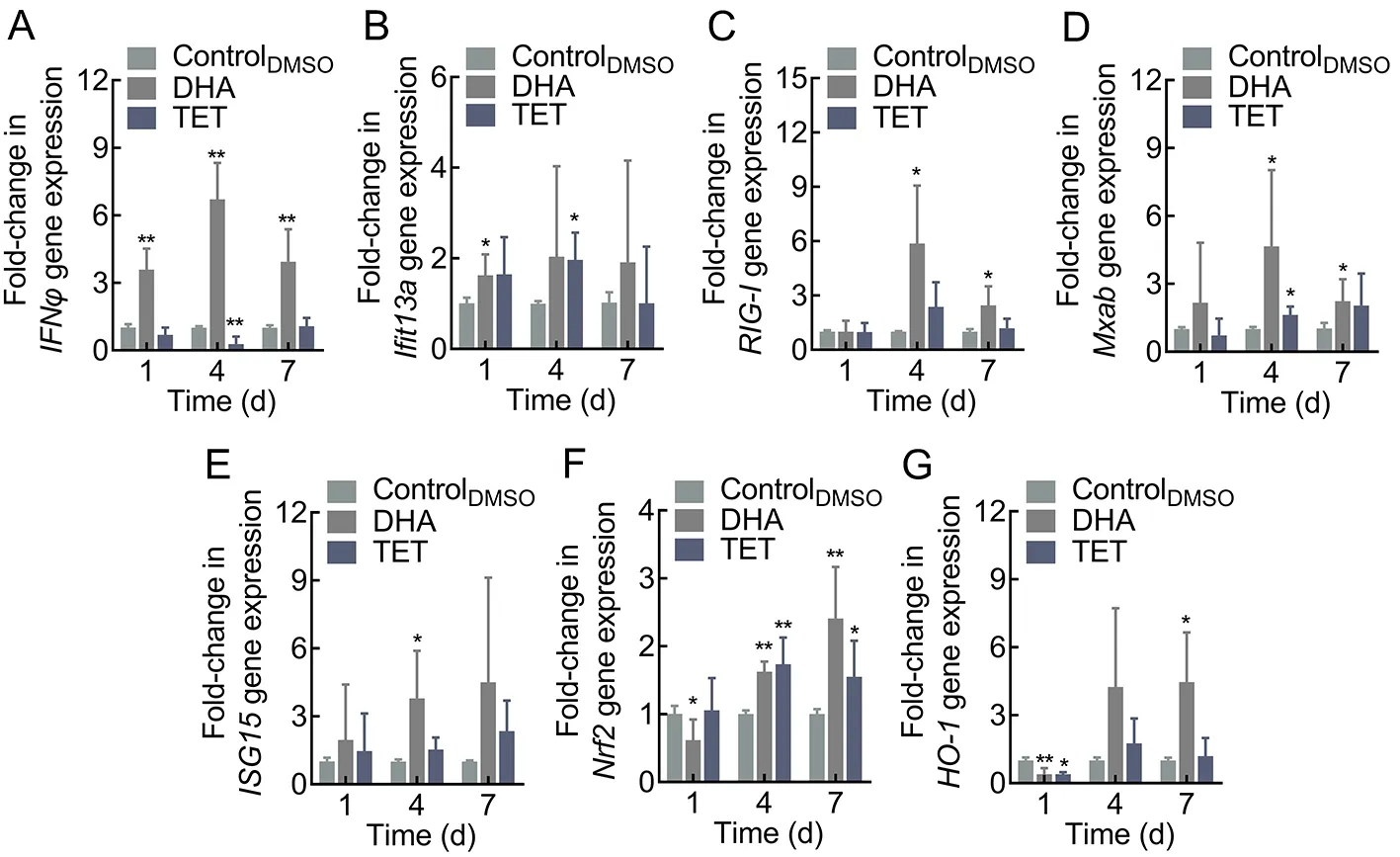
Figure 7 Effects of DHA and TET treatment on antiviral-related gene expression in zebrafish
DISCUSSION
SVC disease occurs in Europe, America, and several countries in Asia (Ashraf et al., 2016). Although efforts have been made to develop a vaccine for the prevention and treatment of SVCV infection, such procedures are difficult and costly to employ on a large scale (Su & Su, 2018). Several agents that interfere with early-stage viral replication have been explored previously and shown to have positive effects on inhibiting horizontal viral transmission (Balmer et al., 2017,2018; Liu et al., 2020a; Vigant et al., 2013). As such, we investigated various natural small molecules capable of interfering with the early replication stage of SVCV to meet the preventive and therapeutic needs of the aquaculture industry.Based on the antiviral activity of 16 natural small molecules,we found that DHA and TET inhibited SVCV replication and virus-induced CPE in EPC cells. Generally, for viruses, host cell apoptosis is favorable for assembled virion release (Li et al., 2019). Rhabdoviruses, including SVCV, can activate apoptosis, leading to structural alterations in host cells, such as apoptotic bodies and nuclear fragments (Li et al., 2019;
Licata & Harty, 2003). Natural small molecules, including flavonoids such as apigenin, baicalin, and luteolin, can inhibit cellular apoptosis caused by viruses, thereby limiting viral release (Lalani & Poh, 2020). As expected, DHA and TET treatment preserved nuclei with a normal spindle structure,reduced apoptotic body production, and inhibited nearly all nuclear damage induced by SVCV in the EPC cells. These findings suggest that DHA and TET can block SVCV-induced apoptosis and restrict virus release, thereby exerting antiviral effects. Furthermore, DHA and TET treatment decreased fish mortality and reduced viral load in SVCV-infected zebrafish,thus implying antiviral activity against SVCVin vivo. Given the importance of preventive measures in aquaculture biosecurity,we also investigated the effectiveness of DHA and TET pretreatment at preventing SVCV infection. Results showed that DHA and TET treatment prior to virus infection had a positive effect on reducing viral loadin vivo, thus demonstrating the ability to protect fish from SVCV infection.As SVCV can be excreted in water by infected fish through feces and urine, horizontal transmission is considered a primary route of infection (Ahne et al., 2002). According to previous studies on coumarin derivatives B4 and C3007,agents capable of reducing mortality and viral load in SVCVinfected fish can limit horizontal viral transmission to some extent (Liu et al., 2020a; Qiu et al., 2020), suggesting that DHA and TET may not completely inactivate the virus, but may reduce SVCV horizontal transmission. These results suggest that DHA and TET can be used not only as treatments in aquatic systems, but also as preventive measures against viral transmission in aquaculture settings.
Rhabdovirus replication requires a series of procedures(Shao et al., 2016). Notably, viral entry requires interactions between cellular receptors and viral proteins to allow the virus to adhere to and enter host cells via the endocytic pathway.Thereafter, the virus is released from the endosome to the cytoplasm, followed by genome replication, particle formation,and progeny release. The impact of antiviral agents on host cell adherence and endocytosis can help reduce host cell damage during viral infection. Various natural small molecules have been found to combat viruses by disrupting the early stages of viral invasion and have shown efficacy in inhibiting viral fusion and viral entry into cells (Musarra-Pizzo et al.,2021). The flavonoid quercetin can inhibit herpes simplex virus type 1 (HSV-1), HSV-2, and Singapore grouper iridovirus(SGIV) infection by blocking the binding of viruses to host cells(Hung et al., 2015; Liu et al., 2020b). Small-molecule antiviral coumarin derivatives occurring in several plants show efficacy in inhibiting the life cycle of viruses, including their binding,internalization, and post-entry transport (Liu et al., 2021; Song et al., 2020a, 2020b, 2021). In this study, TET and DHA both reduced the infectivity of SVCV, but only TET reduced infection by disturbing virus internalization. The pre-incubation of cells with DHA and TET also reduced SVCV replication. As fish rhabdovirus receptors are still understudied, whether fibronectin is associated with SVCV entry into cells and whether TET limits SVCV replication by affecting cell surface receptors such as fibronectin needs to be clarified with indepth study (Bearzotti et al., 1999). Given the dual effects of TET on SVCV and host cells, it could serve as a potential broad-spectrum therapeutic agent for rhabdoviruses.
Enhanced protection against viral diseases can be achieved not only by acting directly on the virus and disrupting its life cycle but also by stimulating the immune response of the host(Pereiro et al., 2021). Innate immunity, also known as nonspecific immunity, is one of the first lines of defense against pathogenic invasion (Beutler, 2004). Immunostimulants exhibit broad-spectrum activity and can improve immunocompetency and disease resistance by enhancing non-specific and specific defense mechanisms in fish. Thus, immunostimulant application is considered a promising method to prevent disease in a pathogen-independent manner. Growing evidence suggests that natural small molecules have the potential to stimulate complement activation, lysozyme activity, antibody response, and immune-related gene expression, thus acting as immunostimulants in aquaculture to enhance immunity and health in fish (Elumalai et al., 2021). As a cytokine, IFN1 plays a key role in innate immune defense,especially against viruses, in both mammals and fish (Chang et al., 2013). Previous studies have shown that coumarin derivatives with anti-SVCV activity can also reduce mortality in SVCV-infected fish by regulating the expression of antiviralrelated genes (Liu et al., 2019; Song et al., 2021). In the present study, antiviral-related genes, including IFNs, ISGs,RIG-Ⅰ, and MAVS, were markedly enhanced after DHA and TET treatment bothin vitroandin vivo. Both TNF-α and IL-1β are potent pro-inflammatory cytokines that participate in the regulation of other molecules and play key roles in regulating inflammation at the early stages of viral infection. Previous research has shown that β-glucan may exert anti-SVCV activity by increasing the expression levels of TNF-α and IL-1β(Medina-Gali et al., 2018). Consistently, we found that DHA and TET also promoted the expression of TNF-α and IL-1β.These findings suggest that DHA and TET can induce the expression of antiviral-related genes to enhance the host immune response and may be used as immunostimulants against viral disease outbreaks in aquaculture.
In conclusion, among the 16 natural small molecules examined, both DHA and TET attenuated SVCV-induced cellular changes and apoptosis and decreased viral load and mortality in zebrafish, with positive antiviral effects against SVCV bothin vitroandin vivo. Furthermore, TET exerted antiviral effects on early-stage viral replication and reduced damage caused by viral infection to the host. In addition, both TET and DHA activated host innate immunity, showing potential as immunostimulants to reduce viral disease outbreaks in aquaculture.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS" CONTRIBUTIONS
J.C. and L.L. designed the study. Y.Z., T.X.Q., and Y.H.performed the experiments. Y.Z. drafted the manuscript with assistance from L.L. All authors read and approved the final version of the manuscript.
杂志排行
Zoological Research的其它文章
- Optimization of sgRNA expression strategy to generate multiplex gene-edited pigs
- Virome in healthy pangolins reveals compatibility with multiple potentially zoonotic viruses
- Chronic lithium treatment ameliorates ketamineinduced mania-like behavior via the PI3K-AKT signaling pathway
- A glimpse into the biodiversity of insects in Yunnan: An updated and annotated checklist of butterflies(Lepidoptera, Papilionoidea)
- Unveiling the functional and evolutionary landscape of RNA editing in chicken using genomics and transcriptomics
- Animal models of Alzheimer’s disease: Applications,evaluation, and perspectives
