Immunotherapy for hepatocellular carcinoma: A promising therapeutic option for advanced disease
2022-11-29GianlucaCasseseHoSeongHanBoramLeeHaeWonLeeJaiYoungChoFabrizioPanaroRobertoIvanTroisi
Gianluca Cassese, Ho-Seong Han, Boram Lee, Hae Won Lee, Jai Young Cho, Fabrizio Panaro, Roberto IvanTroisi
Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, and its incidence continues to increase. Despite improvements in both medical and surgical therapies, HCC remains associated with poor outcomes due to its high rates of recurrence and mortality. Approximately 50% of patients require systemic therapies that traditionally consist of tyrosine kinase inhibitors. Recently, however, immune checkpoint inhibitors have revolutionized HCC management, providing new therapeutic options. Despite these major advances, the different factors involved in poor clinical responses and molecular pathways leading to resistance following use of these therapies remain unclear. Alternative strategies, such as adoptive T cell transfer, vaccination, and virotherapy, are currently under evaluation. Combinations of immunotherapies with other systemic or local treatments are also being investigated and may be the most promising opportunities for HCC treatment. The aim of this review is to provide updated information on currently available immunotherapies for HCC as well as future perspectives.
Key Words: Hepatocellular Carcinoma; Immunotherapy; Hepatocellular Carcinoma management; Hepatocellular Carcinoma therapy; Molecular targeted therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, with an estimated global incidence rate of 9.3 per 100000 persons per year and a corresponding mortality rate of 8.5[1]. While the majority of cases are in Eastern Asia, HCC incidence is widely increasing in northwestern Europe as well as in North America. HCC is also the fastest growing cause of cancer-related deaths in US males, tripling in both incidence and mortality rates[2]. The primary risk factors for HCC include underlying cirrhosis (independent of etiology) and chronic infection with hepatitis B virus or hepatitis C virus[3]. Since diabetes, obesity, and metabolic syndrome are also hypothesized to be risk factors associated with the development of metabolic cirrhosis, HCC is expected to become progressively more concerning as a health problem in the near future[4].
HCC is associated with a poor prognosis. A 5-year overall survival (OS) of 50–70% is only attained if the tumor is still resectable, owing to advances in both surgical and medical therapy[5]. Surgical treatments include liver transplantation (LT) and liver resection, with recurrence rates as high as 20% after LT and 70% after liver resection[6]. LT is the most effective curative treatment for cirrhotic patients, but it is reserved for patients who are ineligible for liver resection or radiofrequency ablation (RFA), as well as transplantable patients with recurrent HCC. Due to organ shortages, long waiting time for donors, and the risk of tumor progression, which leads to patient dropout, this is done[7].
Accordingly, liver resection is considered the first-line treatment for HCC in patients with compensated cirrhosis[8]. Thermal ablation is considered to be effective only for lesions smaller than 3 cm and when technically feasible[9]. Unfortunately, less than 30% of patients with HCC are eligible for these procedures because most patients have advanced disease or impaired liver function at the time of diagnosis, thus limiting aggressive treatment[10].
Trans-arterial chemoembolization (TACE) is the treatment of choice for patients with a suitable performance status[11]. Medical therapy is the only viable option for cases with disseminated disease or when other therapies are not feasible. However, HCC is notoriously resistant to chemotherapy and other systemic treatment modalities[12]. To date, systemic therapy is mainly based on the use of sorafenib, a multitargeted kinase inhibitor that improves survival by only 2.3-2.8 mo[13]. Indeed, the global median survival for patients with unresectable HCC is less than 1 year, highlighting the need for novel therapies to treat this disease.
Owing to an improved understanding of the molecular pathways of HCC carcinogenesis, other molecularly targeted approaches are under investigation. However, the intrinsic drug-metabolizing properties of the liver and other factors likely contribute to the limited efficacy of chemotherapeutics in the treatment of HCC[14]. In this review, we summarize novel immunotherapeutic approaches in HCC, reporting the latest evidence, analyzing their main limitations, and summarizing future perspectives that might overcome these drawbacks.
PHYSIO-PATHOLOGICAL BASIS
Liver immune system
Due to its physiological function, the liver has a peculiar and complex anti-inflammatory immune environment that develops tolerance to harmless foreign molecules, such as food antigens[15]. Kupffer cells (KCs), hepatic stellate cells (HSCs), and liver sinusoidal endothelial cells (LSECs) are the main mediators of this tolerogenic process[16]. KCs produce inhibitory cytokines interleukin 10 (IL-10) and prostaglandins and promote the activation of regulatory T cells (Treg)[17]. HSCs produces hepatocyte growth factor (HGF), that induces the accumulation of Treg cells inside the liver and promotes T-cell apoptosis through programmed death ligand 1 (PDL1) expression[18,19]. LSECs also play a fundamental role in the immune microenvironment of the liver by expressing high levels of PDL1 and actively participating in the induction of Treg cells, mainly through transforming growth factor-β (TGF-β)[20]. KCs, HSCs, and LSECs are antigen-presenting cells (APC)[19,21]. Hepatic dendritic cells (DCs) also contribute to the tolerogenic microenvironment of the liver as they are poor stimulators of effector CD4+ T cells. They express low levels of major histocompatibility complex (MHC) II and co-stimulatory molecules, producing anti-inflammatory prostaglandin E2, which can increase IL-10 secretion and induce Treg cells[22].
The complex physiological immune-tolerating microenvironments of the liver are altered during the formation and progression of HCC. A progressively and persistently downregulated immune gene profile has been identified to occur during HCC progression, which leads to lower tumor immunity in advanced stages of the disease, together with a physical barrier made of collagen and other matrix proteins that protect tumoral cells[23]. Therefore, an interesting strategy could be to use therapeutic compounds to disrupt collagen and promote intratumoral infiltration by CD8+ lymphocytes trapped in the peritumoral zone[24]. Different approaches to modulating this complex immune microsystem are desirable in combination with immunotherapies for HCC.
The tumor microenvironment of hepatocellular carcinoma
The tumor microenvironment (TME) of HCC is the result of complex interactions among hepatic nonparenchymal resident cells, tumor cells, immune cells, and tumor-associated fibroblasts[25]. The TME has important effects on the presence and activity of all signaling molecules, such as cytokines and chemokines, as well as other paracrine factors[26]. This complex cellular interplay has a substantial influence on tumor immune evasion, affecting both innate and adaptive immune responses and often leading to high levels of dysfunctional tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells[27,28].
The peritumoral environment at the forefront of HCC development is also important. KCs at this stage show higher levels of PDL1, and hyperactivation of HSCs in this TME is associated with a poor prognosis[29,30]. Similarly, PDL1 expression is higher in CD8+ T cells, which is associated with a higher risk of cancer recurrence, metastasis, and death[31]. Other molecules involved in the immune checkpoint have been identified in HCC and have been shown to correlate with poor prognosis, such as T-cell immunoglobulin, mucin-domain-containing molecule-3 (Tim-3), and lymphocyte-activation gene 3 in TILs and tumor-associated macrophages (TAM)[32].
Mechanisms of immune evasion in hepatocellular carcinoma
Several molecular mechanisms of immune evasion have been described in HCC, derived from the alteration of different signaling pathways, although much remains to be explored.
TGF-β is abundant in the HCC TME and can be produced by tumor cells, TAMs, and Treg cells[25,33]. TGF-β can reduce or eliminate antitumor responses by blocking T-cells and NK cells, inhibiting APC cells and TAMs, and activating Tregs[34-36]. High TGF-β expression has been shown to be associated with poor prognosis in HCC patients, and circulating levels are associated with clinical response to sorafenib and pembrolizumab[37,38].
Pro-inflammatory cytokines such as tumor necrosis factor and IL-1 are significantly downregulated in the HCC TME and are associated with increased levels of immunosuppressive cytokines, resulting in immune response dysfunction (IL-4, IL-5, IL-8, and IL-10)[23]. This has been associated with poor prognosis and worse clinical outcomes in several cancer types, including HCC[39]. Another pro-inflammatory cytokine, type I interferon (IFN), can activate the immune response; however, it can also trigger anti-inflammatory signals through the production of IL-10[40]. IL-10 is upregulated in HCC and is produced by TAMs and Treg cells. It can impair the capacity of APCs to recruit T cells and promote the upregulation of PDL1 in monocytes[41]. Furthermore, IL-10 Levels are associated with the number of myeloid-derived suppressor cells (MDSC)[42]. Representing the complexity of the TME, low IFNγ levels are associated with a worse prognosis in HCC[43].
Chemokines also play a fundamental role in recruiting Tregs via chemokine receptor 6 (CCR6) and chemokine ligand 20 (CCL20)[44]. The level of both Treg cells and TAMs in the liver is associated with poor prognosis in HCC[45,46]. A rare recently discovered immunosuppressive cell is represented by the T helper 17 cells, that produce high levels of PD1and inhibit NK cell function. Then there are the hepatic neutrophils that can recruit macrophages and Treg cells[47,48].
Vascular endothelial growth factor (VEGF) is a soluble molecule produced by tumor cells and the surrounding stroma[49]. It is known to promote tumor angiogenesis, but it can also act as an immune modulator by inhibiting liver APCs and activating MDSCs and Treg cells[50]. This immunomodulatory action of VEGF inhibitors may play a role into their anti-tumor activity. The presence of many possible immunoregulatory targets in the HCC TME has stimulated the investigation of different immunotherapies in HCC, some of which have been shown to be effective for other malignancies.
IMMUNE CHECKPOINT INHIBITORS
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies directed against extracellular ligands involved in the suppression of antitumor immune responses. These proteins are expressed by both cancer cells and the immune system cells. To date, only two categories of molecular targets have been examined in clinical trials, PD-1 and CTLA-4, and only two checkpoint inhibitors have been approved for use in HCC by the United States Food and Drug Association (FDA)[51,52], while many more promising targets are being investigated.
Nivolumab
Nivolumab is a human anti-PD-1 IgG4 monoclonal antibody targeting PD-1, currently used as a secondline therapy for HCC after approval by the FDA in 2017. Checkmate 040, a phase I/II dose escalation and expansion trial, showed substantial tumor reduction and good tolerability in HCC patients. In the Checkmate040 study, patients showed a median OS of 15 mo (95%CI: 9.6-20.2), with an objective response rate (ORR) of 15% (95%CI: 6-28)[53]. The median duration of response to nivolumab among the 48 patients was 17 mo (95%CI, 6-24 mo), with a 2-year survival rate higher than 80%[53]. As an unexpected result, patients with PD-1- and PD-L1 expression showed better survival outcomes, with a median OS of 28.1 mo (95%CI: 18.2-n.a.) vs. 16.6 mo in the group with low PD-L1 expression (95%CI: 14.2-20.2).
CheckMate 459, a phase III trial, comparied the efficacy of nivolumab vs sorafenib as a first-line treatment in 743 HCC patients[54]. In the Nivolumab cohort, the OS outcomes were not statistically significant (median survival 16.4 vs 14.7 mo, HR 0.85; P = 0.07). However, in this study, patients with PD-L1 expression > 1% in the nivolumab arm had a significantly higher ORR (28.2% vs 12.2%). Furthermore, the rate of grade 3 or 4 adverse events in nivolumab arm was only 22%, compared to 49% after sorafenib[54]. Currently, other clinical trials are investigating the role of nivolumab for HCC, either as monotherapy or in combination with other modalities (Tables 1 and 2).
Pembrolizumab
Pembrolizumab is an anti-PD-1 IgG4 antibody approved by the FDA in 2018 as a second-line systemic therapy for HCC after sorafenib. A phase II trial, KEYNOTE-224, showed high efficacy and tolerability of pembrolizumab in HCC patients with a significant gain of survival (HR, 0.78; P = 0.023), although it did not meet the prespecified statistical threshold[55]. The ORR was 17% (95%CI: 11-26), while1% of the patients showed a complete response to the treatment and 16% experienced a partial response. The median OS was 12.9 mo (95%CI: 9.7-15.5), and the median progression-free survival (PFS) was 4.9 mo (95%CI: 3.4-7.2). Sixty-two percent of patients showed a good disease control (95%CI: 52-71), while the 25% of patients experienced grade 3 or 4 adverse events, with 1 death[55]. More evidences are expected from KEYNOTE-240 and KEYNOTE-394, two phase III trials investigating the role of Pembrolizumab, that are still ongoing.
Similarly, trials evaluating the association between pembrolizumab and other treatments are ongoing. A phase Ib trial showed promising antitumor activity of the combination of pembrolizumab with lenvatinib (a multiple kinase inhibitor against VEGF receptors) as a first-line treatment in patients with unresectable HCC[56]. Furthermore, a phase III trial has investigated the safety and efficacy of pembrolizumab in combination with lenvatinib, while the role of pembrolizumab alone as adjuvant therapy after RFA or radiotherapy is still under evaluation (Tables 1 and 2).
Atezolizumab
Atezolizumab is an engineered IgG1 monoclonal antibody targeting PD-L1 and was recently approved by the FDA and European Medicines Agency in combination with bevacizumab as a first-line treatment for unresectable HCC. In the open-label phase III IMbrave150 clinical trial, 501 patients were randomly assigned at a ratio of 2:1 to receive atezolizumab plus bevacizumab or sorafenib[57]. The combination of atezolizumab and bevacizumab showed significantly higher 12-months OS, 67.2% (95%CI: 61.3-73.1) vs 54.6% (95%CI: 45.2-64.0), respectively, with 52% and 40% of patients surviving at 18 months, respectively. Similarly, the median PFS was improved at 6.8 months (95%CI: 5.7-8.3) vs 4.3 months (95%CI: 4.0-5.6), respectively (P < 0.001).
Further studies investigating the combination of atezolizumab with other treatments are ongoing (Table 2). In particular, the COSMIC-312 phase III study tested atezolizumab plus cabozantinib (an oral tyrosine kinase inhibitor against VEGFR, FLT-3, MET, AXL, KIT, Tie-2, and RET) vs sorafenib as firstline therapy. Two phase III studies are enrolling patients to receive atezolizumab plus bevacizumab in combination with TACE or adjuvant therapy after surgery of RFA. Finally, results from several studies investigating other anti-PD-1 antibodies, such as tislelizumab compared to sorafenib, and anti-PD-L1 antibodies, such as durvalumab or avelumab, are expected (Table 2).
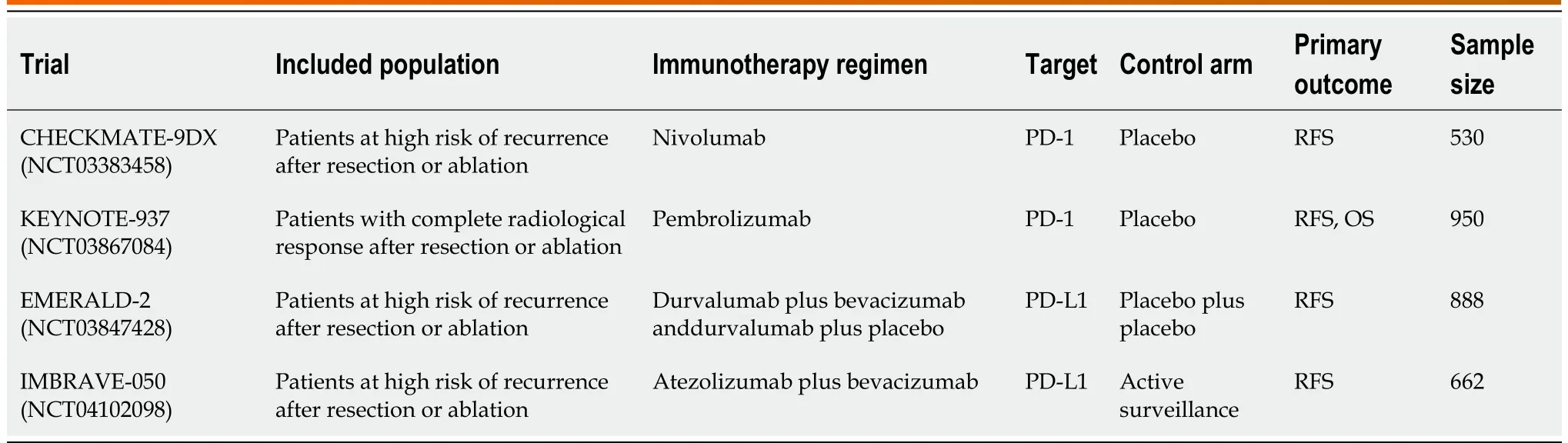
Table 1 Clinical trial involving immunotherapic agents as adjuvant therapy for hepatocellular carcinoma

Table 2 Ongoing clinical trial involving immunotherapic agents as first line therapy for hepatocellular carcinoma
VACCINE THERAPY IN HEPATOCELLULAR CARCINOMA
The development of vaccines against different types of cancer aims to enhance existing tumor-specific responses. Due to altered T cell activity in the HCC TME, vaccine therapy is usually investigated in combination with ICI or other therapies[58]. Although the first HCC vaccines were shown to be safe and have immunologic effects, their clinical efficacy is still limited, possibly because of immunological tolerance to self-antigens that causes them to not be completely tumor-specific[59,60]. Thus, new strategies are currently under investigation.
Alpha-fetoprotein peptide
Alpha-fetoprotein (AFP) is a 70-kDa protein expressed during fetal development and in the adult liver. Serum AFP levels are usually not detectable in the adult. However, AFP levels increase in approx-imately 70% of HCC cases, allowing for its use as a biomarker[61,62]. Butterfield et al[63] constructed a human AFP-expressing replication-deficient adenovirus as a new target for T-cell-based immunotherapy. AFP-based therapies have been evaluated in phase I/II trial with two patients with HCC who had an AFP-expressing tumor and who completed a previous treatment for HCC, and their tolerability and safety were confirmed. Additionally, both patients experienced high levels of AFP-specific CD8+ T cells, further confirming their preexisting immunity[64]. Further studies are required to confirm these results.
Multidrug resistance-associated protein 3
Multidrug resistance-associated protein 3 (MRP3) is a carrier-type protein that is highly expressed in several human cancers, including HCC[65]. Interestingly, MRP3 is also associated with resistance to sorafenib toxicity in HCC cells[66]. The safety and the immune response to the vaccine based on an MRP3-derived peptide (MRP3765) were tested in a phase I study involving 12 HLA-A24-positive HCC patients[67]. The vaccine showed a good safety profile, with an immune response in 72.7% of the treated cases and a median OS of 14.0 mo (95%CI: 9.6-18.5). OS was notably better than in patients undergoing hepatic arterial infusion chemotherapy without the MRP3 vaccination (median OS 12.0 12.6 mo)[68].
Glypican-3
HCC cells sometimes overexpress proteins relative to the surrounding healthy tissue, as is the case for glypican-3 (GPC3)[69]. Therefore, the GPC3-derived peptide vaccine was tested and reported as safe in 33 patients with advanced HCC in a phase I clinical study, showing good results in terms of GPC3-specific immune response[70]. However, only 1 patient developed a partial response, while 19 patients had stable disease. Interestingly, another phase II study investigating the GPC3-derived vaccine as adjuvant therapy showed significantly lower recurrence rates than with surgery alone after 1 year (52.4% vs 61.9%, P = 0. 387) and 2 years (24% vs 48%, P = 0.047)[71].
Oncolytic viruses
Oncolytic viruses are viral units engineered to obtain direct lysis of tumor cells, releasing soluble cancer peptides to induce antitumor neoantigen-specific cytotoxic T lymphocyte responses[72]. A phase II study (NCT00554372) assessed the safety of two doses of JX-594 (Pexa-Vec, by testing both low dose or high dose in 30 patients with HCC. All patients experienced dose-dependent flu-like syndrome with fever, rigor, and vomiting within the first few days[73]. Furthermore, when tested as a second-line treatment, there was no significant survival difference when compared to the standard of care (4.2 vs 4.4 mo, 95%CI: 0.78-1.80; P = 0.428)[74]. Other schemes are currently being tested.
Dendritic cell vaccines
DCs can be activated with a specific antigen in vitro and then injected into patients to enhance the immune response. Wang et al[75] obtained encouraging antitumor effects in murine models treated with DCs activated by tumor cell lysate. A good tolerability profile was reported after a phase I study testing autologous DCs on 10 patients with cholangiocarcinoma or HCC, and after a phase II clinical trial with 35 patients using DCs[76]. Interestingly, DC infusion enhanced a stronger tumor-specific immune responses in combination with TACE, than TACE alone, even if without improved survival outcomes[77]. However, further clinical trials are warranted.
New York esophageal squamous cell carcinoma-1
New York esophageal squamous cell carcinoma-1 (NY-ESO-1) is a promising target antigen owing to its low expression in healthy tissue[78]. A specific CD8+ T cell response to NY-ESO-1b has been reported in 48% of patients with HCC expressing NY-ESO-1 mRNA and HLA-A2[28]. Furthermore, there was a correlation between such response and patient survival. However, no studies have yet been conducted to investigate the clinical response to NY-ESO-1 vaccines in patients with HCC.
对于f2的分析如下,每个电子都受到垂直于导线的分力f2,而导体棒受到向左的力F总则是这些分力f2的合力。我们可以先假设该导体棒的长度为L,其横截面积为S,在单位体积内的电荷数为n,且做定向运动的自由电子的电量为e。
ADOPTIVE CELL THERAPY
Adoptive cell therapy (ACT) is a passive therapy in which lymphocytes are activated and/or expanded ex vivo, and then re-injected into the patient[79]. The treated cells include lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, NK cells, TILs, and redirected peripheral blood T cells. These latter cells are genetically programmed to recognize and attack tumor cells.
Chimeric antigen receptor T cells
An individual’s own T cells can be engineered to recognize tumor cell surface proteins and, in turn, cause cancer cell death, as demonstrated by approximately six chimeric antigen receptor T cell (CAR T cell) therapies already approved by the FDA since 2017 (all of them for blood cancers). A similar effect was observed by targeting GPC3-positive HCC cells in vitro and in mice[80,81]. Takayama et al[82] published excellent results from a trial of 150 patients who were randomly assigned to receive adjuvant adoptive immunotherapy or no treatment. After a median follow-up of 4.4 years, adoptive immunotherapy decreased recurrence rates by 18% compared with that in controls, with a shorter time to first recurrence [33% (95%CI: 22-4) vs 48% (95%CI: 37-59) at 3 years; 22% (95%CI: 11-34) vs 38% (95%CI: 22-54) at 5 years; P = 0.008]. The immunotherapy group had a significantly longer recurrence-free survival (P = 0.01) and disease-specific survival (P = 0.04). Several phase I and phase II clinical studies are currently evaluating CAR-GPC3 T-cell therapy alone or in combination with fludarabine, cyclophosphamide, or other treatment options.
Cytokine-induced killer cells
CIK cells are a heterogeneous population of effector CD3+CD56+NK cells expanded in vitro from peripheral blood mononuclear cells. They are used as pharmacological tools for cancer immunotherapy because they exhibit MHC-unrestricted, safe, and effective antitumor activity. Firsts attempts to develop ACT for HCC were not able to reach the clinical stage owing to the technological complexity and to the low efficacy. A phase II study of 127 patients and a phase III study of 200 patients, in which CIK cells were tested as adjuvant therapy and compared with no adjuvant therapy, showed improved DFS after CIK immunotherapy, although the increase in OS was not statistically significant[83]. Improved OS was observed only in patients diagnosed with tumors > 5 cm in size (P = 0.0002). Furthermore, the combination of CIK immunotherapy and minimally invasive therapies in HCC patients with no history of previous surgery was reported to be safe and feasible, as well[84,85]. As a first-line therapy, the CIK cell treatment followed by TACE and RFA group were compared with those treated with TACE + RFA. Although no significant difference in disease control rates was found between the two cohorts, survival analysis showed that patients in the CIK+TACE+RFA group had a significantly longer median OS of 56 mo (95%CI: 38.09-73.91) compared to 31 mo of TACE+RFA alone (95%CI:24.53-37.47)[85].
TILs can also be created from fresh tumor tissue to produce tumor-reactive expanding cells, screened on the basis of the ability to recognize autologous tumor cells, and further expanded to obtain several billion active cells[86]. The safety and feasibility of adjuvant TIL therapy were demonstrated in a phase I trial on HCC patients[87]. Current challenges include the capability of further expand tumor-specific T cells and scaling up the manufacturing process.
OVERCOMING CURRENT LIMITATIONS TO IMMUNOTHERAPY
Enhancing locoregional therapies
Locoregional therapies can be a strong ally in the immunologic war against HCC, owing to several advantages. Their relatively easy accessibility makes HCC an ideal target for local interventions, such as thermal ablations or intra-arterial therapies, and image-guided interventions are a common practice in this setting of patients. Therefore, these approaches can intratumorally deliver immunostimulant agents, allowing not only more potent immunological responses but also potentially decreasing toxicity. Indeed, such agents are often quite toxic when administered systemically, causing sepsis-like cytokine release syndrome and systemic inflammation[88]. Thermal ablation has been shown to activate immune responses and T cell infiltration in HCC[89]. Furthermore, to obtain stronger immune stimulation, different locoregional therapies can be combined sequentially or simultaneously with systemic immunotherapy[90].
Promising results were obtained from the combination of thermal ablation or TACE plus tremelimumab in patients with advanced HCC, with a reported response rate of 26%, a disease control rate of 89%, an OS of 12.3 mo, and 45% of the stabilizations lasting longer than 6 mo[91]. These encouraging data have prompted new clinical trials combining durvalumab/tremelimumab with TACE or RFA, and these trials are ongoing.
Combination immunotherapy strategies
The combination of multiple immunotherapies could be another option to overcome the limitations of single treatments. Although this approach could increase the risk of high-grade adverse events, the initial results are encouraging. A combination of nivolumab (NIVO) and ipilimumab (IPI) was tested as a second-line therapy after sorafenib. Following treatment with this combination, patients showed an ORR twice that of the NIVO mono-treated patients (31% and 14%, respectively). Thirty-seven percent of the patients had grade 3-4 treatment-related adverse events, but only 5% had an event leading to therapy discontinuation[92]. Similar results were reported in another study in which nivolumab was tested with cabozantinib (CABO) and ipilimumab, both as double and triple therapies. The median PFS was 5.5 mo for patients in the NIVO + CABO and 6.8 mo for those in the NIVO + IPI + CABO groups, while the median OS was not reached. However, the triple combination led to grade 3-4 treatmentrelated adverse events in 71% of patients, with 20% discontinuing therapy[23]. Similarly, a phase II trial investigating the safety and efficacy of a combination of durvalumab and tremelimumab is currently recruiting patients.
Immune checkpoint inhibitors can also be combined with oncolytic viruses; this strategy has been tested in several ongoing randomized trials, but without published results. Another study investigated the combination of activated T cell transfer (ATVAC) with an autologous tumor lysate-pulsed DC vaccine as an adjuvant therapy in HCC patients, showing an improved median PFS of 24.5 mo (95%CI: 7.8-41.2) and OS of 97.7 mo (95%CI: 48.6-146.7), compared to a median PFS of 12.6 mo (95%CI: 6.9-18.3) and OS of 41.0 mo (95%CI: 16.3-65.8) of the other group. No adverse events of grade 3 or more were observed[93]. These encouraging results need to be confirmed in future studies.
Tailoring HCC immunotherapies
As previously discussed, several trials have shown encouraging results in certain subgroups of HCC patients, although the overall outcomes have not improved much. Identification of patient subsets that would benefit from ICI therapy should be a mainstay of current cancer research. Indeed, the identification of the best candidates for immunotherapies and combination therapies can play a fundamental role not only in achieving the best results but also in saving a substantial amount of funding and healthcare resources. At the same time, a better understanding of patient characteristics could help to avoid related toxicities.
Some characteristics of the patients in the KEYNOTE-240 and CheckMate 459 trials have already been reported, identifying Asian patients and those with AFP levels > 200 ng/mL as the patient groups showing the best outcomes[54]. The latter study also showed a better OS among patients with vascular invasion or extrahepatic disease. In the IMbrave150 trial, OS and PFS were worse in patients with a nonviral etiology, high AFP levels, no macro-vascular invasion, and extrahepatic disease[57].
The genetic features of HCC have been identified using next-generation sequencing (NGS), and several biomarkers have been identified as useful for selecting the best candidates for new targeted therapies. NGS analysis detected ten patients with WNT/β-catenin mutations that did not respond to anti–PD-1 or anti–PD-L1drugs, while 50% of CTNNB1 WT cases showed a response[94]. The WNT/βcatenin mutation was also correlated to lower median PFS (2.0 vs 7.4 mo; 95%CI: 2.9-28.8; P < 0.0001) and OS (9.1 vs 15.2 mo; 95%CI: 0.76-8.7; P = 0.11) than WNT/β-catenin wild type. Further studies are needed to determine the clinical implications of NGS in HCC therapy.
CONCLUSION
In conclusion, HCC is a widely studied yet challenging disease. Systemic therapies have shown modest results; however, due to tremendous improvements in basic molecular research on anti-tumor immune responses in the TME, a new class of molecular therapies is emerging and changing the HCC therapeutic landscape. Several clinical trials are ongoing, providing hope for an epochal turning point. In our opinion, the development of synergies between immunotherapies and locoregional or radical therapies is likely to be key in the future of HCC therapy. Similarly, another area where a major shift in HCC management may arise is the role of immunotherapy in adjuvant therapy. In fact, immunotherapy used in an adjuvant setting after surgery showed promising results, affecting recurrence rates, which represents a major challenge following surgical therapy. These results suggest the usefulness of immunotherapy, even in early stages, such as in patients undergoing tumor resection or ablation. Importantly, technological advances and recent evidence have also paved the way for the identification of molecular mechanisms involved in sensitivity and resistance to individual agents or combinations, helping advance the era of personalized medicine. We are convinced that these findings may help in the adoption of and adaptation to different types of therapies for individual patients in the near future. Considering the speed and breadth of discoveries in this field, efforts should be made to embed correlative research studies in every new clinical trial.
FOOTNOTES
Author contributions: Han HS, Cassese G, Troisi RI, and Panaro F conceived and designed the study; Cassese G and Lee B wrote the manuscript; Han HS, Cho JY, Lee HW, and Troisi RI participated in the coordination of the work and in the final revision. All authors approved the final manuscript.
Conflict-of-interest statement: All the authors declare that they have no conflict of interest.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin: South Korea
ORCID number: Gianluca Cassese 0000-0001-9185-2054; Ho-Seong Han 0000-0001-9659-1260; Fabrizio Panaro 0000-0001-8200-4969.
S-Editor: Liu JH
L-Editor: A
P-Editor: Liu JH
猜你喜欢
杂志排行
World Journal of Hepatology的其它文章
- Natural history and management or liver aySuncuon ystorage disorders
- Long-term and non-invasive in vivo tracking of DiD dye-labeled human hepatic progenitors in chronic liver disease models
- Quality of life, depression and anxiety in potential living liver donors for pediatric recipients: A retrospective single center experience
- Hepatic involvement in children with acute bronchiolitis
