Quality Standard of Yi Herb Medicine Millettia dielsiana
2022-11-28MaliniuSHAWenbingLIDongmeiSHAMeiyiTONGJingWANGXinjiaYANYuanLIU
Maliniu SHA, Wenbing LI, Dongmei SHA, Meiyi TONG, Jing WANG, Xinjia YAN*, Yuan LIU*
1. College of Pharmacy, Southwest Minzu University, Chengdu 610041, China; 2.Sichuan Provincial Qiang-Yi Medicinal Resources Protection and Utilization Technology Engineering Laboratory, Chengdu 610225, China; 3.Tibetan Plateau Ethnic Medicinal Resources Protection and Utilization Key Laboratory of National Ethnic Affairs Commission of the People’s Republic of China, Chengdu 610225, China
Abstract [Objectives] The paper was to establish the quality standard of Yi herb medicine Millettia dielsiana. [Methods] Yi herb medicine M. dielsiana was qualitatively identified by microscopic identification and thin layer identification. The content of total isoflavones was determined by UV-Vis spectrophotometry, and the contents of moisture, total ash, acid-insoluble ash and extract were checked according to the method introduced in Chinese Pharmacopoeia (2020 edition). [Results] The microscopic identification features, including stone cells, fiber bundles, calcium oxalate square crystals, starch granules, pigment blocks, ducts, etc., were distinct. A TLC method for identification of M. dielsiana was established. The contents of water, total ash, acid-insoluble ash and extract should be no more than 13%, no more than 7%, no more than 0.5% and no less than 6% in 10 batches of samples, respectively. The total isoflavones of M. dielsiana based on genistein (C15H10O5) should be no less than 0.25%. The linear relationship of genistein was good in the range of 0.002-0.007 mg/mL (R2=0.999 6); the average recovery was 97.01% and the RSD was 2.62%. [Conclusions] The method is simple, accurate and well-reproducible, and can be used for the quality control of Yi herb medicine M. dielsiana.
Key words Yi herb medicine, Millettia dielsiana, Genistein, TLC, UV-Vis spectrophotometry
1 Introduction
Yi herb medicineMillettiadielsianaHarms., belonging toMillettia, Leguminosae, is mainly distributed in south China, southwest and central China. It is featured by warm nature, bitter and sweet taste, and enters to the liver and kidney meridian, with the effects of nourishing liver and kidney, supplementing essence and blood, and be used in the treatment of insufficiency of essence blood, lung asthenia and exhaustion heat, impotence and seminal emission, gonorrhea, irregular menstruation, unhealing ulcer,etc.[1-5]. It is one of the main drugs of Yi herb medicine Tongfeng particles prepared by Sichuan medical institutions. As an important branch of ethnic medicine, Yi herb medicine has its unique characteristics in the treatment of gout, with remarkable clinical efficacy[6]. The earliest records aboutM.dielsianaappeared inAnIllustratedBookofPlants[7]. It has been reported thatMillettiaspp. is rich in flavonoids, terpenoids, lignans, steroids, alkaloids and other compounds, and has immunomodulatory, anti-tumor and anti-inflammatory effects[8].M.dielsianacontains isoflavone[9], isoflavan[10], triterpenes[11]and other active ingredients, and its ingredient genistein compounds have various pharmacological activities of estrogen-like effects, anti-tumor effects, metabolism regulation, preventing osteoporosis, and preventing cardiovascular diseases[12-17]. The current standard inSichuanProvincialStandardforChineseMedicinalMaterials(2010 edition) contains only primitives, characters and identification items, which is not strong in specificity and lack of necessary quantitative control indicators to effectively control the quality of medicinal materials, and there are few relevant reports at present. Therefore, based on the study ofM.dielsianaand its similar species, the qualitative identification and quantitative determination methods of the herb was established in the test, in order to provide laboratory data for the 2022 edition ofSichuanProvincialStandardsforQiang,Yi,andMiaoHerbMedicine.
2 Materials
2.1 InstrumentsTU1810 UV-visible spectrophotometer (Beijing Persee General Instrument Co., Ltd., China); ATY124 electronic balance (SHIMADZU, Japan); AUW220D electronic balance (SHIMADZU, Japan); HWS-26 thermostatic electric water bath (Shanghai Yiheng Scientific Instruments Co., Ltd., China), CAMAG ATS 4 automatic TLC sampler (CAMAG, Switzerland).
2.2 ReagentsReference substance genistein (batch number RP210813, purity 99.36%) was produced by Chengdu Maide Biotechnology Co., Ltd. Silica gel G thin layer plate was manufactured by Qingdao Marine Chemical Plant. All reagents were analytically pure, and the water was ultrapure.
2.3 Medicinal materialsA total of 10 batches of medical materials were identified as the dry stem ofM.dielsianaby Professor Liu Yuan from Southwest University for Nationalities. The information is shown in Table 1.
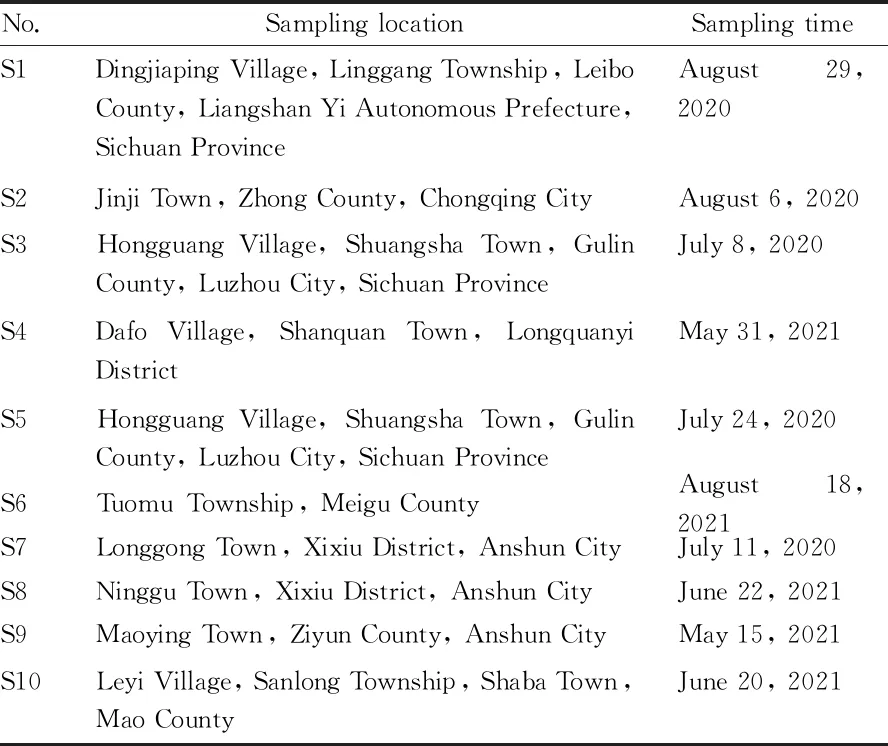
Table 1 Information of Millettia dielsiana samples
3 Methods and results
3.1 Primitive identificationShrubs climbing, 2-5 m long. Bark grayish brown, defoliated; branchlets glabrous or puberulent. Compound leaves pinnate, 15-30 cm long; petiole 5-12 cm long, rachis sparsely pilose, gradually bare, furrowed; stipules linear, 3 mm long. Leaflets 2-paired, 3-5 cm apart, papery, lanceolate, oblong to narrowly oblong, (5-15) cm×(1.5-6.0) cm, apex acute to acuminate, occasionally blunt-rounded, base blunt-rounded, occasionally subcordate, adaxially shiny, subglabrous, abaxially appressed pilose or glabrous. Lateral veins 6-9-paired, midvein slightly concave adaxially, very elevated abaxially, veinlets reticulate, prominent on both surfaces. Petiolule 2-3 mm long; stipulules spiny, 3-5 mm long. Panicles broad, up to 40 cm long, flowering branches extended, 6-15 cm long, suberect when shorter, fanlike and pendulous when long, rachis±yellow-brown pilose. Flowers solitary, 1.2-2.4 cm long; bracts linear, conical at tip, slightly shorter than pedicels, persistent; bracteoles linear, adnate to calyx, caducous. Pedicels ca. 5 mm; calyx broadly campanulate, (3-5) cm×(4-6) mm, pilose as pedicel, calyx teeth shorter than calyx tube, upper 2 teeth nearly connate, others ovate to deltoid-lanceolate, lower 1 tooth longest. Corolla purplish red, vexilla broadly ovate to broadly obovate, densely rust-colored or silvery sericeous, base slightly cordate, with short carpophore, callosum absent; ala wing very short, about half of vexilla, sharp at tip, auriculate at abaxial side, carina falcate. Stamens disomeric; disc shallow dish-shaped. Ovary linear, densely tomentose, style longer than ovary, convoluted, ovules 8-9. Pods linear to oblong, (7-12) cm×(1.5-2.0) cm, flattened, densely gray tomentose; carpel thin, subwoody, lobed, 3-5-seeded. Seeds oblong-convex, ca. 8 cm×6 cm, ca. 2 cm thick. Fl. May-Sept, fr. Jun-Nov (Fig.1)[1].

Note: A. Habitat; B. Whole plant.
3.2 Morphological identificationThe product was oval, quasi-circular or irregularly beveling, 0.5-8.0 cm in diameter. The cortex was rough, taupe to tan, with oval lenticels. There was a ring of reddish-brown to tan resinous material near the xylem. The xylem was pale yellow, with many pores, and the pith was small (Fig.2). The product was featured by hard texture, mild odor and astringent and slightly bitter taste.

Fig.2 Morphological identification of Millettia dielsiana
3.3 Microscopic identification
3.3.1Cross section of stem. The cork layer of cross section of the product was composed of multiple columns of cells, containing light brown red or brown red substances. The cortex was narrow, with more than 10 columns of cells. The phloem was broad and densely packed with small crystalline sclerenchyma cells, with calcium oxalate square crystals in cells; there were numerous transverse oval secretory cells filled with brown to brownish-red secretions; on the lateral side of phloem there were stone cells grous and fiber bundles arranged intermittently in a ring. The cambium formed a ring. The phloem and wood rays were 2-3 rows of cells in width, distinct. The xylem ducts were mostly single and scattered, and the xylem was differentiated to the center without pith part (Fig.3).

Note: A. Full diagram; B. Detail diagram; 1. Cork layer; 2. Cortex; 3.Phloem; 4. Xylem; 5. Stone cells grous; 6. Secretory cell; 7. Phloem ray; 8. Ray.
3.3.2Powder identification. The powder of the product was grayish white to grayish brown. The pigment blocks were interspersed, reddish brown, yellowish brown or tan. The stone cells were colorless or yellowish, quasi-square, polygonal or irregular, 12-74 μm in diameter. The cells around fiber bundles contained calcium oxalate square crystals, forming crystal fibers. The calcium oxalate square crystals were mostly biconical, with a length of 9-35 μm. Starch grains were mostly single, 7-25 μm in diameter. Bordered pit vessel can be seen (Fig.4).

Note: 1. Pigment block; 2. Stone cell; 3. Fibre bundle; 4. Calcium oxalate square crystal; 5. Starch grain; 6. Duct.
3.4 Thin layer chromatographyApproximately 1 g of powder of the product was added with 10 mL of methanol and treated by ultrasound for 30 min, and the filtrate obtained was used as the test solution. Approximately 1 g of powder of reference medicinal material was prepared into reference solution by the same method. According to TLC test (General rule 0502), 2-5 μL of each of the above two solutions were absorbed and respectively dotted on the same silica gel G thin layer plate. With gyclohexane-ethyl acetate- glacial acetic acid (15∶3∶0.5) as the developing agent, the solutions were unfolded, taken out, dried, sprayed with 10% sulfuric acid ethanol solution, and heated at 105 ℃ until the spots developed clear color. The solutions were examined under the ultraviolet lamp (365 nm). In the chromatogram of the test sample, fluorescent spots of the same color appeared at the position corresponding to the chromatogram of the reference medicinal material (Fig.5).

Note: 1. Reference medicinal material of M. dielsiana; 2-10. Samples of M. dielsiana.
3.5 Determination of moisture, ash and extract contentAccording to the four general rules ofChinesePharmacopoeia(2020 edition)[18], the moisture (General Rule 0832), total ash (General Rule 2302), acid-insoluble ash (General Rule 2302) and extract (General Rule 2201) were examined, and the results are shown in Table 2.

Table 2 Determination results of moisture, ash and extract content %
3.6 Determination of total isoflavone content[19-21]
3.6.1Selection of detection wavelength. The genistein reference was scanned at full wavelength (200-400 nm) on a UV detector, and the maximum absorption was observed at 260 nm (Fig.6).
3.6.2Preparation of test solution. Accurately 1.0 g of powder of the product (pass through No. 3 sieve) was added with 50 mL of 70% ethanol, and weighed. After heat reflux extraction in water batch for 1 h, the solution was cooled down and weighed again. The lost weight was made up with 70% ethanol, and the solution was shaken well and filtered. Accurately 500 μL of the solution was absorbed by a pipette gun and then diluted in a 10 mL volumetric flask to the constant volume as reserve liquid.
3.6.3Investigation of linear relation. Accurately 10.02 mg of genistein reference was weighed and placed in a 50 mL volumetric flask, and the volume was fixed to the scale by adding 70% ethanol. Accurately 0.10, 0.15, 0.20, 0.25, 0.30, 0.35 mL of the solution were placed in 10 mL volumetric flasks, added with 70% ethanol to the scale, and shaken well. The absorbance was measured at the wavelength of 260 nm by UV-Vis spectrophotometry (General Rule 0401) with 70% ethanol as the blank. Taking absorbance (A) as the ordinate and genistein content (X, mg/mL) as the abscissa, the regression equation of genistein wasY=1.162×102X+6.56×10-2,R2=0.999 6, indicating that genistein had a good linear relationship in the range of 0.002-0.007 mg/mL.
3.6.4Precision test. Appropriate amount of genistein reference solution was accurately absorbed and diluted properly. The absorbance of genistein was measured by repeated injection for 6 times at the wavelengths described in Section3.6.1since 70% ethanol was used as blank. TheRSDof genistein absorbance was 0.374%, indicating that the instrument had good precision.
3.6.5Stability test. Appropriate amount of test solution (S1) was accurately absorbed. The absorbance was measured at 0, 1, 2, 3 and 4 h at the wavelengths described in Section3.6.1, and theRSDof absorbance was 1.228%, indicating that the solution had good stability.
3.6.6Reproducibility test. Six samples of fine powder (S1), each about 1.0 g, were accurately weighed, and diluted to prepare the test solution according to the above method. At the wavelengths described in Section3.6.1, theRSDof absorbance was 2.569%, suggesting that the method had good reproducibility.
3.6.7Recovery test. Six samples of fine powder (S1), each about 0.5 g, were accurately weighed and placed in a conical flask with cover, and appropriate amount of reference was precisely added. The test solution was prepared according to the method described in Section3.6.2, and the absorbance was measured at the wavelengths described in Section3.6.1. The average recovery of genistein was 97.01%, and theRSDwas 2.62% (Table 3).

Table 3 Results of recovery test for genistein (n=6)
3.6.8Determination of sample content. The above 10 batches ofM.dielsianasamples were taken, and the test solution was prepared according to the method described in Section3.6.2. The absorbance was measured at the wavelengths described in Section3.6.1, and the content was calculated by the external standard method. The genistein contents of S1-S10 samples were 0.337 7%, 0.349 7%, 0.428 9%, 0.430 6%, 0.473 7%, 0.317 0%, 0.310 2%, 0.348 9%, 0.422 0%, 0.338 6%, with an average of 0.375 7%.
4 Discussion and conclusions
4.1 Moisture, ash and extractBased on the four general rules ofChinesePharmacopoeia(2020 edition) and the relevant requirements ofGeneralRulesfortheVerificationofMedicinalMaterialsandDecoctionPieces, it was tentatively determined that the contents of moisture, total ash, acid-insoluble ash and extract should be no more than 13%, no more than 7%, no more than 0.5% and no less than 6%, respectively.
4.2 Thin-layer identificationThe development systems of trichloromethane-acetone (12∶1), ethyl acetate-methanol-water (9∶3∶1), cyclohexane-ethyl acetate-glacial acetic acid (10∶3∶0.5), cyclohexane-ethyl acetate-glacial acetic acid (15∶3∶0.5) were investigated. With gyclohexane-ethyl acetate- glacial acetic acid (15∶3∶0.5) as the developing agent, the solutions were unfolded, taken out, dried, sprayed with 10% sulfuric acid ethanol solution, and heated at 105 ℃ until the spots developed clear color. When the solutions were examined under the ultraviolet lamp (365 nm), it was found that the spots were clear with the best resolution. Therefore, the TLC method was established by using this condition.
4.3 Total isoflavone content and limitScholars at home and abroad have dedicated much effort to chemical components ofM.dielsiana. The main chemical components include flavonoids, alkaloids, triterpenes and sterides, and there are more flavonoids, such as daidzein, genistein and formononetin. But due to the characteristics of more components and less content, it is not easy to determine the content of each component.
The content of total isoflavones is quite different among different producing areas and batches, probably attributed to factors such as harvesting area and time. The method established in this test is simple and feasible, and can evaluate the quality ofM.dielsianaaccurately and scientifically. The total isoflavone content inM.dielsianais tentatively calculated as genistein (C15H10O5), which should not be less than 0.25%.
4.4 SummaryAs the source of Chinese patent medicine, whether the quality of Chinese medicinal materials is uniform and stable directly affects the quality of Chinese patent medicine products, thus affecting the clinical effectiveness and safety[22]. Therefore, it is very important to control the quality of medicinal materials. The quality standard ofM.dielsiana, a Yi herb medicine, was established in the study, and a comprehensive and specialized quality control method was established, which is beneficial to the safety and effectiveness of the medicinal material in clinical application. Moreover, it will provide a basis for the formulation of quality standard and the development and utilization of resources of this medicinal material.
杂志排行
Medicinal Plant的其它文章
- Establishment of Quality Standard for Freeze-dried Tablets of Polygonatum sibiricum and Study on Anti-tumor Activity of Diosgenin
- Herbal Textual Research of Inulae Flos in Chinese Classic Prescription
- Quality Standard of Zijinbiao
- Quality Standard of Yi Medicinal Material Anaphalis margaritacea
- Serum Metabolomic Characteristics of Primary Dysmenorrhea Rat Model Induced by Estradiol Benzoate Combined with Oxytocin
- Prescription Design and Preparation Process of Paeonol Bead Popping Gum with Hypoglycemic Effect
