Quality Standard of Yi Medicinal Material Anaphalis margaritacea
2022-11-28CigaDIJIUJianlongLANYuebuHAILAIZhengmingYANGYuanLIU
Ciga DIJIU, Jianlong LAN, Yuebu HAILAI, Zhengming YANG, Yuan LIU
1. College of Pharmacy, Southwest Minzu University, Chengdu 610041,China; 2.Sichuan Provincial Qiang-Yi Medicinal Resources Protection and Utilization Technology Engineering Laboratory, Chengdu 610225,China; 3. Tibetan Plateau Ethnic Medicinal Resources Protection and Utilization Key Laboratory of National Ethnic Affairs Commission of the People’s Republic of China; 4. Institute of Qinghai-Tibetan Plateau Research, Southwest Minzu University, Chengdu 610041,China; 5. Ethnic Medicine Institute, Southwest Minzu University, Chengdu 610041, China
Abstract [Objectives] To establish quality standards for traditional Yi medicine of the Anaphalis margaritacea. [Methods] The original plants, traits, identification and TLC was used to identify L. franchetii Beauv. water content, total ash, acid-insoluble ash and extract were checked according to the Chinese Pharmacopoeia (2020 Edition). The content of 3,5-dicaffeoylquinic acid was determined by HPLC. [Results] The microscopic identification features are obvious, epidermal cells, stomata, non-glandular hairs, annular vessel, conduit wood fiber, pollen grains, starch grains, calcium oxalate clusters were observed, etc. TLC showed clear spots and good resolution. The extract content of acid-insoluble ash aqueous solution in 16 batches of samples was 5.22%-9.33%, 6.79%-14.47%, 0.39%-3.30%, 20.49%-32.93%, respectively. 3,5-O-dicafeylquinic acid showed good linear relationships within their own ranges (R2=1.0), whose average recoveries were 99.11% with the RSD of 1.35%, respectively. [Conclusions] The method adopted was reasonable and feasible, and could be used for quality control of A. margaritacea.
Key words Anaphalis margaritacea, Quality standard, 3,5-O-dicafeylquinic acid, TLC
1 Introduction

There are many reports on the chemical constituents ofLeontopodium, the main components are organic acids, flavonoids, volatile oil, sesquiterpenes, phenylpropanoids and other compounds[5-10]. And studies have shown that 3,5-O-dicafeylquinic acid also has a certain antioxidant effect[11-14].A.margaritaceais mainly distributed in southwest China, such as Sichuan, Yunnan and Guizhou. It grows on hillsides, shrubs, grasslands and forest margins at an altitude of 1 600 to 3 200 m. It is rich in reserves in the Yi area, but there is no quality standard of medicinal materials and clinical scientific verification. Therefore, this paper intends to study its original plant, character identification, microscopic identification, TLC identification, content determination and routine items in Pharmacopoeia, in order to provide laboratory support data for the establishment of the standard of traditional Chinese medicine in Sichuan Province, to provide a scientific basis for the improvement of the quality standard of Yi medicine, and to provide a reference for the quality evaluation of other ethnic medicinal materials and the development and utilization of national traditional preparations.
2 Materials
2.1 InstrumentsAgilent 1260, American Agilent; ME104/02 electronic balance, Mettler-Toledo Instruments (Shanghai) Co., Ltd.; ME55/02 electronic balance, Mettler-Toledo Instruments (Shanghai) Co., Ltd.; KQ-300DE CNC ultrasonic cleaner, Kunshan Ultrasonic Instrument Co., Ltd.; HWS-12 electric thermostat water bath, Shanghai Yiheng Scientific Instruments Co., Ltd.
2.2 Reagents and drugs3,5-O-dicafeylquinic acid reference substance (Chengdu MUST Biotechnology Co., Ltd., batch number: MUST-21102611); silica gel G thin layer plate; acetonitrile, methanol and phosphoric acid solution were chromatographically pure; the water was ultra-pure and the other reagents were analytically pure.
2.3 Medicinal materials16 batches of herbs were collected by the team led by Aerlazi, attending traditional Chinese physician in Sichuan Yi Medical Hospital/Liangshan Hospital of Traditional Chinese and Western Medicine, Liangshan Yi Medicine Research Institute, and were identified as the dry aboveground part ofA.margaritacea(L.) Benth. et Hook. f. by Professor Liu Yuan in Southwest Minzu University. The information is shown in Table 1.

Table 1 Sample information of Anaphalis margaritacea
3 Methods and results
3.1 Identification
3.1.1Original plant. It was perennial herb, 30-70 cm high. All of it is densely covered with white woolly hairs, and some of hairs fall off when it is old. Its rhizome was slender and horizontal, woody, and there were short creeping branches with brown scales. Its stem was erect and its base was semi-woody. Its leaves were alternate and sessile. The leaves were strip-lanceolate or lanceolate, 8-11 cm long, 1.3-1.6 cm wide, tapering upward. The apex was obtuse, the base was slightly narrow suborbicular, entire, dark green above with white woolly hairs, and gray below with dense white woolly hairs. Single vein or 3-5 veins. Most of the flower heads were dense, the branches were arranged into terminal corymbs, and the involucre was wide bell-shaped or hemispherical, with a diameter of 8-13 mm and a length of 5-8 mm; there were 5-7 rows of involucral bracts, which were elliptical and ovate, membranous and white, with short outer rows and woolly hairs; flowers were few and all were tubular flowers; there were many rows of female flowers at the edge, bearing fruit; the middle part is bisexual flower, seldom bearing fruit. Achenes were long elliptic, with white pappus, 1-rowed, feathery.
3.1.2Character identification. It was densely covered with white hairs. The stem was cylindrical, brittle, easy to break, and the central pith was grayish white or perforated. The leaves were sessile, striate-lanceolate or linear, base tapering, tip acuminate, entire; the main vein of leaf was obvious, with single vein or 3-5 veins from base; the leaves were dark green above and grayish white below. Most of them were flower heads, and the bracts were membranous and white. It was fragrant and bitter.
3.1.3Microscopic identification. The microscopic characteristics of the medicinal powder (chloral hydrate for permeation, thin glycerin for mounting) ofA.margaritaceawere identified and observed.
The powder was yellowish brown. Non-glandular hairs were common, curved and twined, with a diameter of 20 μm. There were many pollen grains, spherical, with a diameter of 30-45 μm, 3 germination holes, and the surface was densely covered with short spiny carvings. The diameter of starch granules was 25-40 μm, round or oval, and the hilum was cracked or punctate. The diameter of calcium oxalate cluster crystal was 30-50 μm, and its edges and corners were unclear. The surface of epidermal cells was polygonal or irregular, with wavy anticlinal wall, closely arranged, stomata were indeterminate, and there were 4-6 accessory cells. The vessel was a threaded vessel with a diameter of 5-18 μm. The fibers were bundled and lignified. The pappi were branched hairs in many rows with a diameter of 6-15 μm (Fig.1).
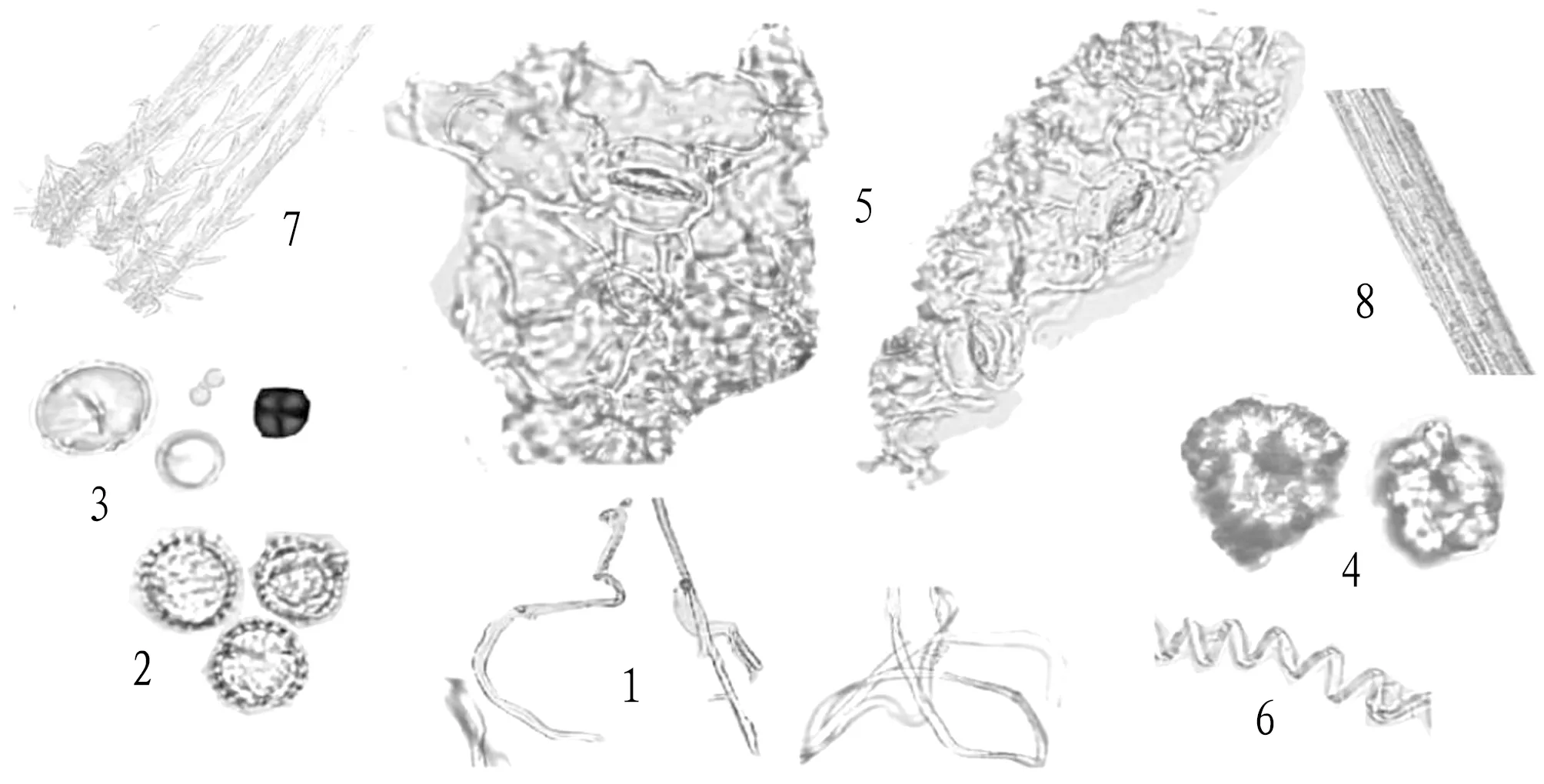
Note: 1-7: non-glandular hairs, pollen grains, starch grains, calcium oxalate cluster crystals, epidermal cells and stomata, vessel and pappi.
3.1.4TLC qualitative identification. 1.0 g of powder of this product was taken, 60 mL of dilute ethanol was added, ultrasonic treatment was carried out for 45 min, and the filtrate was used as the test solution. In addition, chlorogenic acid reference substance and 3,5-O-dicafeylquinic acid reference substance were taken, and dilute ethanol was added to prepare the 0.5 mg/mL solution, which was used as the control solution. According to the TLC (General Rule 0502) test, 5 μL of each of the above two solutions was absorbed and placed on the same silica gel G thin layer plate,respectively. The upper solution of butyl acetate-formic acid-water (7∶2.5∶2.5) was used as the developer for development, removed, dried and examined under ultraviolet lamp (365 nm). In the chromatogram of the sample, the fluorescent spots of the same color were shown in the corresponding position of the chromatogram of the reference substance (Fig.2).

Note: 1,11: chlorogenic acid reference substance; 2,12: 3,5-O-dicafeylquinic acid reference substance; 3-10,13-20: 16 batches of medicinal materials.
3.2 Conventional substance examination and content determination of extractAccording to the 2020 edition ofChinesePharmacopoeia(General Rule 4)[6], water content (fourth method of General Rule 0832), total ash (General Rule 2302), acid-insoluble ash (General Rule 2302) and extract (General Rule 2201) were examined. The results are shown in Table 2.

Table 2 Determination results of water content, ash content and extract of samples (%)
3.3 Content determination of 3,5-O-dicafeylquinic acid
3.3.1Chromatographic conditions. Agilent5 TC-C18chromatographic column (250 mm×4.6 mm, 4 μm); mobile phase acetonitrile (A)-water (containing 0.2% phosphoric acid) (B), gradient elution (0-5 min, 10%-15% A; 5-10 min,15%-20% A;10-35 min,20% A; 35-40 min,20%-30% A; 40-50 min,30% A); detection wavelength 327 nm; column temperature 30 ℃; volume flow 1.0 mL/min; injection volume 10 μL. The chromatogram is shown in Fig.3.

Note: A. margaritacea; B.3,5-O-dicafeylquinic acid.
3.3.2Preparation of sample solution. 1.0 g of powder was taken, weighed accurately, placed in a conical flask with stopper. 60 mL of dilute ethanol was added precisely, weighed, treated by ultrasound (power 600 W, frequency 40 kHz) for 45 min, cooled, weighed again. Dilute ethanol was used to make up for the lost weight, and it was shaken and filtered. Then the filtrate was taken to obtain the test solution.
3.3.3Investigation of linear relationship. For the preparation of reference solution, 0.028 25 g of 3,5-O-dicafeylquinic acid reference substance was precisely weighed, dilute ethanol was added to a constant volume of 25 mL, and a solution of 3-dicaffeoylquinic acid at a concentration of 1.13 mg/mL was prepared. The reference solutions of 0.25, 0.50, 1.00, 2.00, 2.50, 5.00 mL were precisely pipetted and put into the 5 mL volumetric flask, respectively, and dilute ethanol was added to a constant volume and shaken well. According to the chromatographic conditions mentioned above, the peak area was determined by injecting the solution into liquid chromatograph. The peak area (Y) was used as ordinate and the content of 3,5-O-dicafeylquinic acid (X, mg/mL) as abscissa for regression. The regression equation for 3,5-O-dicafeylquinic acid wasY=34 084X-23.965(R2=1.0). The results showed that there was a good linear relationship in the range of 0.056 5-1.13 mg/mL for 3,5-O-dicafeylquinic acid.
3.3.4Precision test. For the same reference solution, the peak area of 3,5-O-dicafeylquinic acid was determined for 6 times, and theRSDwas 1.42%, indicating that the precision of the instrument was high.
3.3.5Stability test. The DHC13 sample solution was taken and the peak area of 3,5-O-dicafeylquinic acid was determined at 0, 4, 6, 8, 10, 12, 18 and 24 h according to the chromatographic conditions under Section3.3.1. TheRSDwas 1.31%, indicating that the sample was stable within 24 h.
3.3.6Repeatability test. 6 groups of DHC13 powder were precisely weighed (1.0 g each), and determined according to the chromatographic conditions under Section3.3.1. The peak area of 3,5-O-dicafeylquinic acid was determined. The average content was 8.60 (mg/g) andRSDwas 1.06%, indicating that the method had good reproducibility.
3.3.7Sample recovery test. A total of 6 groups of DHC13 powder (each about 0.25 g) were precisely weighed, and 2 mL of reference solution of 3,5-O-dicafeylquinic acid was precisely added to the sample. The test solution was prepared according to the method in Section3.3.2, and the sample was injected under the chromatographic conditions under Section3.3.1. The content of 3,5-O-dicafeylquinic acid was determined, and the recovery rate was calculated. Results showed that the recovery rate was 97.35%-100.87%, and the average recovery rate was 99.11%. TheRSDwas 1.35%, indicating that the accuracy was good. The results are shown in Table 3.

Table 3 Results of recovery test of 3,5-O-dicafeylquinic acid
3.3.8Content determination of 3,5-O-dicafeylquinic acid in samples. The above 16 batches ofA.margaritaceasamples were taken to prepare the test solution according to the method of Section3.3.2, and the samples were injected under the chromatographic conditions under Section3.3.1. The peak area was determined and the content was calculated by external standard method. The content of 3,5-O-dicafeylquinic acid in DHC1-DHC16 was 2.16%, 1.39%, 1.30%, 3.27%, 2.41%, 2.23%, 3.14%, 2.64%, 5.62%, 3.72%, 4.81%, 3.95%, 0.86%, 0.90%, 0.54%, 0.35%, respectively, with an average of 2.46%.
4 Discussion
4.1 TLC identificationIn TLC identification, the effects of different solvent systems on TLC were investigated, including butyl acetate∶formic acid∶water (14∶7∶8; 7∶2∶3; 7∶2.5∶2.5; 14∶5∶3), ethyl acetate∶acetone∶formic acid∶water (7∶3∶1∶1.2), ethyl acetate∶methanol∶formic acid∶water (10∶1∶1∶1), ethyl acetate∶butanone∶formic acid∶water (10∶6∶1∶2). The results showed that butyl acetate∶formic acid∶water (7∶2.5∶2.5) had the best developing effect, and theRfvalue of the extract of the medicinal material was similar to that of the reference solution of 3,5-O-dicafeylquinic acid. The results indicated that 3,5-O-dicafeylquinic acid was contained inA.margaritacea, the identification was simple and the reaction was easy to observe, which could be used as the basis for qualitative identification of chemical constituents ofA.margaritacea.
4.2 Water content, ash, extractIn accordance with the four general rules of theChinesePharmacopoeia(2020 edition) and the relevant requirements of the general rules for the determination of medicinal materials and herbal pieces, it was tentatively determined that the water content ofA.margaritaceashould not exceed 13%, the total ash content should not exceed 15%, the acid-insoluble ash content should not exceed 4%, and the extract should not be less than 16%.
4.3 The content and limit of 3,5-O-dicafeylquinic acidThere was a significant difference in the content of 3,5-O-dicafeylquinic acid in 16 batches ofA.margaritaceas samples (P<0.01). According to the dried products ofA.margaritacea, it was tentatively determined that the content of 3,5-O-dicafeylquinic acid inA.margaritaceashould not be less than 0.4%.
5 Conclusion
Through the above experimental research, it filled the gap in the quality standard of the raw material of Yi medicine—A.margaritacea, and provided the basic research for the establishment of the quality standard of traditional Chinese medicine in Sichuan Province. 3,5-O-dicafeylquinic acid was used as the quality control constituent to reduce the testing cost, increase the accuracy and improve the feasibility of quality control.
杂志排行
Medicinal Plant的其它文章
- Quality Standard of Zijinbiao
- Herbal Textual Research of Inulae Flos in Chinese Classic Prescription
- Serum Metabolomic Characteristics of Primary Dysmenorrhea Rat Model Induced by Estradiol Benzoate Combined with Oxytocin
- Establishment of Quality Standard for Freeze-dried Tablets of Polygonatum sibiricum and Study on Anti-tumor Activity of Diosgenin
- Protective Effects of Six PPTs on Hypoxia/Reoxygenation Induced Cardiomyocyte Injury by Different Treatments
- Prescription Design and Preparation Process of Paeonol Bead Popping Gum with Hypoglycemic Effect
