Foxtail millet supplementation improves glucose metabolism and gut microbiota in rats with high-fat diet/streptozotocin-induced diabetes
2022-11-26XinRnLinxunWngZnglongChnMinZhngDinzhiHouYongXuXinminDioRuihiLiuQunShn
Xin Rn, Linxun Wng, Znglong Chn, Min Zhng, Dinzhi Hou,Yong Xu, Xinmin Dio, Ruihi Liu, Qun Shn,*
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Engineering and Technology Research Center of Food Additives,Beijing Technology and Business University, Beijing 100048, China
b Key Laboratory of Plant Protein and Grain processing, National Engineering and Technology Research Center for Fruits and Vegetables,College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
c State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China
d Chinese Academy of Agricultural Sciences, Beijing 100081, China
e Institute of Comparative and Environmental Toxicology, Department of Food Science, Cornell University, Ithaca, NY, United States
Keywords:
Foxtail millet
Glucose metabolism
Gut microbiota
Short-chain fatty acids
A B S T R A C T
Foxtail millet (FM) whole grain has received special attention in recent years.To confirm the hypoglycemic effects of FM, we investigated the effects of FM supplementation on glucose metabolism and gut microbiota in rats with high-fat diet/streptozotocin (HFD/STZ)-induced diabetes.Specifically, we fully assessed the blood biochemical profiles, pancreatic histopathology, insulin-glucagon immunofluorescence, short-chain fatty acids,and gut microbiota composition of rats with HFD/STZ-induced diabetes before and after FM supplementation.Results showed that both 30% and 48% FM supplementation significantly decreased concentrations of fasting blood glucose, 60-min blood glucose, and blood triglycerides (P < 0.05); additionally, 48% FM supplementation significantly improved blood glucose tolerance and insulin resistance (P < 0.05).However,FM supplementation could not effectively repair damage to β-cells over a short period of time.In addition,4 weeks of 48% FM supplementation siginificantly increased the relative abundance of Bifidobacterium and concentration of butyrate, suggesting that the hypoglycemic effects of FM supplementation might be partially mediated by gut microbiota.Collectively, we found a dose-dependent relationship between FM supplementation and improvement of blood glucose metabolism, but did not find a synergistic effect between FM supplementation and metformin (Met) treatment.Our findings provide further support that consuming more whole-grain FM might be beneficial to individuals suffering from type 2 diabetes.
1.Introduction
Over the past few decades, both the prevalence of diabetes and the number of diabetics have been steadily increasing.For many individuals with diabetes, the most challenging part of the treatment plan is determining what to eat [1].Thankfully, the integral role of nutrition therapy in overall diabetes management has been recognized [2].Among the established risk factors, balanced diet and rational nutrition play important roles in improving glycemic control and reducing the risk of type 2 diabetes (T2D) [3,4].Recent studies have enhanced our understanding that increased consumption of whole grains, instead of individually isolated nutrients or supplements,could significantly reduce the risk of T2D [5-7].For instance, the latest prospective cohort study following 194 784 people for 24 years showed that participants in the highest quintile for total whole-grain consumption had a 29% lower rate of T2D than those in the lowest quintile [7].Based on such scientific evidence, several governments,including those of China and the United States, have recommended increasing whole-grain consumption as part of a healthy diet for the prevention of T2D [2,8].
Millet is a generic term for a series of small seeded cereals, such as foxtail millet (Setaria italica), proso millet (Panicum miliaceum),and finger millet (Eleusine coracana).Foxtail millet (FM), as a good source of whole grain, has been playing an important role in global food security, especially in arid and semiarid areas of Asia and Africa [9].Because of its various nutrients and phytochemicals, scientists and nutritionists have been paying increasing attention to its potential health benefits.Specifically, several studies have suggested the hypolipidemic effects of FM protein [10], theα-glucosidase-inhibitory activity of FM peptide fractions [11]and the anti-proliferative activity of FM phenols [12].The hypoglycemic effect of FM was first recorded in traditional Chinese medicine classics more than 400 years ago [9].Our previous studies have demonstrated the relatively low starch digestibility of FM and moderate glycemic indices of FM-derived products [13,14], increasing the consumption of FM might benefit individuals suffering from T2D [15].
In addition, recent work has highlighted the increasingly important role of gut microbiota in metabolic disorders.Several studies have shown that gut microbiota play a vital role in the occurrence and development of T2D [16,17].The gut microbiome can rapidly respond to alterations in diet [18,19].It is generally believed that T2D is one result of gut microbial dysbiosis caused by an unbalanced diet [18,20].Whole grain has a prebiotic effect on the human gut microbiota [21].It has been reported that bound polyphenol from FM bran can restore the gut microbiota disorder caused by colitisassociated carcinogenesis [22].The specific relationship between whole-grain FM and gut microbiota in regulating glucose metabolism,however, remains unclear.
Therefore, in the present study, we conducted 4 weeks of wholegrain FM intervention in rats with high-fat diet/streptozotocin(HFD/STZ)-induced diabetes.The effects of FM supplementation on glycemic metabolism, insulin secretion,hepatic and pancreatic histopathology, gut microbiota, and short-chain fatty acids (SCFAs) were fully investigated.We hoped to 1) clarify the hypoglycemic effect of FM; 2) illustrate the effect of FM supplementation on gut microbiota; and 3)analyze the relationship among FM supplementation, gut microbiota, and blood glucose metabolism.
2.Materials and methods
2.1 Animals
Male Sprague Dawley (SD) rats (8 weeks old;SCXK(J)2019-0004; Vital River Laboratories Co.Ltd., Beijing,China) were kept in a climate-controlled room ((22 ± 2)°C, (55 ± 5)% relative humidity and a 12 h light/dark cycle) with free access to food and water.All animal procedures were approved by Beijing Laboratory Animal Center and performed in accordance with the principles of laboratory animal care.
The experimental protocol was shown in Fig.1A.After 1 week of acclimatization, we randomly grouped 8 rats into the normal control (NC) group, which were fed a 10% low-fat control diet(D12450J; Research Diets, New Brunswick, NJ, USA) throughout the study.The 60 remaining rats were fed a 60% HFD (D12492;Research Diets) for 4 weeks to induce obesity and insulin resistance.Next, after a 12 h fast, rats were injected with a low dose of STZ(35 mg/kg, dissolved in 0.1 mmol/L sodium citrate buffer to a concentration of 1%; pH 4.4) intraperitoneally (i.p.), and the NC rats were injected with vehicle citrate buffer (3.5 mL/kg).Three days later,rats with non-fasting plasma glucose concentration of ≥ 16.7 mmol/L were considered diabetic [23]and randomly divided into 5 groups of 8 rats each based on glucose concentration for an additional 4 weeks of intervention.Specifically, the study included a diabetic control (DC)group, a 30% FM group, a 48% FM group, a 48% FM combined with Metformin (48% FM + Met) group, and a Met positive control group.In order to maximize the amount of FM, the diabetic rats were fed low-fat diets.FM supplementation of 30% was similar to the median amount of whole-grain consumption recommended by the Dietary Guidelines for Chinese Residents (2016) [24].The maximum FM addition was 48%, which could guarantee consistent energy ratios among all intervention diets and 10% low-fat diet.FM was purchased from Dongfangliang Life Technology Co.Ltd.(Shanxi, China).It was milled, sieved, and extruded according to reference [13].Here, foxtail millet flour was first mixed with other materials according to the formula shown in Table 1.The next steps were consistent with routine procedures.Rats in the 48% FM + Met and Met groups were treated with Met (50 mg/kg once daily, dissolved in 0.9% sterile saline) by intragastric (i.g.) administration [25], and the remaining rats were treated with 0.9% sterile saline only.At the end of the intervention period, rats (18 weeks old) were euthanized by decapitation under isoflurane anesthesia, and we collected samples of blood, tissues(liver and pancreas) and feces.The rats were stimulated to defecate by massaging their abdomen with fingers and the faecal samples were collected into a sterile tube and frozen immediately at −80 °C until analysis.

Fig.1 Experimental protocol (A) and effects of FM supplementation on (B) body weight and (C) food intake.Data were represented as mean ± SEM (n = 8).Means with different letters on the bar charts indicate significant differences (P < 0.05).
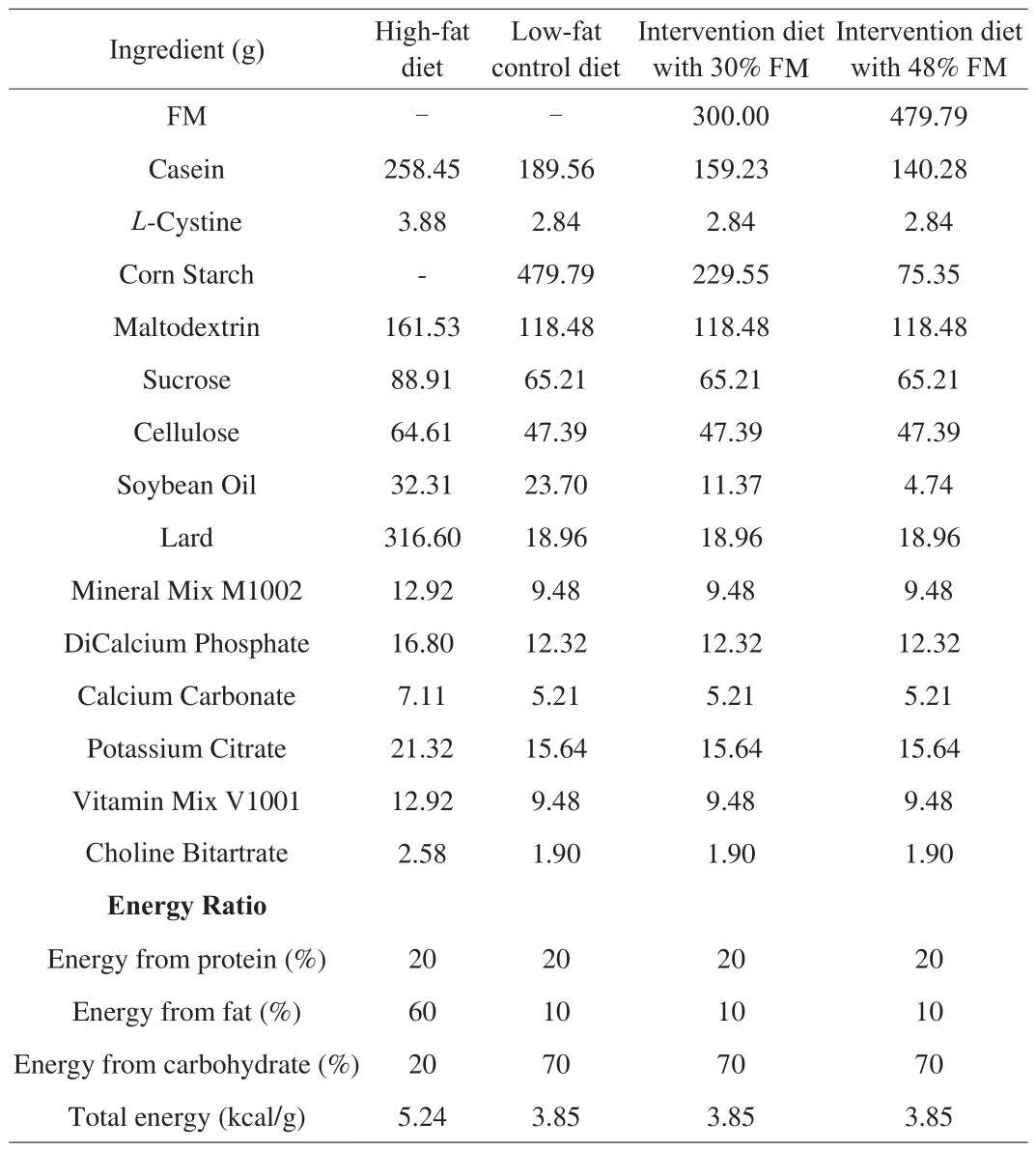
Table 1Ingredient composition and energy ratio of experimental diets.
2.2 Blood analysis
Fasting blood from the orbital-vein plexus before (week 0)and after (week 4) intervention were collected.Fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC),high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and glycated serum protein were measured using a COBAS INTEGRA 800 auto-analyzer(Roche, Basel, Switzerland) per the manufacturer’s protocols.Free fatty acids (ml003228) and fasting insulin (ml302840) were determined using commercial kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China).Then,viahomeostasis model assessment, the insulin resistance index (HOMA-IR)and islet β-cell function index (HOMA-β) were calculated [26].In addition, we performed i.p.glucose tolerance tests (GTTs) at weeks 0(14 weeks old) and 4 (18 weeks old).In detail, after a 12-h fast, all rats were administered 50% glucose solution (2.0 g/kg body weight),and blood samples from the tail veins were collected at 0, 30, 60, 120,and 180 min successively.
2.3 Histopathology and immunofluorescence staining
Isolated pancreatic tissues were fixed in paraformaldehyde,embedded in paraffin and stained with hematoxylin and eosin (H&E)for microscopic histological observation.Immunofluorescence staining was also performed on dewaxed pancreatic sections [27].Insulin was identified by incubation first with monoclonal anti-insulin antibody (I2018; 1:1000; Sigma-Aldrich, St.Louis, MO, USA) and then with tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-mouse immunoglobulin (IgG).Glucagon was identified by incubation first with monoclonal anti-glucagon antibody (G2654;1:2 000; Sigma-Aldrich) and then with fluorescein isothiocyanate(FITC)-conjugated anti-rabbit IgG.Next, we counted cells and calculated the cell areas using quantitative image analysis.
2.4 Gut microbiota
The gut microbiota of rats in the 48% FM group were investigated before and after intervention.In brief, total bacterial DNA were extracted from fecal samples using MoBio Power Soil htp-96 extraction kits (MoBio Laboratories, Carlsbad, CA, USA).The DNA concentration and purity were determined with NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA).The 338F-806R primer pair targeting the V3-V4 region of the 16S rRNA gene was chosen for PCR amplifications.The resulting PCR products were purified using a QIAquick PCR Purification Kit (QIAGEN,Valencia, CA, USA).Finally, qualified DNA samples were sequenced and analyzed on an Illumina MiSeq platform (Illumina, Inc., San Diego, California, USA).
2.5 SCFAs
The effect of FM supplementation on SCFAs were also investigated in rats’ fecal samples.In brief, the pH of fecal suspensions (about 17%,m/m) were adjusted to 2–3 by adding 5 mol/L HCl.After 10 min intermittent shaking, the suspensions were centrifuged and the resultant clear supernatants were injected into a gas chromatography system equipped with a flame ionization detector(6890N; Agilent Technologies, Palo Alto, CA, USA).SCFAs were detected using a fused-silica capillary column (30 m × 0.53 mm) with a free fatty acid phase (DB-FFAP 125-3237; Agilent).The specific experimental conditions of gas chromatography was set according to the method developed by Zhao et al.[28].
2.6 Statistical analyses
Statistical analyses were conducted using SPSS software version 20.0 (IBM Corp., Armonk, NY, USA) and graphs were plotted using GraphPad Prism version 6.01 (GraphPad Software, San Diego, CA,USA).Data were represented as mean ± standard error of the mean(SEM).Significance of treatment effect among different groups were examined according to one-way analysis of variance (ANOVA).Differences before and after intervention of a specific group (weeks 0–4) were examined using a paired-samplet-test.All statistical tests were two-sided, andP< 0.05 was considered statistically significant.
3.Results
3.1 FM supplementation reduced FBG
After 4 weeks of FM supplementation, the FBG of diabetic rats in all FM supplementation groups was significantly lower than that of DC rats (P< 0.05, Fig.2A).Compared with their FBG before intervention, there was no significant difference in the FBG of DC rats, but the FBG of diabetic rats in all FM supplementation groups was decreased significantly, by 41.4%–67.2% after 4 weeks(P< 0.05).The decrease was highest in 48% FM rats.Moreover, there was no significant difference in FBG among NC, Met, and all FM supplementation groups.However, FM supplementation did not cause significant improvement in glycated serum protein, body weight and food intake (Figs.2B, 1B and 1C).
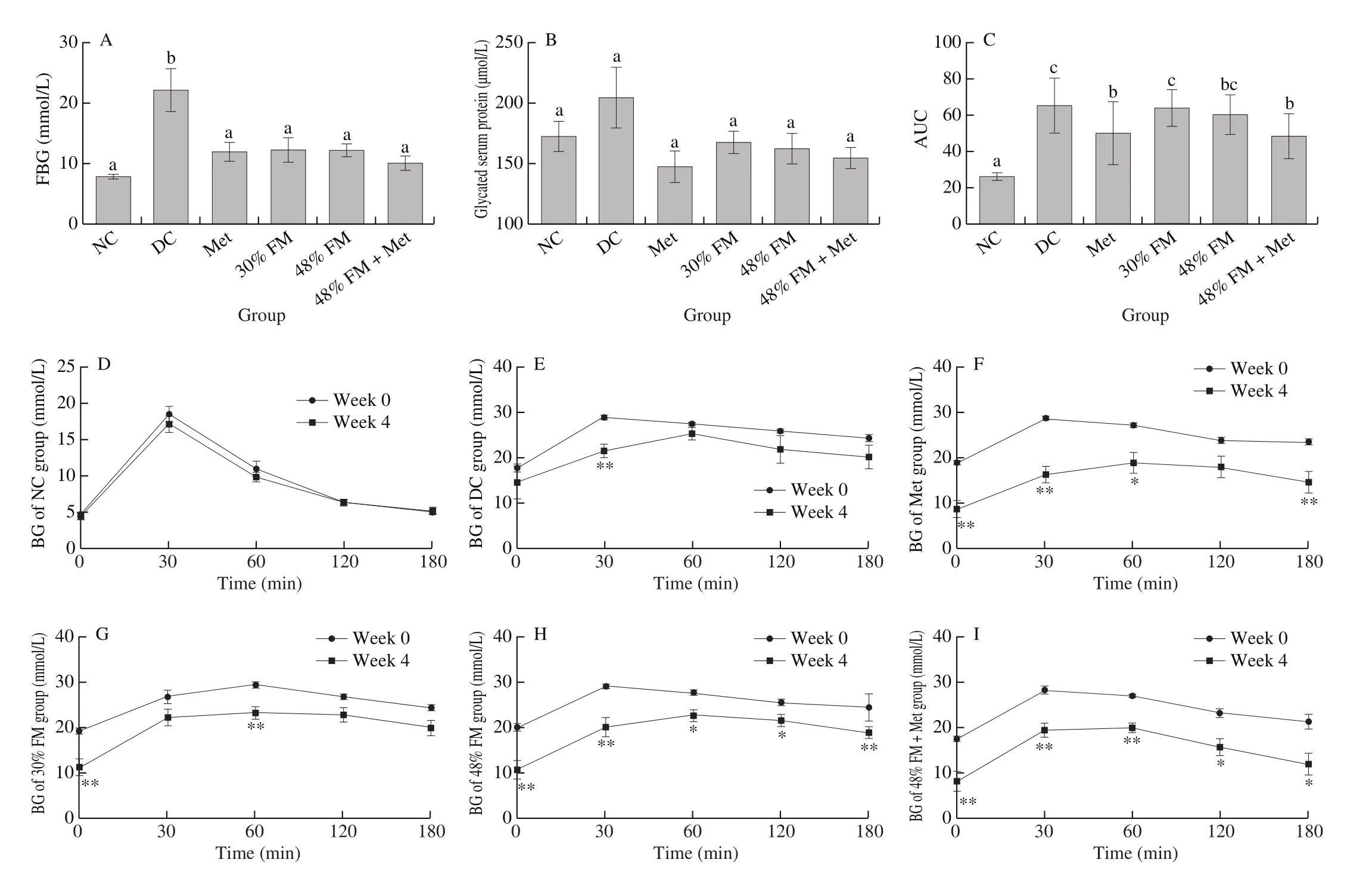
Fig.2 Effects of FM supplementation on (A) fasting blood glucose, (B) glycated serum protein, (C) area under the GTT curve, and (D–I) GTT curve.Data were represented as mean ± SEM (n = 8).*P < 0.05 and **P < 0.01 show significant differences before and after intervention of a specific group (weeks 0–4).
3.2 FM supplementation improved glucose tolerance
Before FM supplementation (week 0), the blood glucose (BG)of diabetic rats in all groups at all time points during the GTT were significantly higher than those of NC rats (P< 0.05).As shown in Figs.2D–2I, 4 weeks of FM supplementation significantly decreased the BG of rats with HFD/STZ-induced diabetes.Specifically,compared with their BG at baseline, the BG of 30% FM rats decreased significantly at 0 min (P= 0.004) and 60 min (P= 0.001).The BG of rats from the 48% FM, 48% FM + Met, and Met (except 120 min)groups at all time points throughout the GTT were significantly lower than their corresponding baseline data (P< 0.05).Notably, although there was a decrease of BG of GTT in low-fat diet fed DC rats, the significant decrease was only observed at 30 min.After BG peaked,however, the subsequent hypoglycemic rate of diabetic rats was still obviously slower than that of NC rats [23], meaning that at 180 min BG was still higher than FBG in all diabetic groups.In addition, after 4 weeks of FM supplementation, the areas under the curve (AUC)for GTTs of rats from the 48% FM + Met and Met groups were significantly lower than those of DC rats (P< 0.05, Fig.2C).
3.3 FM supplementation nonsignificantly improved pancreatic-islet injury
After 4 weeks of FM supplementation, the HOMA-IR of rats from the 48% FM and 48% FM + Met groups were significantly lower than that of DC rats.There were no significant differences in fasting insulin concentration or HOMA-β among all groups (Table 2).In consideration of the selective toxicity of STZ to pancreatic β-cells, we investigated the histopathology of pancreatic tissue (H&E staining)to analyze degree of lesions and conducted immunofluorescence staining to analyze insulin secretion characteristics.The results showed that pancreatic-islet injury was most serious in DC rats, with the lowest average pancreas weight ((1.30 ± 0.24) g) and islet number(10.33 ± 4.63), and the highest degree of pancreatic lesions (1.00 ± 0.82;Table 3, Fig.3).Specifically, the number of pancreatic cells and islet cells were decreased, and watery degeneration and eosinophilic degeneration were observed in DC rats.There was no significant difference among different intervention groups.To a certain extent,the pancreatic-islet status of rats in the 48% FM group was better than that of DC rats but still worse than that of NC rats.Moreover, β-cell numbers in single islets, total β-cell number, and β-cell area in single islets of rats from the DC, Met, and all FM supplementation groups were still significantly lower than those of rats from the NC group(P< 0.05; Table 3, Fig.3).These results suggested that islet β-cells were still severely damaged.FM supplementation could not effectively repair this damage in a short time; such repair might require a relatively long process of gradual accumulation.

Fig.3.Effects of FM supplementation on pancreatic islets (upper) and pancreatic histopathology (below; original magnification, × 400; scale bar, 50 μm).Means with different letters on the bar charts indicate significant differences.

Table 2Effects of FM feeding on blood insulin and blood lipid‡.
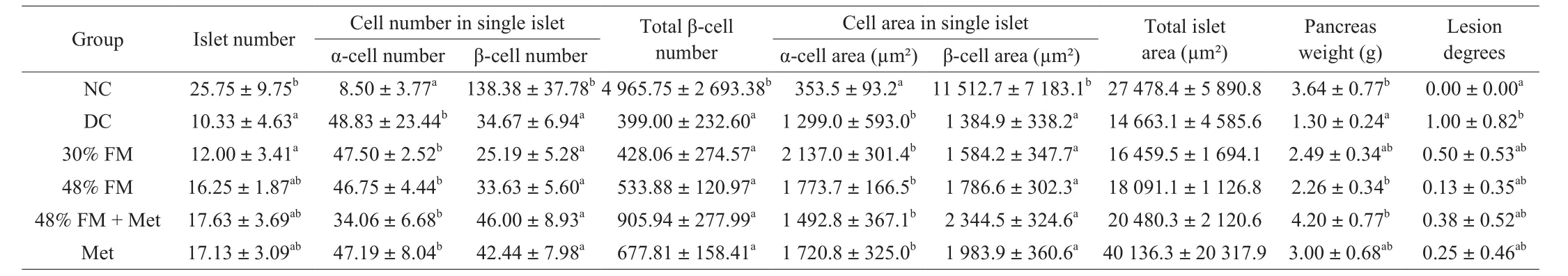
Table 3Effects of FM feeding on the islet β-cell number and β-cell area‡.
3.4 FM supplementation reduced blood TG
After 4 weeks of FM supplementation, average TG concentrations of diabetic rats in all FM supplementation groups and the Met group were significantly lower than those of DC rats (P< 0.05), and no significant difference was found among NC, Met and different intervention groups.The low-density lipoprotein cholesterol concentrations of 48% FM rats were significantly lower than those of DC rats.However, 4 weeks of FM supplementation did not induce significant changes in TC, HDL-C, and free fatty acid in rats with HFD/STZ-induced diabetes (Table 2).
3.5 Effect of FM supplementation on gut microbiota and SCFA
Based on this biochemical analysis, we chose the 48% FM group,which experienced the best hypoglycemic effect, to investigate the changes in gut microbiota and SCFAs before and after FM supplementation.After removing low-quality sequences, 743 762 qualified reads were subjected to the following analysis.The rarefaction and Shannon curves (data not shown) indicated that sequencing depth met the requirements for data analysis.As shown in Figs.4A and 4B, gut microbiota between week 0 and week 4 could be well separated, with a percentage of cumulative explained variance of 64.25% at the phylum level and 46.64% at the genus level.This indicated that FM supplementation could significantly influence the gut microbiota.Specifically,Firmicutes, Bacteroidetes,Proteobacteria, and Verrucomicrobia were the 4 main phyla in the rats,up to 74.5% of which harbored Firmicutes; the next most common species was Bacteroidetes (11.8%; Fig.4C).Although there was no statistically significant difference, 4 weeks of FM supplementation induced a trend toward higher relative abundance of Firmicutesand a lower relative abundance of Bacteroidetes.The ratio of Firmicutesto Bacteroidetes increased nonsignificantly from 5.60 ± 0.73 at week 0 to 10.45 ± 1.68 at week 4 in the 48% FM group (P= 0.059; Fig.4D).With respect to family level, Ruminococcaceae and Lachnospiraceae were two main family in the rats, with 23.12% and 20.31% of relative abundance respectively.After 4 weeks of FM supplementation, the relative abundance of Lachnospiraceae was decreased significantly(P= 0.001).With respect to genus level (Figs.4E, 4F), the relative abundance of unclassifiedLachnospiraceae decreased significantly(P= 0.003) and that ofBifidobacteriumincreased significantly(P= 0.035).Meanwhile, we also investigated the effect of FM intervention on the relative amount of SCFAs.From Figs.4G–4I,it can be seen that the relative amount of butyric acid increased significantly from (5.9 ± 1.11)% at week 0 to (16.24 ± 3.28)% at week 4 (P= 0.032), accompanied by significant decreases in isobutyric acid(P= 0.01) and isovaleric acid (P= 0.003).There were no significant differences in the relative amounts of acetate and propionate.In addition, the results from Spearman’s correlation analysis (Fig.5)showed that unclassifiedLachnospiraceaewas significantly positively correlated with 2 h-glucose, isobutyric acid and isovaleric acid, and significantly negatively correlated with butyric acid;Ruminococcuswas significantly positively correlated with AUC; Unclassified S24-7 andAllobaculumwere significantly negatively correlated with FBG;AkkermansiaandBlautiawere significantly negatively correlated with butyric acid; andBifidobacteriumwas significantly positively correlated with butyric acid.

Fig.4 Effects of 4 weeks of FM supplementation on gut microbiota and SCFAs in rats with HFD/STZ-induced diabetes.(A) PCA at the phylum level; (B) PCA at the genus level; (C) gut microbiota composition at the phylum level; (D) ratio of Firmicutes to Bacteroidetes; (E–F) significantly changed bacteria at the genus level; and (G–I) significantly changed SCFAs.
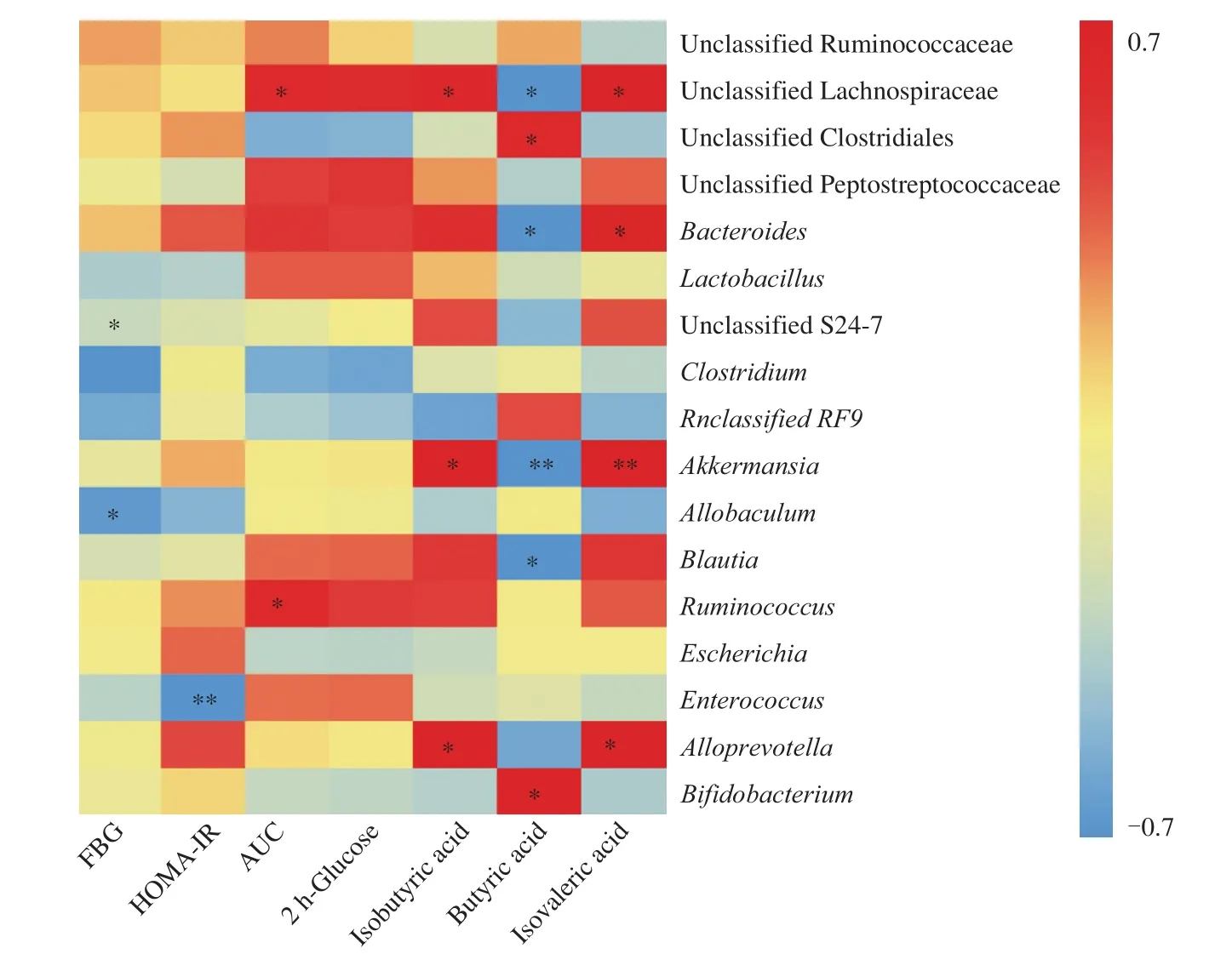
Fig.5 Heatmap of Spearman’s correlation analysis between gut microbiota and glucose metabolism-related indexes.The intensity of the colors represented the degree of association (red, positive correlation; blue, negative correlation).Significant correlations are marked by *P < 0.05; ** P < 0.01.
4.Discussion
T2D is a major health issue that has reached alarming levels.According to the latest edition of the International Diabetes Federation (IDF) Diabetes Atlas[29], the estimated prevalence of diabetes in people ages 20–79 years old has risen to 463 million(9.3%) today and will jump to a staggering 700 million (10.9%) by 2045.This accelerating pandemic has a significant economic impact on individuals, societies, and national economies.The cornerstone of T2D management is promotion of a lifestyle that includes a healthy diet rich in whole grains.Numerous studies have shown that a high intake of whole grains is associated with improvement in glycemic control and a reduced risk of T2D [3,4,7].In the present study, we fully investigated the hypoglycemic effect of whole-grain FM on rats with HFD/STZ-induced diabetes.
Blood glucose control is the primary problem in diabetes management.Well-controlled blood glucose can improve quality of life and even save lives.For example, T2D is a major comorbidity of coronavirus disease 2019 (COVID-19).Zhu et al.[30]found that well-controlled FBG was associated with markedly lower mortality than poorly controlled FBG in patients with COVID-19.In the present study, our result showed that 4 weeks of FM supplementation significantly reduced FBG and improved the blood glucose tolerance of rats with HFD/STZ-induced diabetes.Taking both FBG and glucose tolerance into consideration, the hypoglycemic effect of 48% FM was better than that of 30% FM, which suggested a dose-dependent relationship between FM supplementation and improvement in blood glucose metabolism.However, there was no significant difference between 48% FM and 48% FM + Met,suggesting no synergistic effect between FM supplementation and Met treatment.Previous study has shown that FM has a lower starch digestibility and glycemic index than wheat [13].Replacing part of wheat flour with millet flour (25%) significant lowers the rapidly digestible starch level and glycemic index of bread [31].Combined with those of previous trials in human subjects [15], the results of this study suggested that increasing whole-grain FM consumption could improve blood glucose metabolism.
Whole grains have been widely recognized as healthy hypoglycemic foods because of their high fiber, polyphenol, and phytochemical content.Dong et al.[11]found that soluble dietary fiber from FM bran had strongα-amylase inhibition, delaying hydrolysis of starch to oligosaccharides.Pradee et al.[32]and Karás et al.[33]found that both peptide fractions and phenolic antioxidants of FM strongly inhibitα-glucosidase, which leads to the release of a large amount of glucose.In addition, Nishizawa et al.[34]found that supplementation with Japanese millet protein or Korean proso millet protein reduced FBG by about 24% in T2D mice [35].But the hypoglycemic effect of millet protein was less pronounced than that of whole-grain FM, with a 41.4%–67.2% reduction in FBG observed in the present study.This suggested a potential synergistic effectin vivoamong various nutrients and phytochemicals of whole-grain FM.We need further experiments to verify this synergistic effect and clarify the specific process.
Hyperlipidemia, especially hypertriglyceridemia, is particularly common in T2D patients.In the present study, 4 weeks of high-dose FM supplementation significantly decreased concentrations of TGs and low-density lipoprotein cholesterol in rats with HFD/STZ-induced diabetes.A similar hypolipidemic effect of whole-grain FM has been reported in rats fed a HFD [36].In addition, a millet-supplemented diet also improves hyperlipidemia and hepatic lipid accumulation [37].Whole grain FM is abundant in health-promoting nutrients and phytochemicals, such as unsaturated fatty acids, dietary fiber, and polyphenols.The amount of unsaturated fatty acid in FM is quite high,especially linolenic acid (C18:2, account for 66.68%) and oleic acid(C18:1, account for 16.11%) [38].Several studies have suggested that replacing saturated fat with unsaturated fatty acid leads to significant improvements in the lipid profiles of hyperlipidemic patients [39].Dietary fiber, a typical feature of whole grains, has been shown to reduce risk factors associated with T2D, including hypertriglyceridemia [40].Furthermore, polyphenol is considered to be the other important functional component in FM’s effect on lipid metabolism.Li et al.[36]found that ethanol extract of FM containing mainly polyphenols could alleviate lipid accumulation in HepG2 cells.
It has been known for decades that T2D is influenced by many risk factors.Over the past decade, it has become clear that the influence of gut microbiota on T2D might be profound.Numerous studies have indicated that gut microbiota play a vital role in the occurrence and development of T2D [17,41-43].Patients with T2D are characterized by a moderate degree of gut microbial dysbiosis, a decrease in abundance of some universal butyrate-producing bacteria and an increase in various opportunistic pathogens [16].In addition,our diet could shape our gut microbiota and affect their compositions and functions.Gut microbiota might act as bridges between dietary intervention and blood glucose metabolism [19].
To illustrate the effect of FM supplementation on gut microbiota,we investigated variations in gut microbiota composition and relative abundance of bacteria of rats in the 48% FM group.Four weeks of FM supplementation in the present study could mitigate negative gut microbiota variations in rats with HFD/STZ-induced diabetes.Although the Firmicutes/Bacteroidetes ratio is frequently cited in the scientific literature, a great number of contradictory results are reported [17,44].Larsen et al.[43]observed a significant decrease in Firmicutes/Bacteroidetes ratio in T2D patients compared with controls, but Zhao et al.[42]observed a significant increase.According to a systematic review, out of 13 case-control studies that examining association between T2D and Firmicutes/Bacteroidetes ratio, 3 reported negative associations, 4 reported positive associations, and 6 reported no associations [17].Similar contradictory results also were found in obese patients [44].In the present study,we observed a slight increase in Firmicutes/Bacteroidetes ratio on FM supplementation, which was consistent with that of a low linoleic acid diet in rats with HFD/STZ-induced diabetes [41]but was inconsistent with that of whole-grain millet in dyslipidemic rats [36].In addition to the differences in animal subjects, the contradictory results are probably due to many environmental factors, including diet and physical activity [44].
With recent advances in our understanding of SCFAs’ functions,it is plausible that both the gut microbiota and SCFAs play vital roles in regulating T2D.They interact with dietary constituents; modulate inflammation; and affect gut permeability, insulin sensitivity and overall energy homeostasis in mammalian hosts [17,18].Dietary fiber and resistant starch could be fermented by gut microbiota to generate SCFAs.Studies in humans have pointed out that individuals with T2D have reduced abundances of fiber-degrading and butyrate-producing bacteria [16].A diet rich in fiber (e.g.whole grain) contributes to the maintenance of healthy gut microbiota associated with increased production of SCFAs [18].In the present study, 4 weeks of wholegrain FM intervention significantly increased the relative abundance ofBifidobacteriumand concentration of butyrate.Bifidobacterium, a fiber-degrading bacteria, is one of the most widely used probiotics; a substantial body of literature has provided evidence for its beneficial role in glucose metabolism in humans and experimental animals[17,36,45].Furthermore,Bifidobacteriumappears to be the most commonly reported and consistently supported gut microorganism that is potentially protective against T2D [17].In addition to the abovementioned common mechanism of gut microbiota,Bifidobacteriumalso could increase muscle glycogen synthesis and decrease hepatic gluconeogenesis [45].SCFAs are mainly composed of acetate (C2), propionate (C3), and butyrate (C4).Recent studies show that butyrate provides the primary energy source for colonic epithelial cells [18]and can reduce inflammation and promote repair of intestinal mucosal cells [46].Increased relative abundance of butyrate-producing bacteria and a high amount of butyrate have been proven to be associated with improvements in several metabolic disorders, including T2D [16,18].Thus, based on our findings, we suggest that the hypoglycemic effect of FM could be achieved by increasing the relative abundance ofBifidobacteriumand stimulating butyrate production.
Our positive results provide novel evidence for the health effects of whole-grain FM.Although, except forBifidobacterium,we didn’t find significant increase in other famous bacteria, e.g.Akkermansia,Blautia, and some other butyrate-producing bacteria,we did find improvement in glucose metabolism targeted by wholegrain FM.It suggested that other components except dietary fiber in whole-grain FM also contributed to its hypoglycemic effect.Further experiments to verify this meaningful suggestion need to be carried out.Besides, a better understanding of diet-microbiota interactions will help researchers develop a nutritional approach that will target and more efficiently reduce the incidence of T2D [18].Our current findings do not sufficiently demonstrate the causal role of gut microbiota in glucose metabolism improvement.To further explore the hypoglycemic function of improved gut microbiota, a fecal-microbiome transplantation experiment will be needed [47].In addition, after 4 weeks of intervention, the morphology and function of pancreatic islets were nonsignificantly improved.Whether or not prolonging study duration will enhance this positive effect needs further investigation.There are still some limitations in our study.To maximize the FM addition amount and guarantee consistent energy ratio, low-fat diets were used during the intervention period.Although diabetes control (DC) and positive control (Met) groups were set,there was still a risk of self-recovery.Moreover, it would be better if a group of normal rats fed with FM was set, which could be guarantee the safety of FM supplementation.
Conclusions
The present study confirmed the hypoglycemic effect of wholegrain FM.Four weeks of FM supplementation significantly decreased concentrations of FBG and TGs, increased the relative abundance ofBifidobacteriumand concentration of butyrate and improved the blood glucose tolerance of rats with HFD/STZ-induced diabetes.The prevention of T2D by FM supplementation was at least partially mediated by structural modulation of gut microbiota.Although we investigated FM in the present study, it should not be ignored that other whole-grain foods also have similar hypoglycemic effects.Our findings provide further support for the current recommendations that promote increased consumption of whole grains as part of a healthy diet for the prevention of T2D.
Conflict of Interest
There is no conflict of interest to declare.
Acknowledgement
This work was supported by the National Key R&D Program of China (2017YFD0401200), China Agriculture Research System(CARS-07-13.5-A17), General S&T project of Beijing Municipal Commission of Education (KM202010011006), and BTBU Youth Fund (PXM2019_014213_000007).The authors declare no conflict of interest.
杂志排行
食品科学与人类健康(英文)的其它文章
- Docosahexaenoic acid-rich fish oil prevented insulin resistance by modulating gut microbiome and promoting colonic peptide YY expression in diet-induced obesity mice
- Verification of Lactobacillus brevis tolerance to simulated gastric juice and the potential effects of postbiotic gamma-aminobutyric acid in streptozotocin-induced diabetic mice
- Safety assessment of monosodium glutamate based on intestinal function and flora in mice
- Dose-dependent effects of apple pectin on alleviating high fat-induced obesity modulated by gut microbiota and SCFAs
- Sulforaphane attenuates dextran sodium sulphate induced intestinal inflammation via IL-10/STAT3 signaling mediated macrophage phenotype switching
- Structural characterization of oligosaccharide from Spirulina platensis and its effect on the faecal microbiota in vitro
