Scales in the Early Cretaceous bird Gansus from China provide evidence on the evolution of avian scales
2022-11-24ToZhoZhiHengLiHeZhngYnHongPn
To Zho , Zhi-Heng Li , He Zhng , Yn-Hong Pn ,*
a State Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Centre for Research and Education on Biological Evolution and Environment and Frontiers Science, Center for Critical Earth Material Cycling, Nanjing University, Nanjing 210023, Jiangsu Province, China
b Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China
c CAS Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Beijing 100044,China
Abstract Most modern birds have scales covering feet, but our knowledge of early avian scales is limited,mainly due to their scarcity in the fossil record. Here we describe the morphological details of scutellate and interstitial scales preserved in IVPP V15077,a specimen of the Early Cretaceous bird Gansus from the Changma Basin in northwestern Gansu Province, Northwest China. These results, combined with previous reports of scutate and reticulate scales, show that all four types of scales present in modern birds already appeared in the Early Cretaceous.The phylogenetic distribution of skin appendages of feet,including feathers and scales,shows that non-avian dinosaurs already evolved scales resembling those in modern birds, and that scales can coexist with feathers on feet, suggesting that avian scales may be homologue with scales of non-avian dinosaurs. However, to further test this hypothesis, more research combined with a strengthened focus on detecting specimens with soft tissue preservation is necessary.
Keywords Foot scale, Avian scale, Feather, Bird, Dinosaur, Changma Basin, Early Cretaceous
1. Introduction
The majority of modern birds have scales covering the foot (tarsometatarsus and toes) and feathers covering most of the rest of the body. Some wild species (e.g., owls, grouses, and ptarmigans) and some breeds of domestic pigeon and chicken display foot feathering (ptilopody) (Kelso and Kelso, 1936; Lucas and Stettenheim, 1972; H¨ohn, 1977; Bartels, 2003;Boer et al., 2017). The scales in modern birds can be categorized into four types:1)scutate scales that are large, rectangular or polygonal, somewhat overlapping, and located on the cranial surfaces of the tibiotarsus and tarsometatarsus and the dorsal surfaces of the toes; 2) scutellate scales that are similar to scutate scales but smaller, located on the caudal surfaces of the tibiotarsus and tarsometatarsus; 3)reticulate scales that are small, radially symmetrical,and located on the plantar surfaces of the toes;and,4)interstitial scales that are morphologically similar to reticulate scales but more variable in shape, and located on the entire surface of the ankle joint and the lateral and medial surfaces of the tarsometatarsus(Sawyer et al.,1986).Reticulate scales consist only of α-keratins, while other avian scale types resemble avian epidermal structures such as feathers, beaks,and claws which are composed of both α-keratins and corneous beta-proteins (CBPs; formerly referred to as“β-keratins”) (Sawyer et al., 1986; Di-Poï and Milinkovitch, 2016; Holthaus et al., 2019). Because of the morphological similarity,interstitial scales are also sometimes referred to as reticulate scales in the literature(Lucas and Stettenheim,1972;Brush,1985;Homberger and Brush, 1986). However, as interstitial scales are chemically different from reticulate scales,we regard them as two separate types, following Sawyer et al. (1986). The grooves between the scales are referred to as sulci(Lucas and Stettenheim,1972).
Histological and molecular studies show that reptilian scales, avian scutate scales, feathers, and mammal hairs exhibit an anatomical placode in early development,and suggest that most skin appendages in amniotes are homologue and evolved from their shared reptilian ancestor's skin appendages characterized by an anatomical placode and associated signaling molecules(Di-Poï and Milinkovitch,2016).But the homology between the skin appendages is controversial. Homology between avian scales and reptilian scales has long been proposed(Lucas and Stettenheim,1972).Another viewpoint is that avian scales have evolved secondarily from feathers (Dhouailly, 2009; Wu et al., 2018). A detailed assessment of fossil avian scales might provide useful information for understanding their origin.However, fossilized scales are rarely preserved in comparison to feather remains, and our knowledge of scales in ancient birds to date is limited to only very few taxa (Li et al., 2011; Zheng et al., 2013; Falk et al.,2016; Xing et al., 2017, 2019a). As a result, the pattern and timing of acquisition of avian scales are poorly resolved.
The specimen IVPP V15077 examined in this study is deposited at the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences,Beijing,China.This specimen belongs to the Early Cretaceous bird Gansus yumenensis and has scales well preserved in situ around the ankle joint(Li et al., 2011). To date, all reported specimens of Gansus yumenensis are from the Lower Cretaceous Xiagou Formation of the Changma Basin, Northwest China (e.g., Hou and Liu, 1984; You et al., 2006;Li et al., 2011). Stable isotope chemostratigraphy dates the fossil-bearing part of the Xiagou Formation to the early Aptian age (Suarez et al., 2013), which is possibly a crucial time for our understanding of the transition from feathered feet to scaled feet in birds.Despite the exceptional preservation of the skin in IVPP V15077, no investigation on the ultrastructure and chemistry of the scales has been performed yet.The aim of the present study is to investigate what details, and how the morphological and chemical details are preserved in the fossil scales by employing multiple techniques, including scanning electron microscopy (SEM), energy dispersive X-ray spectrometry(EDS), Raman spectroscopy, and X-ray powder diffraction(XRPD);and to discuss their implications for the evolution of the scales in ancient birds.
2. Material and methods
2.1. SEM and EDS mapping
For scanning electron microscopy (SEM) observations and energy dispersive X-ray spectroscopy (EDS)analysis,a piece of the fossil containing several scales was removed from IVPP V15077 using a sterile blade and mounted on a stub with carbon tape. No sputter coating was performed. SEM imaging was performed using a Sigma 500 Field Emission Scanning Electron Microscope (FE-SEM) with ZEISS SmartSEM software.All pictures were taken with a secondary electron(SE)detector at an accelerating voltage of 1.5 keV at the Key Laboratory of Surficial Geochemistry, Ministry of Education, Nanjing University. EDS analysis was performed using a Tescan MAIA3 FE-SEM equipped with Aztec software.Elemental mappings were carried out using an energy dispersive X-ray spectrometry at accelerating voltages of 8 keV and 20 keV,at the State Key Laboratory of Palaeobiology and Stratigraphy,Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. The elemental mapping results were acquired at accumulation times of 36 min at 8 keV and 16 min at 20 keV.

Fig.1 Morphological details of the skin preserved in IVPP V15077,a referred specimen of Gansus yumenensis.A)Photo of IVPP V15077;B-C)Close-up showing the scales preserved around the joint between the right tibiotarsus and tarsometatarsus; D-I) SEM images of the scales.
2.2. Raman spectroscopy
Raman analysis of specimen IVPP V15077 was performed using a LabRAM HR Evolution Raman spectrometer with Horiba LabSpec 6 software at the Key Laboratory of Surficial Geochemistry, Ministry of Education,Nanjing University.A 532 nm laser was used as the excitation source. The neutral density (ND) filter was set at 1%-5% to prevent sample damage. The grating groove density was 600 grooves/mm. The
Raman spectrometer was calibrated with silicon peak at 520.7 cm-1.The 10×and the long working distance(LWD) 50 × microscope objective lens were used.Spectra were acquired with four accumulations, and accumulation times of 4 s-8 s.
2.3. X-ray powder diffraction(XRPD)analysis
To characterize the mineral composition of the sediment, one sediment sample (about 1 g) was scraped from the bedding plane surface adjacent to the fossil and powdered for X-ray powder diffraction(XRPD) analysis. The XRPD analysis of the sediment sample was performed using a Bruker D8 ADVANCE diffractometer with Cu Kα radiation(λ=1.5418 ◦A)at 40 kV and 40 mA at the State Key Laboratory for Mineral Deposits Research, Nanjing University. The scanning angle ranged from 3°to 90°of 2θ(diffraction angle, the angle between incident and reflected Xray). XRPD data acquisition was conducted with the software DIFFRAC.COMMANDER and phase identification was conducted with the software DIFFRAC.EVA.
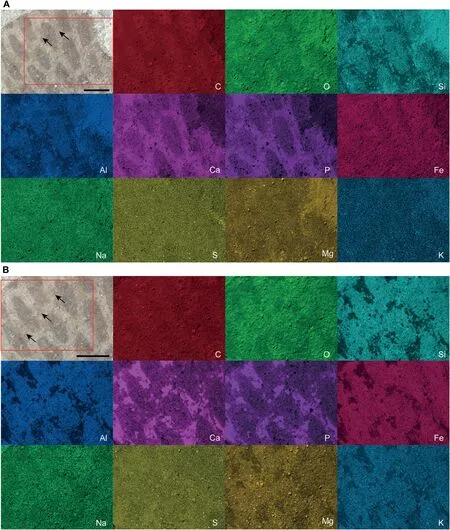
Fig.2 Elemental maps for areas indicated on IVPP V15077 at A)8 keV and B)20 keV.Arrows indicate where the layer of sediment covering the sulci was removed. C, O, Si, Al, Ca, P, Fe, Na, S, Mg, and K refer to the concentration of carbon, oxygen, silicon, aluminum, calcium,phosphorus, iron, sodium, sulfur, magnesium, and potassium in the sample, respectively. At 8 keV, the concentration of C is higher in the scales than in the sulci;this discrepancy is less distinct at 20 keV.The sediment covering the sulci has an elevated concentration of Ca and P.Where the layer of sediment was removed(arrows),the elements Si,Al,Fe,Na,S,Mg,and K show no spatial partitioning between scales and sulci at either 8 keV or 20 keV. Scale bars are 500 μm.
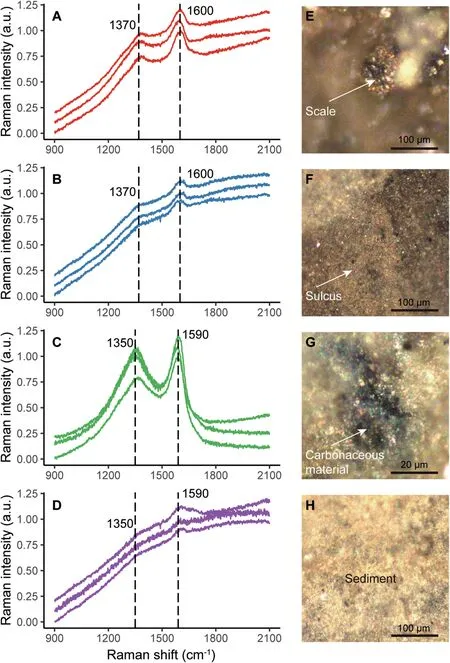
Fig.3 Raman analysis of IVPP V15077.Raman spectra of A)scales,B)sulci,C)carbonaceous material in sediment,and D)the sediment with no evident carbonaceous material; photomicrographs showing E) a scale, F) a sulcus, G) carbonaceous material in sediment, and H) the sediment with no evident carbonaceous material.
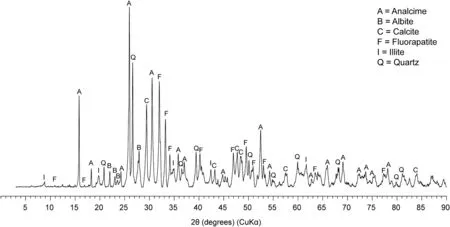
Fig. 4 X-ray powder diffraction analysis of the sediment from the bedding plane surface on which IVPP V15077 is preserved. Analcime,fluorapatite, quartz, calcite, illite, and albite are identified.
2.4. The phylogenetic tree
A phylogenetic tree was constructed based on previous work. The phylogeny of birds follows Wang et al. (2015). Non-avian dinosaurs were added based on the phylogenetic framework of Dinosauria in Xu et al. (2014) and on other sources giving phylogenies for particular non-avian dinosaur clades (Ji et al.,2007; Godefroit et al., 2014).
3. Results
Specimen IVPP V15077 contains skin preserved around the caudal part of the right ankle joint(Fig.1),and shows a series of aligned and interlocking scales,which decreases in the size distally.Overall,two types of scales, scutellate and interstitial scales (Fig. 1B),can be distinguished based on their morphologies.The larger and elongate proximal scales are aligned in parallel rows, ranging in shape from quadrilateral to hexagonal. The middle and distal scales are smaller and rounder than the proximal ones(Fig.1B),and show no parallel arrangement. All scales are nonoverlapping. The sulci between the scales are lighter in color than the scales (Fig. 1C).
Scanning electron microscopy (SEM) reveals impressions of ovoid and rod-like microbodies on scattered fragments of the skin (Fig. 1F and G). Such microbodies likely represent melanosomes, based on recent studies on fossil feathers and reptile skin(Vinther et al.,2008;Li et al.,2010,Zhang et al.,2010,Li et al.,2012;Colleary et al.,2015;McNamara et al.,2016; Rossi et al., 2019). Unfortunately, SEM imaging did not reveal any other possible biological microstructures within the skin(Fig.1D,E,H,I).
Elemental mappings were performed at two accelerating voltages of 8 keV and 20 keV to assess how elemental distribution changes over depth within the sample.The elemental mapping at 8 keV(Fig.2A)shows an elevated concentration of C in the scales compared to the sulci.This discrepancy is less distinct in the elemental mapping at 20 keV (Fig. 2B), which indicates that the element C is distributed near the surface,as the electron beam penetrates deeper into the specimen at 20 keV. The sediment covering the sulci has an elevated concentration of Ca and P,indicating the presence of calcium phosphate.Where this layer of sediment was removed(arrows in Fig.2),the elements Si, Al, Fe, Na, S, Mg, and K show no spatial partitioning between scales and sulci at either 8 keV or 20 keV.
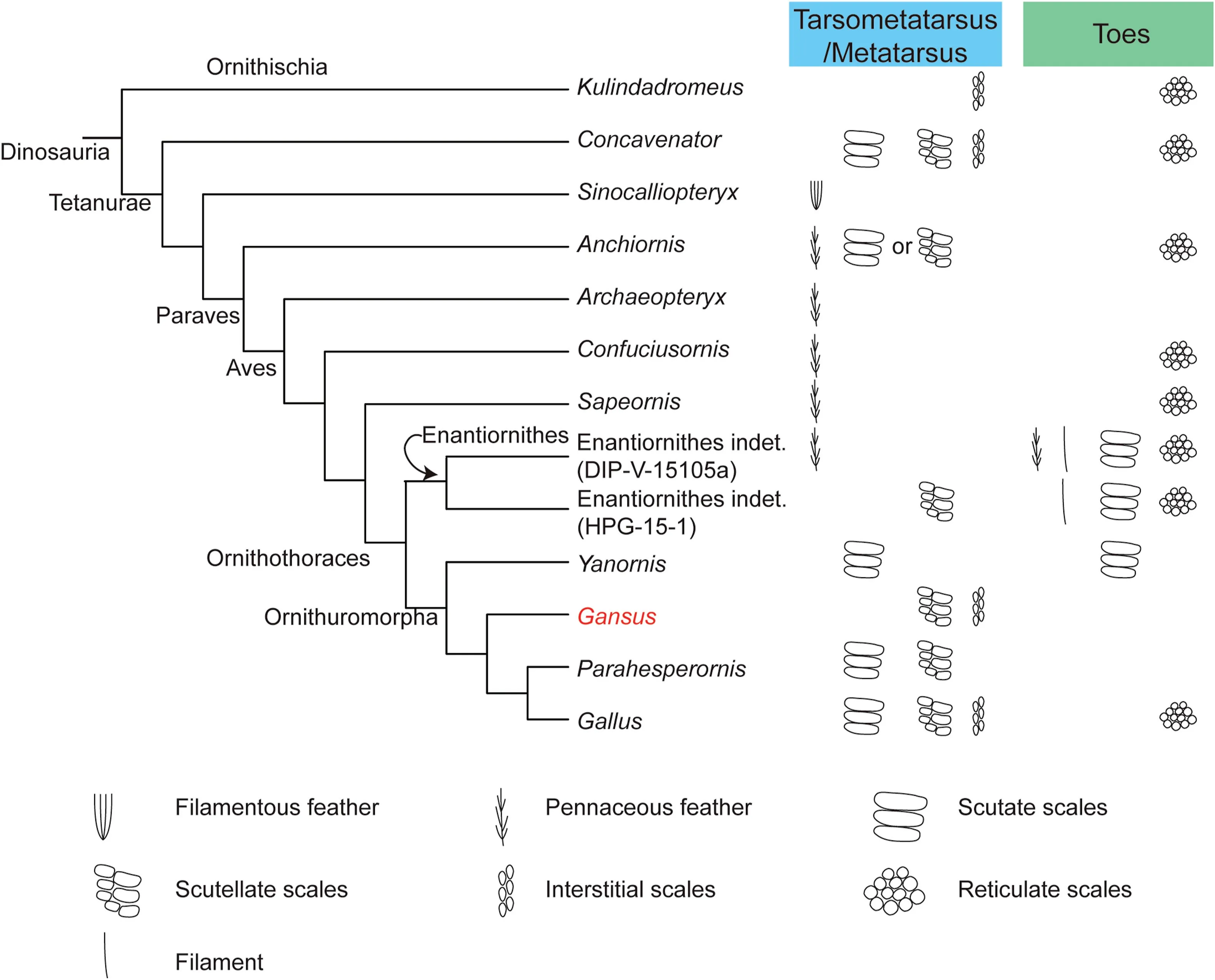
Fig. 5 Schematic phylogeny of selected dinosaurs showing the distribution of skin appendages of the foot (tarsometatarsus and toes).Available fossil evidence shows that feathers and scales can coexist on the foot.Filaments on the toes observed in enantiornithines stemmed from scales. indet. = indeterminata.
The scales, the sulci between the scales, and the sediment were evaluated by taking at least three Raman spectra from different locations, and in all cases the replicate spectra were fairly similar to one another.The Raman spectra of the scales and the sulci display a G-band near 1600 cm-1and a D-band near 1370 cm-1(Fig. 3A and B). The Raman spectra of the carbonaceous material in the sediment and the sediment without carbonaceous material display a G-band near 1590 cm-1and a D-band near 1350 cm-1(Fig. 3C and D).The two bands are less prominent in the Raman spectra of the sulci and the sediment without carbonaceous material (Fig. 3B, D).
XRPD analysis shows that the sediment consists mainly of analcime, fluorapatite, quartz, calcite,illite, and albite (Fig. 4).
4. Discussion
4.1. Interpretation of the scales in the Early Cretaceous bird Gansus
The larger scales preserved in IVPP V15077 are rectangular to hexagonal in shape and do not overlap each other.They can be identified as scutellate scales present in modern birds(Lucas and Stettenheim,1972;Sawyer et al., 1986). The smaller and rounder scales preserved on the tarsometatarsus in IVPP V15077 are also non-overlapping and they are more similar to interstitial scales(Sawyer et al.,1986).The record of these two scale types in Gansus complements earlier descriptions of scutate scales covering the cranial surface of the tarsometatarsus and the dorsal surfaces of toes in Yanornis from the Early Cretaceous Jehol Biota (Zheng et al., 2013). Furthermore, reticulate scales have been found in the basal birds Sapeornis(Pu et al., 2013) and Confuciusornis (Falk et al., 2016).These results show that all four types of scales present in modern birds were already present in their Early Cretaceous relatives.
SEM observations suggest that the scales of Gansus might bear melanosomes and thus were likely pigmented. However, the possibility that these melanosome-like impression molds were from decaying feathers nearby cannot be excluded.
4.2. Origin and evolution of avian scales
The exceptionally preserved scales in the Early Cretaceous bird Gansus, as well as recent reports on scales and feathers in other ancient birds and nonavian dinosaurs, provide us a rare chance to infer the origin and early evolution of scales in birds by taking the new paleontological evidence.Previous reports on metatarsal feathers in non-avian dinosaurs, the basal birds Archaeopteryx and Sapeornis, and enantiornithines imply that feathered metatarsi are a plesiomorphic state for the avian clade(Zheng et al.,2013;Foth et al., 2014; Xing et al., 2019a) (Fig. 5). Phylogenetic analysis places Gansus and Yanornis in the Ornithuromorpha (Fig. 5), the clade that contains all taxa closer to modern birds than to the Enantiornithes(Wang et al., 2015). The discovery of scutate scales covering the anterior surface of the tarsometatarsus and the dorsal surfaces of toes in Yanornis (Zheng et al., 2013), and the presence of both scutellate scales and interstitial scales covering the ankle joint in Gansus,suggest that scaled metatarsi in modern birds evolved in the Early Cretaceous basal ornithuromorphs(Zheng et al., 2013). The discovery of both feathered and scaled metatarsi in enantiornithines suggests that scaled metatarsi evolved independently in enantiornithines (Xing et al., 2017, 2019a).
However,if the available data on foot scales in nonavian dinosaurs are considered, the scenario becomes more complicated.Recent studies indicate that scales resembling scutate, scutellate, and interstitial scales of extant birds may not be restricted to the avian clade. Scutellate scales associated with tibiae have been found in the ornithischian dinosaur Kulindadromeus (Godefroit et al., 2014, 2020), and scutate,scutellate, and interstitial scales associated with the metatarsus have been found in the tetanurae Concavenator(Cuesta et al.,2015).Reticulate scales have been found even more frequently in the Dinosauria,including in birds(Fig.5).They have been reported not only in basal birds,e.g.,Sapeornis(Pu et al.,2013)and Confuciusornis (Falk et al., 2016), but also within the paravian Anchiornis (Wang et al., 2017). Similar reticulate scales have even been found in ornithischians, e.g., Kulindadromeus (Godefroit et al., 2014,2020) and Psittacosaurus (Vinther et al., 2016).
Are avian scales homologous with scales in nonavian dinosaurs? To answer this question, we need to know whether the foot scales were completely lost in avian ancestors. It has been found that the paravian Anchiornis and an enantiornithine bird have scutate/scutellate scales, reticulate scales, and feathers on their feet (Hu et al., 2009; Wang et al., 2017; Xing et al., 2019a; Hendrickx et al., 2022), which indicates that the presence of metatarsal feathers does not necessarily mean the complete loss of foot scales.Coexistence of reticulate scales with metatarsal feathers has also been evidenced by basal birds Sapeornis and Confuciusornis (Pu et al., 2013; Zheng et al., 2013; Falk et al., 2016).
It is also instructive to integrate insights from these morphological studies with recent progress in development of foot scales using molecular data of modern birds.Among modern birds,some wild birds and some breeds of domestic pigeon and chicken evolved foot feathers instead of scales(Kelso and Kelso,1936;Lucas and Stettenheim, 1972; H¨ohn, 1977; Bartels, 2003;Boer et al., 2017). Recent studies show that the foot feathering in domestic pigeon and chicken is determined by the same set of genes,PITX1 and TBX5,which encode a key transcription regulator of hindlimb identity and development and a key transcription regulator of forelimb identity and development,respectively (Domyan et al., 2016; Bortoluzzi et al.,2020). Foot feathering in pigeon and chicken is associated with ectopic expression of TBX5, which alters the identity of the hindlimb rather than individual epidermal placodes (Domyan et al., 2016; Bortoluzzi et al., 2020). In the paravian Microraptor, the leg feathers, including those associated with the femur,tibia, and the metatarsus, are arranged in a pattern similar to wing feathers (Xu et al., 2003), also suggesting a partial shift in limb identity (Domyan et al.,2016). If true, the foot feathering in basal birds and non-avian dinosaurs does not mean that avian scales evolved from feathers, just as foot feathering in modern birds does not mean that foot feathers evolved from foot scales. Rather, it is likely that avian scales are homologue with scales of non-avian dinosaurs.This scenario is consistent with the independent evolution of avian scale and feather β-keratins (Greenwold and Sawyer, 2013).
However, some studies proposed that avian scales evolved secondarily from feathers (e.g., Prin and Dhouailly,2004;Dhouailly,2009;Wu et al.,2018).One argument in favor of this view is that the development of scales in extant birds requires the inhibition of the development of feathers (Prin and Dhouailly, 2004;Dhouailly, 2009), which led to the hypothesis that the feet of the ancestors of modern birds were almost entirely covered with feathers. Though the foot feathering in paravians was cited in support of this hypothesis (Dhouailly, 2009), the available fossil evidence shows that foot feathers can coexist with scales and therefore cannot confirm this hypothesis.Another argument in favor of this viewpoint is that at the early placode stage, the chicken scutate scales are more similar to chicken feathers than to alligator scales in morphology and molecular profile(Wu et al.,2018).If avian scales were inherited from the dinosaurian antecedents, the similarity between chicken scutate scales and feathers should have a much deeper origin,and avian scales and foot scales in non-avian dinosaurs should share some chemical similarity.Research on the chemical composition of the scales in non-avian dinosaurs and the coexistence of foot scales with metatarsal feathers will help further clarify the homology between avian scales and the foot scales in non-avian dinosaurs.
4.3. Evolutionary significance of scaled tarsometatarsi in birds
The elongated pedal phalanges,unrecurved unguals with large flexor tubercula, and webbed feet indicate that Gansus was adapted to life in aquatic environments(Hou and Liu,1984;Ji et al.,2006;You et al.,2006;Li et al., 2011). Water-based niches have also been suggested for multiple other early ornithuromorphs,including Hongshanornis, Yixianornis, Yanornis, Ichthyornis, and hesperornithiformes (Galton and Martin,2002; Clarke, 2004; Zhou, 2004; Zhou and Zhang,2005). It has been suggested that ornithuromorphs rapidly shifted from terrestrial/arboreal niches to aquatic ones during early evolution (Zhou and Zhang,2005; You et al., 2006). The evolution of scaled tarsometatarsi in ornithuromorphs could therefore represent an adaptation to their new environment, because scaled tarsometatarsi are better suited to an aquatic lifestyle than feathered tarsometatarsi.
However, scaled tarsometatarsi evolved independently in enantiornithines (Xing et al., 2017), which are predominantly arboreal (Wang et al., 2014; Xing et al., 2019b). How scaled tarsometatarsi benefited an arboreal lifestyle remains to be determined, as arboreal habits have been inferred for some taxa with metatarsal feathers such as Microraptor (O'Connor et al.,2011)and Archaeopteryx(Feduccia, 1993).
4.4. Taphonomy
Finally, it is intriguing to ask why foot scales are preserved so rarely in the fossil record. Although thousands of exceptionally preserved birds and feathered dinosaurs have been reported from the Early Cretaceous Jehol Biota, known occurrences of foot scales remain limited.Raman spectroscopy,elemental mappings, and XRPD analysis reveal potential clues to the preservation mode of scales in Gansus. Raman spectra imply that organic materials are preserved within the scales, but have been diagenetically altered. The pattern of elemental distribution indicates that the sulci are covered by calcium phosphate. This accumulated calcium phosphate suggests that the decay of the scales and sulci generated steep chemical gradients in and around the skin,which led to the dissolution of fluorapatite in the sediment and then the reprecipitation in and near the skin(Sagemann et al., 1999). The spatial distribution implies that Ca2+and PO43-permeated into the skin mainly at the sulci.Even when the sulci were seriously decayed, the scales were complete enough to resist infiltration of Ca2+and PO43-from the outside and diffusion of decay products from the inside, which could be important for the formation of steep chemical gradients in an alkaline environment (Briggs and Kear, 1993; Mcnamara et al., 2009).
Sedimentary facies analysis showed the fossilbearing Xiagou Formation in the Changma Basin are dominated by lacustrine deposits (Xue et al., 2013).The primary or early diagenetic dolomites in this formation suggest that the lake in which the deposits were formed was closed and alkaline to saline(Suarez et al., 2017). The identification of analcime in the sediment agrees with this interpretation of the lake geochemistry,because alkaline lake water and detrital clay can react to form analcime (Zhu et al., 2020).
The resistance of the scales to decay can be partly explained by the recalcitrance of keratins(e.g.,Wang et al.,2016).However,instances of keratinous tissues replicated in calcium phosphate exist (e.g., Lindgren et al., 2011; McNamara et al., 2018). It is likely that the taphonomic environment of the Changma Basin also played a role in the formation of fossil carbonaceous compression.Recent work suggested that Al and Fe are important elements for the preservation of soft tissues (e.g., Wilson and Butterfield, 2014; Naimark et al., 2016, 2018; Anderson et al., 2018). The underlying mechanism can be described as taphonomic tanning induced by elements Al and Fe, a concept borrowed from the leather industry, in which the secondary cross-linking of structural biomolecules induced by tanning agents, including multivalent Fe and various aluminosilicate minerals, can protect the structural biomolecules from bacterial degradation(Petrovich, 2001; Wilson and Butterfield, 2014). The elemental distribution of Al and Fe in our studied specimen is consistent with this hypothesis.
5. Conclusions
It seems promising to focus more attention on the evolution and biological significance of foot scales in ancient birds, despite their scarcity in the fossil record. While comparable scales have been described from non-avian dinosaurs, presently available information indicates that the earliest known birds (e.g.,Archaeopteryx) had feathered tarsometatarsi, in contrast to the majority of extant birds. In this context, it is particularly interesting that scaled tarsometatarsi seem to appear in Early Cretaceous ornithuromorphs with aquatic lifestyles, in which feathered feet are less advantageous. Further research in this area could potentially detect potential links of this evolutionary adaptation with large-scale environmental changes. Climatic and tectonic changes led to the formation of extensive lake and wetland habitats in China during the Late Jurassic-Early Cretaceous period. It is tempting to propose that scaled feet are positively selected under the aquatic ecosystems in early ornithuromorphs,giving them completive advantage as well in other habitats,and eventually become the dominant trait in modern birds. However, this hypothesis needs considerably more evidence and is difficult to adequately test at present, given the very low number of fossil bird specimens with remaining traces of feathers,skin,and/or scales.Moreover,scaled feet have also evolved in enantiornithines, which are predominantly arboreal. More research combined with a strengthened focus on detecting specimens with soft tissue preservation is still necessary.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Authors'contributions
All the authors have actively participated in the preparation and revision of the manuscript. TZ contributed to data curation,software operation,and original draft preparation. ZL contributed to data curation and provided materials. HZ contributed to data curation. YP contributed to the design of the study and original draft preparation. All authors read and approved the final proof.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank Ya-Xiao Wang(Key Laboratory of Surficial Geochemistry, Ministry of Education, School of Earth Sciences and Engineering,Nanjing University)for assistance during SEM observations and Raman analysis, Yan Fang (State Key Laboratory of Palaeobiology and Stratigraphy,Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences)during SEM-EDS analysis, Yu-Guan Pan (State Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Nanjing University)during XPRD analysis. We are also grateful to Dr. Corwin Sullivan and other two anonymous reviewers for their efforts reviewing the manuscript.
Funding
杂志排行
Journal of Palaeogeography的其它文章
- Genesis and mechanisms of organic matter enrichment of the Hongshuizhuang Formation in the Zhoukoudian area of Jingxi sag, North China
- A new oospecies of Shixingoolithus(Shixingoolithus qianshanensis oosp. nov.)from the Qianshan Basin, Anhui Province, East China
- Sedimentology and foraminiferal paleoecology of the exposed Oligocene-Miocene Ogwashi-Asaba Formation in Issele-Uku area, Anambra Basin, southern Nigeria: A paleoenvironmental reconstruction
- Application of remote sensing in the description of fluvial system in dryland: A case study of Golmud distributive fluvial system,Qaidam Basin, NW China
- Mid-Cretaceous Hainan back-arc basin: record of the sustained extension of South China margin
- The control of Pleistocene palaeogeography on the distribution of sandy patches in a silty Holocene lagoon (central Netherlands)
