Research Progress on The Effect of Astrocytes on Neurodegenerative Diseases
2022-11-22WANGYiYuan
WANG Yi-Yuan
(Department of Biomedical Engineering,The Hong Kong Polytechnic University,Hong Kong 999077,China)
Abstract Neurodegenerative diseases are common and incurable diseases that cause great inconvenience to patients’lives.Astrocytes play an important role in neurodegenerative diseases.In the nervous system of patients with neurodegenerative diseases,damaged glial cells can have toxic effects on the surrounding neurons,causing neuronal dysfunction and thus death.At the same time,some reactive astrocytes produced by the disease can protect neurons,remove harmful substances around neurons,and temporarily slow down the disease progression.This review will discuss the role of astrocytes in some common neurodegenerative diseases,including amyotrophic lateral sclerosis(ALS),Alzheimer’s disease(AD),and Parkinson’s disease(PD).The role played by astrocytes in these diseases is summarized,aiming to further promote the progress of research in neurodegenerative diseases.
Key words neurodegenerative diseases,astrocytes,amyotrophic lateral sclerosis,Alzheimer’s disease,Parkinson’s disease DOI:10.16476/j.pibb.2022.0484
Astrocytes are a class of cells widely present in the mammalian nervous system,including fibrous astrocytes and protoplasmic astrocytes,and play a key role in maintaining the homeostasis of the central nervous system[1].Astrocytes have many projections and can interact with surrounding cells and thus perform their functions.Different astrocytes play different roles in neural signalling processes[2].In addition,astrocytes are closely related to neurodegenerative diseases.The ageing,death,and abnormal proliferation of astrocytes may lead to the development of related diseases.Meanwhile,the growth process of astrocytes is regulated by the nervous system.It has been found that astrocytes are damaged to varying degrees during the development of neurodegenerative diseases[3].
Neurodegenerative disease is a disorder associated with neurons and the cells surrounding them.The main pathogenic triggers include oxidative stress,mitochondrial dysfunction,excitotoxins,and immune inflammation. Some common neurodegenerative diseases include amyotrophic lateral sclerosis(ALS),Parkinson’s disease(PD),Alzheimer’s disease(AD),stroke,and so on.These diseases pose a serious threat to human health,especially to the health of the population.These diseases are serious health risks,especially for the elderly,but the pathogenesis is not fully understood.ALS is a neurodegenerative disease that affects motor neurons,causing muscle paralysis and death due to respiratory failure[4],with symptoms including weakness of the extremities and trunk muscles,which in turn affects other body functions[1].Sporadic ALS accounts for approximately 90% of patients,whereas hereditary ALS accounts for approximately 10% of patients,and the main characteristics of PD include bradykinesia,tonicity,and postural instability.These features are associated with a large reduction of dopaminergic cells in patients with PD.Current research suggests that astrocytes have both a facilitative and inhibitory effect on PD.AD is the leading cause of dementia,accounting for 80% of patients with dementia[5].The main features include memory and cognitive impairment[6].The occurrence of AD is influenced by both environmental and genetic factors.Only 5% of AD patients are inherited in families.Ageing is a major factor in the development of AD.The incidence of AD increases significantly with age and with cellular ageing and apoptosis[7].
It has been shown that there are glial cell responses in the substantia nigra pars compacta in patients with various neurodegenerative diseases.This article summarizes the research progress of astrocytes in neurodegenerative diseases such as ALS,PD,and AD by reviewing the literature,and then analyzes the relationship between astrocytes and these three diseases,aiming to further promote researchers’understanding of neurodegenerative diseases,as well as clinical innovations in the treatment of related diseases.
1 The effect of astrocytes on amyotrophic lateral sclerosis(ALS)
ALS is a fatal neurodegenerative disease whose representative pathological features include degeneration and reactive gliosis of upper and lower motor neurons from the cerebral cortex to the brainstem and spinal cord[8].In addition,the physiological features of ALS include muscle atrophy,spasticity,speech impairment,and respiratory failure[9].It has been found that most ALS patients will die within five years of onset and that 10% of ALS is dominantly inherited[5].
Previous studies have found that there are approximately 25-30 genes associated with sporadic ALS and hereditary ALS[9].Common genes closely associated with ALS disease include the copper-zinc superoxide dismutase geneSOD1,C9orf72,43-ku transactive response(TAR)-DNA-binding protein gene(TDP-43),the fusion sarcoma geneFUS,andVCP[1].Among them,mutations in theSOD1gene,which encodes Cu2+/Zn2+binding,are the most common,accounting for 20% of patients with hereditary ALS[10].TheSOD1gene has been extensively studied in recent decades and it was the first identified ALS risk gene[11].Also,SOD1has been suggested as a target for the treatment of ALS[12].In addition,1% to 2% of inherited ALS is due to mutations in the chromosome where theVCPgene is located[13].TheVCPgene is involved in a variety of cellular activities and can express ATPases.The geneC9orf72mainly encodes a protein whose function has not been discovered so far[9].TheFUSgene can encode an RNA/DNA binding protein,which regulates gene expression.In addition,environmental factors may also lead to damage to glial cells,which may induce ALS[9].
Previous studies have found that glial cells are closely associated with the onset and progression of ALS[1].Astrocytes can release toxins that cause damage and death of motor neurons.When glial cells in mice carry a mutant phenotype,an ALS phenotype occurs[8].At the same time,astrocytes in ALS mice develop functional abnormalities and release neurotoxins[10].The appearance of abnormal astrocytes can be observed in both the cortex and medulla of patients with sporadic ALS cases and hereditary ALS[14].It was demonstrated thatSOD1mutant astrocytes,when transplanted into the spinal cord of healthy mice,can differentiate to form neurotoxic glial cells and cause motor neuron damage.However,transplantation of normal astrocytes intomSOD1mutant mice alleviates ALS and extends the life span of the mice[10].In addition,in studying the production of neurotoxins by astrocytes,researchers found that antioxidants attenuated the toxicity ofSOD1mutant astrocytes,thereby increasing motor neuron survival[15].It was demonstrated that mice with mutations in theSOD1gene in the late stages of ALS disease have reduced levels of membrane proteins in the spinal cord and lack the protein membrane intracellular glutamate transporter(Glysporter1,GLT-1),which directly leads to increased glutamate concentrations in the environment in which astrocytes are located[10].
It was found that a decrease in Membralin membrane protein in astrocytes also contributes to ALS[16].The membrane protein is involved in the intracellular mechanism of clearing misfolded proteins from the endoplasmic reticulum[17].Anotherin vitrostudy experiment,in which astrocytes lacking membrane proteins were co-cultured with motor neurons,found elevated glutamate concentrations,which may lead to impaired synaptic growth of motor neurons in this system[13].Also,the survival rate of motor neurons co-cultured withSOD1mutant astrocytes overexpressing membrane proteins was increased.Taken together,the Membralin membrane protein is considered a target for the treatment of ALS to reduce motor neuron damage due to astrocytes[16].
In addition,astrocyte connexin 43(Cx43)is associated with the release of neurotoxins from astrocytes.Cx43 promotes cellular connections and information exchange[18].High expression of Cx43 can be detected in astrocytes from bothSOD1mutant mice and ALS patients[10].Co-culture ofSOD1mutant astrocytes with motor neurons was able to suppress the expression of Cx43,thus effectively protecting motor neurons[19].
2 Effects of astrocytes on Parkinson’s disease(PD)
The pathology of PD is characterized by the massive death of dopamine neurons in the compact part of Substantia nigra(SNpc)of patients[20].Patients with PD experience symptoms such as slowed movement,resting tremors,and tonicity[20].Studies have found that muscular ageing is a major trigger for PD and that the incidence of PD increases significantly with age[21].To date,researchers have identified approximately 20 genes associated with PD[22].
The presence of α-synuclein is found in neurons in the substantia nigra,cerebral cortex,and other sites.This protein is a soluble protein located at both ends of the synapse,and its formation is attributed to impaired cellular autophagy[23]and oxidative stress[24],and so on.Impaired α‑synuclein degradation has been found in astrocytes of PD patients[25],which leads to incomplete degradation of α‑synuclein aggregates,resulting in deposit formation and mitochondrial dysfunction[26],which in turn leads to the deterioration of PD.The α‑synucleinogenesis mutations lead to an increase in the number of astrocytes,which affect normal cellular function and lead to the expression of the glutamate transporter protein GLT-1,which in turn causes impaired synaptic growth in motor neurons[1],demonstrating that the accumulation of α‑synuclein in astrocytes has a negative impact on neuronal growth and proliferation.Thus,the clearance of α‑synuclein by astrocytes in the early stages of PD disease plays a protective role for motor neurons[27].However,the formation of deposits due to the accumulation of α‑synuclein and the resulting mitochondrial dysfunction can negatively affect astrocyte function,leading to the release of neurotoxins from astrocytes.
DJ-1is a gene that encodes a multifunctional protein associated with PD.The protein encoded by this gene inhibits oxidative stress[28]and reduces the rate of neuronal apoptosis[29].Mullettet al.[30]culturedDJ-1mutant astrocytes together with dopaminergic neurons and found that the mutant astrocytes were unable to properly protect against neurons subjected to oxidative stress and reduced the tolerance of dopaminergic neurons to oxidative stress.Meanwhile,deletion of theDJ-1gene reduces α‑synuclein degradation in microglia[31],which leads to α-synuclein aggregation.It directly causes damage to neurons and also affects the normal function of astrocytes,which in turn,again causes negative effects.In addition,astrocytes lacking theDJ-1gene or with reduced gene expression have reduced activity of the glutamate transporter protein GLT-1[32],and therefore cellular glutamate uptake is reduced,leading to increased glutamate concentrations in the cellular environment,which can cause impairment of neuronal synaptic growth.
In addition,astrocytes play an important and active role in the brain in neurodegenerative diseases.When brain structures are damaged,some astrocytes are activated,increase in size and release cytokines,which are called “reactive astrocytes”.DJ-1gene expression is increased in reactive astrocytes in the PD brain,while normal expression in neurons also protects neurons.It was found that microglia can release TNF and IL-1 lymphokines,both of which can provide nutrients to neurons.Also,IL-1 can promote astrocyte proliferation,which in turn induces the release of astrocyte nerve growth factor(NGF)[33].In addition,striatal native astrocytes increase neuronal survival and reduce mortality[9].
3 Effects of astrocytes on Alzheimer’s disease(AD)
The pathological features of AD include extracellular β‑amyloid deposition,intracellular neurofibrillary tangles(NFT),neuroinflammation,neuronal degeneration,and abnormal brain function[6].Patients usually present with symptoms of memory[34].AD is a disease influenced by a combination of environmental,genetic,and other factors and can be divided into hereditary AD and sporadic AD.Sporadic AD is also influenced by genetic factors in addition to environmental factors,such as astrocyte and microglia gene mutations[35].
Neuroinflammation occupies an important place in the pathology of AD.Common neuroinflammatory factors are interleukin 1β(IL-1β),interleukin 1α(IL-1α),interleukin 6(IL-6),and tumor necrosis factor(TNF‑α)[36].Neuroinflammation is mainly characterized by the massive proliferation of glial cells around β-amyloid deposits.Inflammatory factors promote the expression of β‑amylopectin deposits,neuronal fibre entanglement,and precipitating β‑amyloid peptides[37],which in turn cause cellular harm and affect the normal growth,proliferation,and differentiation of neurons[38].Researchers have found a rapid increase in the number of pro-inflammatory cells in the nervous system of AD patients and that patients gradually develop symptoms such as neuronal degeneration and memory loss[36].When large amounts of β-amyloids are present around astrocytes,neuronal mortality around astrocytes increases[36].This is since β-amyloid causes activation of caspase3,which in turn promotes apoptosis of neurons.
The β‑amyloid degrading enzymes include neprilysin(NEP),endothelin-converting enzyme(ECE),and insulin-degrading enzyme(IDE)[39].These enzymes can reduce protein deposition and neuroinflammatory dystrophy[40].In early AD patients,the level of NEP is reduced in the brain,leading to a large deposition of β-amyloid protein,which triggers memory impairment.Therefore,patients with early AD may be able to mitigate the disease progression by enhancing the expression of NEP or other β‑amyloid degrading enzymes[41].Studies have shown that astrocytes play an important role in the degradation of β‑amyloid.Previous studies have shown that β‑protein degrading proteases are produced by reactive astrocytes[39].In the presence of microglia,the morphology and function of astrocytes are altered,allowing the conversion of astrocytes into reactive glial cells,which in turn produce β‑protein degrading proteases.
Previous studies have demonstrated dysfunction of the glutamate transporter proteins GLAST and GLT-1 and reduced expression of related mRNAs in the nervous system of AD patients[39].In the nervous system,astrocytes convert glutamate to glutamine through amidation reactions and catalysis by glutamine synthetase,which then releases glutamine to the extracellular compartment.Neurons can take up glutamine and simultaneously synthesize glutamate,forming a cycle[42](Figure 1).The massive accumulation of extracellular glutamate leads to the release of neurotoxins from astrocytes and causes neuronal deformation and necrosis[43].At the same time,dysregulation of glutamate homeostasis in astrocytes leads to abnormalities in glucose metabolism and affects the normal function of astrocytes,thereby disrupting the normal protective effect of astrocytes on neurons[43].

Fig.1 Astrocytic and neuronal glutamate cycles[44]
Currently,most treatments for AD focus on inhibiting the production and deposition of β-amyloid and promoting the expression of β‑amyloid proteins such as NEP[45].In addition,some researchers have also treated it by inhibiting the production of reactive astrocytes that release neurotoxins.This therapeutic approach is not only effective for AD but also has therapeutic implications for other neurodegenerative diseases[46-48].
4 Conclusion
Neurodegenerative diseases involve different regions of the nervous system.During disease development,astrocytes play an important role,which has been verified by many experiments[9].Under genetic and environmental influences,astrocytes evolve into reactive astrocytes that release neurotoxins and inflammatory factors,which in turn negatively affect the physiological activity of neurons.At the same time,damaged astrocytes have dysfunctional glutamate transporters,leading to increased environmental glutamate concentrations,which result in neuronal damage and death.At the same time,different neurodegenerative diseases produce similar protein aggregates around neurons.Astrocytes scavenge protein aggregates and maintain environmental homeostasis for neuronal survival[49].
Astrocytes play an important role in maintaining neurological homeostasis(Table 1).In the nervous system of patients with neurodegenerative diseases,reactive glial cells protect neurons to carry out normal physiological activities and remove harmful substances from the surrounding area,such as neurotoxins.Astrocyte-induced production of inflammatory factors also negatively affects neurons and is a key cause of some diseases.In addition,genetic mutations are also important reasons why astrocytes do not function properly.Also,some genetic mutations and environmental factors can cause astrocytes to differentiate into phenotypes that produce harmful substances.Therefore,researchers can study the genes or proteins of astrocytes inpatients with postmortem neurodegenerative diseases or animal models to explore the disease principles.This has important implications for inherited neurodegenerative diseases.In addition,the specific role played by astrocytes in neurodegenerative diseases,the role of different glial cells,and the effect of the interaction between glial cells and neurons on neurons need to be continued to be investigated.
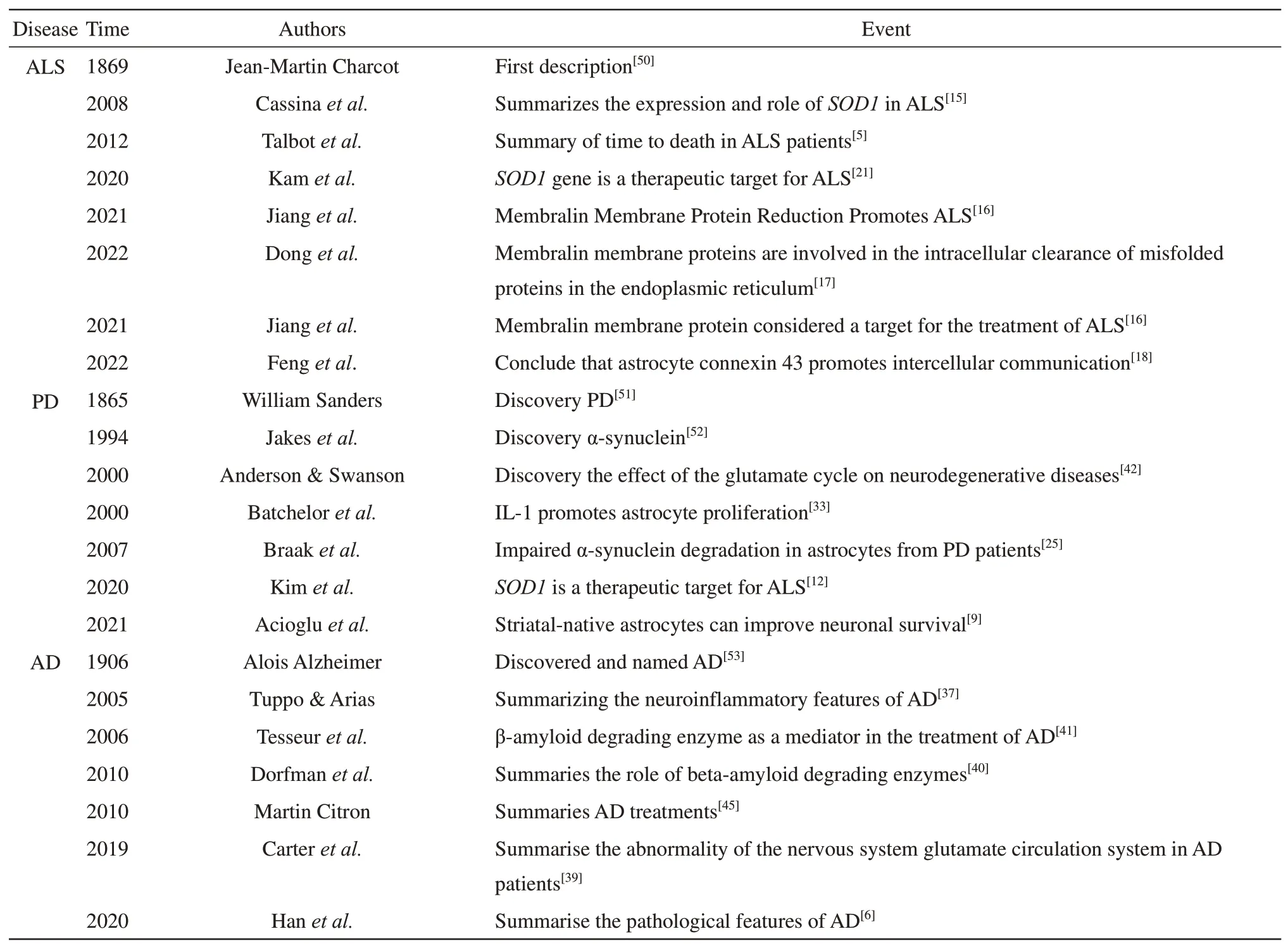
Table 1 Chronological table for the research of neurodegenerative diseases associated with astrocytes
