CoN/g-C3N4 0D/2D复合结构及其光催化制氢性能研究
2022-11-04陈瀚翔周敏莫曌宜坚坚李华明许晖
陈瀚翔, 周敏, 莫曌, 宜坚坚, 李华明, 许晖
CoN/g-C3N40D/2D复合结构及其光催化制氢性能研究
陈瀚翔1, 周敏1, 莫曌2, 宜坚坚3, 李华明2, 许晖2
(1. 江苏大学 环境与安全工程学院, 镇江 212013; 2. 江苏大学 能源研究院, 镇江 212013; 3. 扬州大学 环境科学与工程学院, 扬州 225127)
在光催化产氢反应中引入助催化剂可促进光生电子快速转移, 是提高光催化活性的有效方法。而目前, 高效助催化剂主要仍然是贵金属, 其高昂的价格极大限制了实际应用。本研究探讨了构筑非贵金属助催化剂CoN与g-C3N40D/2D紧密界面对光催化制氢性能的影响。负载非贵金属助催化剂CoN可以有效提高2D g-C3N4的光催化制氢活性, 负载量对其活性也有影响。构筑的0D/2D紧密界面有利于光生电子快速传输。两者的共同作用使得10% CoN/2D g-C3N4复合物光催化制氢效率达到403.6 μmol·g–1·h–1, 是2D g-C3N4单体的20倍。在CoN/2D g-C3N4复合材料中, 负载CoN作为析氢助催化剂可以显著促进电荷转移过程, 从而大幅提高光催化析氢活性。
非贵金属; CoN; 助催化剂; 光催化; 产氢
氢能作为清洁能源已被广泛应用于原油精炼、合成氨和甲醇、燃料电池等领域[1-4]。通过水煤气转化和甲烷热解得到氢气是传统的制氢方法之一, 但生产过程需要消耗大量不可再生能源。相比而言, 利用太阳光将水分解产生氢气, 能源消耗小, 无二次污染等问题, 是众多学者的研究目标。将太阳能直接转化为清洁的氢能已成为水分解制氢的研究热点[5-6]。
氮化碳(C3N4)于1834年被发现, 是最古老的有机共轭聚合物之一。氮化碳具有可见光响应、热稳定性和化学稳定性好等优点[7-10]。然而, g-C3N4存在氧化还原能力低、光激发载流子复合率高等问题, 单体g-C3N4的光催化活性较低, 限制了其广泛应用[11-13]。考虑到光催化产氢主要利用光催化剂所产生的电子, 提高电子的利用效率即可提高光催化产氢的速率。二维薄层结构的g-C3N4具有良好的电子传递路径和高比表面积, 是一类很有应用前景的催化剂。与之对应, 贵金属具有较大的功函数和合适的氢吸附脱附自由能, 是最合适的助催化剂。但贵金属昂贵的价格限制了其工业化应用, 因此, 需要探索合适的替代材料。研究表明, 过渡金属氮化物因为优秀的导电性及电催化性能, 在提升光催化活性助催化剂领域具有巨大的应用潜能。过渡金属钴基材料中若引入氮元素, 可使过渡金属d带电子密度增大并收缩, 使其电子结构类似贵金属且反应能垒更低[14-16], 有利于降低光催化反应的能量。而且引入氮元素会占据过渡金属钴基材料的层间隙, 从而提升耐腐蚀性[17-18]。其次, 材料的结构也会在一定程度上对催化反应产生影响, 优质的0D/2D结构可以在界面上与反应物接触良好和优化活性位点分布[19-22]。实验通过选择研究CoN的助催化性能, 旨在探索一种低成本、高效的可替代贵金属基助催化剂的材料。在光催化反应中, CoN能够有效地提取氮化碳产生的电荷, 提高e–-h+对的分离效率, 最终达到提高光催化性能的目的。
为了构筑具有高效HER反应活性的光催化体系, 达到提高催化剂光催化性能的目的, 本研究利用简单的水热法和氨气煅烧法, 构筑了由超小尺寸CoN纳米点修饰2D g-C3N4纳米片的0D/2D光催化复合材料。并深入分析了CoN提升光催化性能的机制, 通过多种表征测试方法探究了构效关系。
1 实验方法
1.1 材料合成
2D g-C3N4的制备:将2 g三聚氰胺置于坩埚, 在马弗炉中以2 ℃/min升温到550 ℃, 保温4 h, 获得黄色C3N4。研磨后取400 mg C3N4置于方舟, 在马弗炉中, 以10 ℃/min升温到550 ℃, 保温1 h, 获得白色2D g-C3N4。
Co(OH)2/2D g-C3N4的制备:将50 mg 2D g-C3N4、22.8 mg Co(NO3)2∙6H2O和22.1 mg 六亚甲基四胺(Hexamethylenetetramine, HMT)溶解在12 mL去离子水中并搅拌0.5 h, 将混合溶液转移到25 mL不锈钢高压釜中, 在120 ℃烘箱中加热12 h。反应结束后冷却至室温, 离心得到的混合液用蒸馏水和乙醇各洗涤3次, 在55 ℃真空干燥箱下干燥12 h, 得到Co(OH)2/2D g-C3N4。
Co(OH)2的制备:将0.5036 g Co(NO3)2∙6H2O和0.488 g六次甲基四胺(Hexamethylenetetramine, HMT)溶解在12 mL去离子水中并搅拌0.5 h, 而后转移到25 mL不锈钢高压釜并放入烘箱中, 在120 ℃下加热12 h。冷却至室温, 对得到的混合液进行离心处理, 用蒸馏水和乙醇各洗涤3次, 在55 ℃真空干燥箱内干燥12 h, 可得Co(OH)2。
CoN的制备:称取50 mg Co(OH)2置于方舟, 在管式炉中通入100 mL/min的氨气, 以5 ℃/min速率加热到380 ℃, 保持3 h, 获得氮化钴。
CoN/2D g-C3N4的制备:称取50 mg Co(OH)2/ 2D g-C3N4置于方舟, 在管式炉中通入100 mL/min氨气, 以5 ℃/min速率升温到380 ℃, 保持3 h, 获得10% CoN/2D g-C3N4。再按比例调整得到5% CoN/2D g-C3N4和15% CoN/2D g-C3N4复合物。
1.2 光催化制氢性能测试
光催化制氢速率测试:向反应器中加入10 mg光催化剂、10 mL三乙醇胺(Triethanolamine, TEOA)以及90 mL水, 混合超声30 min。将反应器与光催化制氢反应仪相连接, 混合液持续搅拌。在反应开始前对测试系统抽真空, 去除系统内的空气, 并在配备有10 ℃的恒温冷却水循环的条件下进行测试, 避免光致热效应。模拟太阳光的光源为配备400 nm滤光片的300 W氙灯, 光催化实际制氢量通过气相色谱进行在线分析(Ar载气、TCD检测器、0.5 nm分子筛)。
光催化循环实验:光催化循环实验的测试步骤类似于制氢速率测试步骤。在每轮循环反应结束后重新对装置抽真空, 然后开始新一轮的测试。
1.3 光电化学测试
将2 mg催化剂溶解在1 mL乙二醇和1 mL乙醇的混合液中并超声0.5 h。移取20 μL混合液, 悬滴在氧化锡铟(ITO)玻璃(1 cm× 0.5 cm)上,红外灯下烘干, 并在80 ℃烘箱中加热2 h确保接触紧密。光电流循环测试时, 照射和遮光各20 s为一个循环, 阻抗测试时保持遮光。
2 结果与讨论
2.1 结构及形貌分析
通过X射线衍射(X-ray diffraction, XRD)和红外光谱(Fourier transform infrared spectroscopy, FT-IR)表征2D g-C3N4和CoN/2D g-C3N4的晶体结构和化学结构。如图1(a)所示, 2D g-C3N4在2=27.7°处存在单独的衍射峰, 对应于2D g-C3N4的(002)晶面, 这是层间堆叠造成的[23-24]。位于2=36.2°、42.2°、61.3°、73.3°和76.8°处的衍射峰, 分别对应于CoN的(111)、(200)、(220)、(311)和(222)晶面, 这表明CoN制备成功, 且具有立方晶体结构。与此同时, 随着CoN含量增大, 2D g-C3N4的主峰强度逐渐减小, (111)、(200)和(220)晶面的特征峰逐渐增强, 这表明2D g-C3N4的结构未发生改变, 并与CoN成功复合。如图1(b)所示, FT-IR光谱图中, 复合材料的特征峰与2D g-C3N4相似, 与XRD结果一致。800 cm–1处的吸收峰归属于-三嗪单元的振动拉伸[25];1000~1800 cm–1之间的吸收峰归属于CN杂环的振动[26-27];其他在3000~3500 cm–1之间的吸收峰归属于O–H和N–H的振动[28-29]。以上结果表明成功制备了复合物, 并且引入CoN没有破坏2D g-C3N4的结构。由紫外–可见漫反射光谱(UV-visible diffuse reflectance spectroscopy, DRS)(图1(c))可见, CoN可以吸收可见光, 但无法被激发, 说明它的主要作用是充当捕获电子的角色。但引入CoN导致复合物由白色变为灰色, 在一定程度上增强了对太阳光的吸收。测试2D g-C3N4以及10% CoN/2D g-C3N4复合催化剂的比表面积, 如图1(d)所示。复合材料的比表面积降低, 这可能是由于CoN以及煅烧对氮化碳结构产生了一定的破坏。结合上述结果说明, 引入CoN可以促进对太阳光的吸收。

图1 (a)2D g-C3N4, CoN/2D g-C3N4复合催化剂和CoN的XRD图谱, (b)2D g-C3N4和CoN/2D g-C3N4复合催化剂的 FT-IR谱图, (c)2D g-C3N4, CoN/2D g-C3N4复合催化剂和CoN的紫外-可见光漫反射吸收光谱图, (d)2D g-C3N4和10% CoN/2D g-C3N4的氮气吸附–脱附等温线
Colorfulfigures are available on website
为了研究2D g-C3N4和CoN/2D g-C3N4复合催化剂的形貌结构和组成, 对其进行扫描电镜(Scanning electron microscope, SEM)及元素能谱(Energy dispersive spectrometer mapping, EDS mapping)、透射电镜(Transmission electron microscope, TEM)和原子吸收分光光度法(Atomic absorption spectrophotometry, AAS)测试。图2(a, b)中, 2D g-C3N4单体呈现卷曲的褶皱结构, CoN单体则是团聚的纳米点结构。图2(c)中复合物的SEM形貌依旧保持2D g-C3N4所具有的褶皱结构, 但出现了明显的点状颗粒。TEM照片(图2(d))中, 10% CoN/2D g-C3N4复合物依旧保持了2D g-C3N4的二维结构, 并且纳米点锚定在2D g-C3N4的表面。增加放大倍数, 图2(e)中, CoN纳米点均匀分布在超薄2D g-C3N4上, 成功构建了优质的0D/2D界面。复合物的高分辨率透射电镜(High resolution transmission electron microscope, HR-TEM)照片(图2(f))中观察到明显的晶格条纹, 间距为0.25 nm, 对应CoN的(111)晶面, 0D的CoN纳米点的直径为5~6 nm。
为了了解材料的元素组成和金属元素含量, 对复合材料进行扫描电镜的元素能谱测试和原子吸收分光光度测试, 10% CoN/2D g-C3N4中CoN的实际质量分数为8.6%。图S1中, 10% CoN/2D g-C3N4含有C、N和Co元素且分布均匀, 证明成功制备了CoN/2D g-C3N4复合材料。
2.2 可见光光催化水分解测试分析
首先, 为了评估引入CoN是否影响2D g-C3N4的光催化活性, 对2D g-C3N4以及不同比例的CoN/2D g-C3N4复合催化剂在可见光照射下光分解水制氢性能进行测定。如图3(a), 引入CoN纳米点有效促进了2D g-C3N4光催化分解水制氢的活性, 而且不同CoN的负载量对制氢活性存在一定影响。引入CoN后, 复合物的制氢活性显著提升, 其中最优负载量10% CoN/2D g-C3N4的制氢速率达到了403.6 μmol·g–1·h–1, 约为2D g-C3N4制氢速率的20倍。已报道的类似光催化材料的析氢性能如表 S1[45-51]所示, 与同类光催化材料比较, CoN/2D g-C3N4的平均析氢效率较高。然而, 当CoN的负载量超过10%, CoN/2D g-C3N4催化剂的光催化活性发生衰减, 其原因是过多CoN影响了2D g-C3N4对光的吸收, 同时CoN也发生了自团聚现象。其次, 对复合光催化剂10% CoN/2D g-C3N4进行四个周期的循环稳定性测试(图3(b)), 样品前三次产氢量较为稳定, 第四次循环的产氢量略微降低, 约为第一次循环产氢量的85%, 说明复合材料的性能整体较稳定[30-32]。
CoN/2D g-C3N4的单波长产氢表观光合量子效率(Apparent quantum yield, AQY)测试结果显示其在420 nm单波长条件下的AQY为0.13%, 而在435 nm单波长条件下的AQY为0.09%, 由此可见CoN/2D g-C3N4的单波长产氢表观光合量子效率随着材料的光吸收能力减弱而逐渐降低。这说明在CoN/2D g-C3N4催化剂体系中, 只有2D g-C3N4才能受到激发产生光生电子, 而CoN的作用是捕获电子并且完成质子得到电子生成氢气的过程。
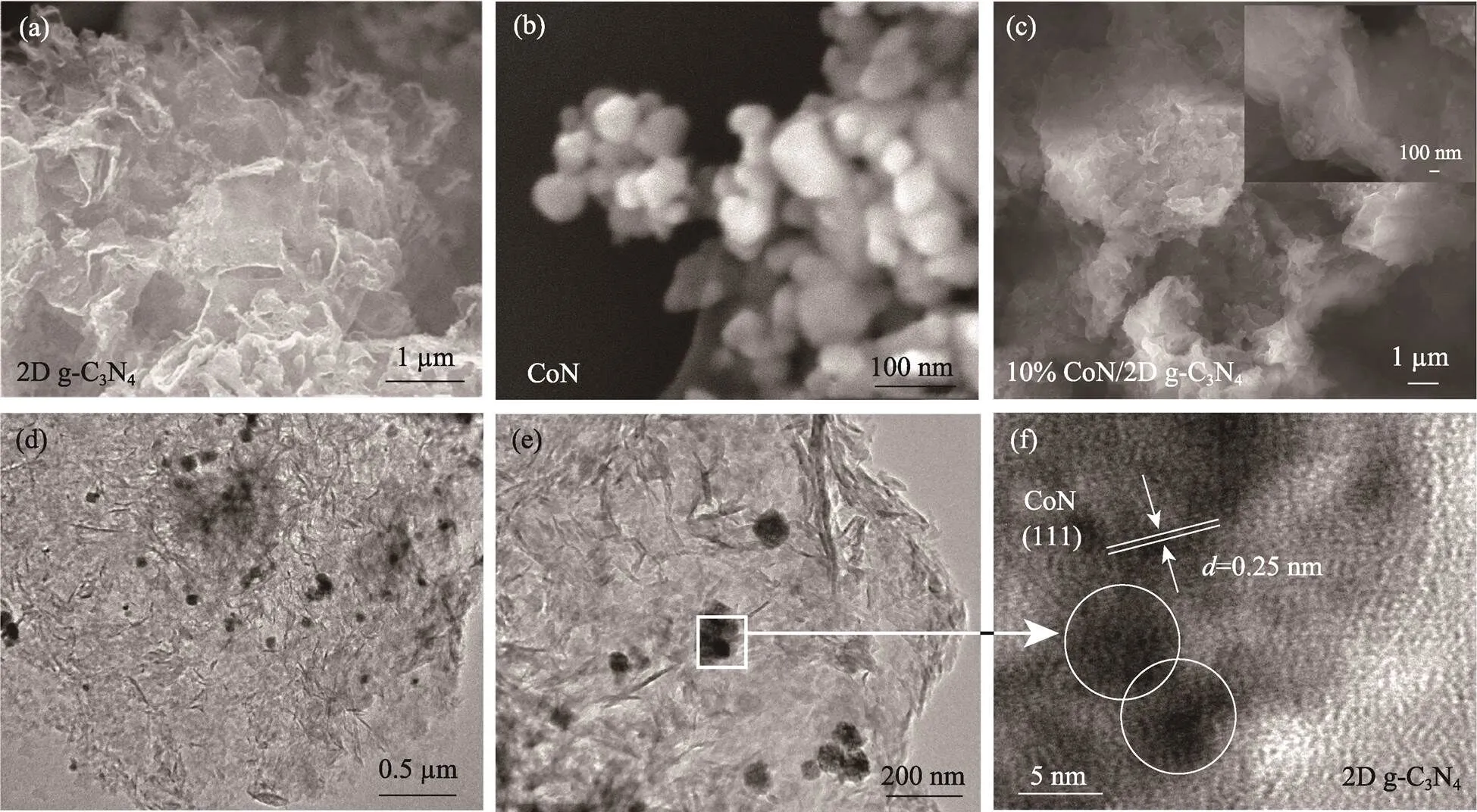
图2 (a)2D g-C3N4, (b)CoN和(c)10% CoN/2D g-C3N4的SEM照片, 10% CoN/2D g-C3N4的 (d, e)TEM照片和(f)高倍TEM照片
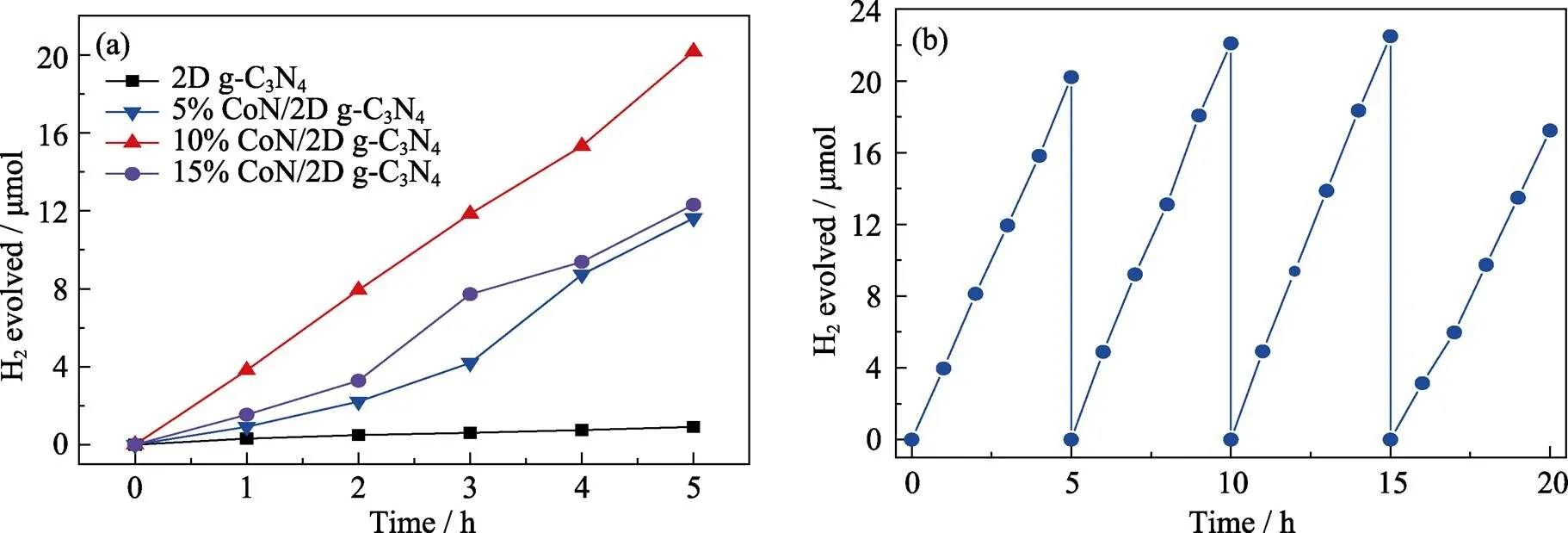
图3 (a)2D g-C3N4和CoN/2D g-C3N4复合催化剂的光催化制氢速率测试, (b)10% CoN/2D g-C3N4复合催化剂的制氢稳定性测试(10% TEOA作为牺牲剂, 10 mg催化剂, 氙灯作为光源, λ>400 nm)
Colorfulfigures are available on website
除了CoN, 氮化铁、氮化镍等也可有效促进基体催化剂的性能[33-34], 表明金属氮化物可有效促进电荷分离, 提升光解水活性。
2.3 复合材料的光学及光电性能分析
为进一步研究材料光生载流子的分离与转移, 进行了稳态荧光光谱(Photoluminescence spectroscopy, PL)、光电流以及电化学阻抗(Electrochemical impedance spectroscopy, EIS)等测试。如图4(a)所示, 相比于2D g-C3N4, 10% CoN/2D g-C3N4复合催化剂的荧光强度发生显著淬灭, 光生载流子复合减少[35-36]。这些结果表明, 负载CoN可以有效抑制2D g-C3N4的电荷复合。与此对应的是图4(b)中光电流测试结果, 相较于2D g-C3N4, 10% CoN/2D g-C3N4的光电流有一定提升, 说明更多电子被激发到氧化铟锡(Indium tin oxide, ITO)玻璃表面。图4(c)中阻抗结果也显示, 引入CoN可以有效促进电荷传输。电化学测试结果表明, 在CoN/2D g-C3N4复合材料中, 负载CoN作为析氢助催化剂可以显著促进电荷转移过程, 从而大大提高光催化析氢活性。为了研究材料负载CoN前后的导带(Conduction band, CB)变化, 进行莫特肖特基(Mott Schottky, MS)测试, 从(图4(d)) Mott Schottky曲线可知, 2D g-C3N4的平带电位为–0.84 V (. Ag/AgCl, pH6.8), 对应于–0.1 V (NHE, pH0)。而CoN/2D g-C3N4复合材料的平带电位为–0.82 V (. Ag/AgCl, pH6.8), 这说明CoN与2D g-C3N4复合后, 复合材料的CB没有明显改变[42]。
2.4 光催化机理研究
为了进一步探索光催化反应机理, 进行电子自旋共振(Electron spin resonance, ESR)光谱测试[37-39]。测试时在溶液中加入自由基捕获剂5, 5-二甲基-1-吡咯啉--氧化物(5,5-dimethyl-1-pyrroline--oxide, DMPO)抑制或减缓光氧化过程, 可以稳定超氧自由基(•O2−)和羟基自由基(•OH), 分别在甲醇溶液和水溶液中测试, 得到DMPO-•O2−和DMPO-•OH的ESR信号。如图5所示, 2D g-C3N4和10% CoN/2D g-C3N4在黑暗条件下均未出现自由基信号。在光照条件下, 2D g-C3N4仅出现自由基•O2−的信号, 而没有自由基•OH信号。10% CoN/2D g-C3N4的谱图中两种自由基的信号均存在, 且•O2−的信号强于纯2D g-C3N4。•O2−的信号变化说明引入CoN能够加速导出光生电子, 促进光生电子和空穴分离。基于上述结果, 本研究提出该体系的光催化制氢机制: 在可见光照射下, 2D g-C3N4受光激发产生光生电子, 电子由价带跃迁至导带, 由牺牲剂三乙醇胺(Triethanolamine, TEOA)消耗了价带上留下的空穴, 而CoN可以捕获并有效转移电子。同时, CoN起到了电子陷阱的作用, 电子很难回到2D g-C3N4中,不能与空穴重新结合。并且由于CoN具有优良的导电性, 电子可以快速转移到催化剂表面并被H+捕获, 完成光催化HER中H2的生成过程[2,13,30-31,44]。

图4 2D g-C3N4和10% CoN/2D g-C3N4的(a)稳态荧光光谱图(激发波长为384 nm), (b)光电流响应图, (c)电化学阻抗谱图和(d)2D g-C3N4和10% CoN/2D g-C3N4的莫特肖特基曲线
Colorfulfigures are available on website
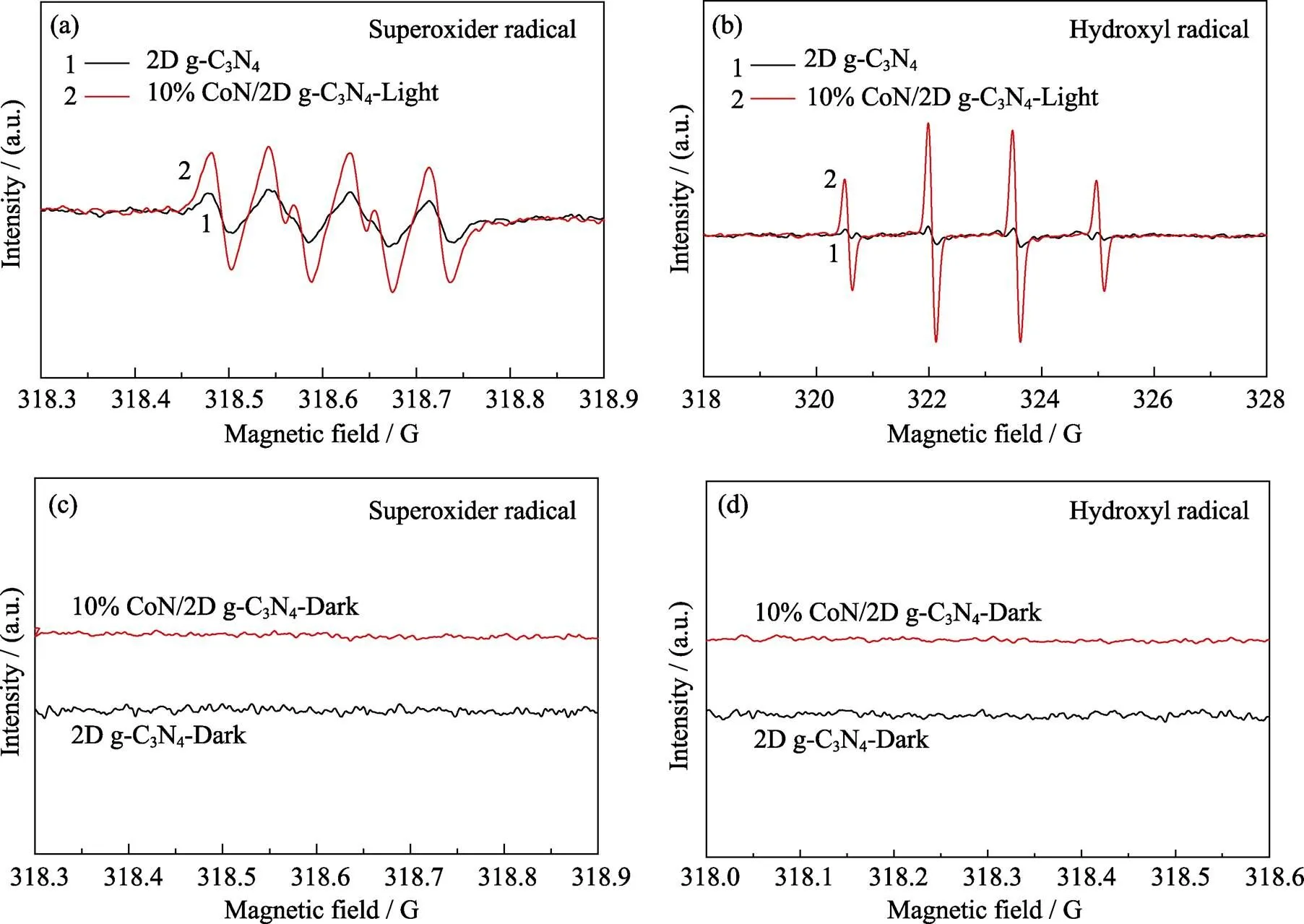
图5 2D g-C3N4和10% CoN/2D g-C3N4在(a, b)可见光照射和(c, d)暗处的(a, c)超氧自由基和(b, d)羟基自由基ESR谱图
Colorfulfigures are available on website
3 结论
本研究以CoN为助催化剂, 设计了由CoN纳米点修饰2D g-C3N4纳米片的0D/2D高效光催化剂, 构筑了具有高效HER反应活性的光催化体系。其中最优负载量10% CoN/2D g-C3N4的制氢速率达到403.6 μmol·g–1·h–1, 约为2D g-C3N4的20倍。活性提升的原因可以归为以下2点: (1)该体系的原位合成策略保证了界面紧密接触; (2)负载小尺寸助催化剂CoN保证了活性单元覆盖最大化, 而没有影响光吸收, 良好的导电性有利于抽取光生电荷, 提高光生载流子的分离效率。
本文相关补充材料可登陆 https://doi.org/ 10.15541/jim20210806 查看。
[1] YAN Z, JI M, XIA J,Recent advanced materials for electrochemical and photoelectrochemical synthesis of ammonia from dinitrogen: one step closer to a sustainable energy future.,2019, 10(11): 1902020.
[2] CHISALITA D A, PETRESCU L, CORMOS C C. Environmental evaluation of european ammonia production considering various hydrogen supply chains.,2020, 130: 109964.
[3] APOSTOLOU D, XYDIS G. A literature review on hydrogen refuelling stations and infrastructure. current status and future prospects.,2019, 113: 109292.
[4] STAFFELL I, SCAMMAN D, ABAD A V,The role of hydrogen and fuel cells in the global energy system.,2019, 12(2): 463–491.
[5] WANG Y C, LIU X Y, WANG X X,Metal-organic frameworks based photocatalysts: architecture strategies for efficient solar energy conversion.,2021, 419: 129459.
[6] CHEN Y W, LI L L, XU Q L,Controllable synthesis of g-C3N4inverse opal photocatalysts for superior hydrogen evolution.,2021, 37(6): 2009080.
[7] NIU P, LI L. Overall photocatalytic water splitting of crystalline carbon nitride with facet engineering.,2020, 6(10): 2439–2441.
[8] VOROBYEVA E, GERKEN V C, MITCHELL S,Activation of copper species on carbon nitride for enhanced activity in the arylation of amines.,2020, 10(19): 11069–11080.
[9] GUO H, NIU C G, LIANG C,Highly crystalline porous carbon nitride with electron accumulation capacity: promoting exciton dissociation and charge carrier generation for photocatalytic molecular oxygen activation.,2021, 409: 128030.
[10] MO Z, DI J, YAN P,An all-organic D-A system for visible- light-driven overall water splitting.,2020, 16(48): 2003914.
[11] FU J J, MO Z, CHENG M,An all-organic TPA-3CN/ 2D-C3N4heterostructure for high efficiency photocatalytic hydrogen evolution.,2020, 589: 124397.
[12] YI J J, EL-ALAMI W, SONG Y H,Emerging surface strategies on graphitic carbon nitride for solar driven water splitting.,2020, 382: 122812.
[13] YANG W, WANG Y. Enhanced electron and mass transfer flow- through cell with C3N4-MoS2supported on three-dimensional graphene photoanode for the removal of antibiotic and antibacterial potencies in ampicillin wastewater.,2021, 282: 119574.
[14] GAO J F, ZHANG F D, XUE H Q,synthesis of novel ternary CdS/PdAg/g-C3N4hybrid photocatalyst with significantly enhanced hydrogen production activity and catalytic mechanism exploration.,2021, 281: 12.
[15] LI Y, ZHANG M, ZHOU L,Recent advances in surface- modified g-C3N4-based photocatalysts for H2production and CO2reduction.,2020, 37(6): 2009030.
[16] LIAO Y W, YANG J, WANG G H,Hierarchical porous NiO as a noble-metal-free cocatalyst for enhanced photocatalytic H2production of nitrogen-deficient g-C3N4.,2021, 41(2): 396–405.
[17] KIM D, YONG K. Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production.,2021, 282: 119538.
[18] LIU C, FENG Y, HAN Z T,Z-scheme N-doped K4Nb6O17/ g-C3N4heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production.,2021, 42(1): 164–174.
[19] XUE Z L, KANG J Y, GUO D,Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting.,2018, 273: 229–238.
[20] ZHU D D, ZHOU Q X. Nitrogen doped g-C3N4with the extremely narrow band gap for excellent photocatalytic activities under visible light.,2021, 281: 119474.
[21] LIU W W, PAN J, PENG R F. Shape-dependent hydrogen generation performance of PtPd bimetallic co-catalyst coupled with C3N4photocatalyst.,2021, 40(12): 3554–3560.
[22] SHEN R C, HE K L, ZHANG A P,construction of metallic Ni3C@Ni core-shell cocatalysts over g-C3N4nanosheets for shell–thickness–dependent photocatalytic H2production.,2021, 291: 120104.
[23] MIAO H, ZHANG G W, HU X Y,A novel strategy to prepare 2D g-C3N4nanosheets and their photoelectrochemical properties.,2017, 690: 669–676.
[24] XU H T, XIAO R, HUANG J R,construction of protonated g-C3N4/Ti3C2MXene Schottky heterojunctions for efficient photocatalytic hydrogen production.,2021, 42(1): 107–114.
[25] KUMAR S, GAWANDE M B, KOPP J,P- and F-co-doped carbon nitride nanocatalysts for photocatalytic CO2reduction and thermocatalytic furanics synthesis from sugars.,2020, 13(19): 5231–5238.
[26] LAN H C, LI L L, AN X Q,Microstructure of carbon nitride affecting synergetic photocatalytic activity: hydrogen bonds. structural defects.,2017, 204: 49–57.
[27] ZHAO C X, TANG H, LIU W,Constructing 0D FeP nanodots/2D g-C3N4nanosheets heterojunction for highly improved photocatalytic hydrogen evolution.,2019, 11(24): 6310–6315.
[28] QIU P, XU C, CHEN H,One step synthesis of oxygen doped porous graphitic carbon nitride with remarkable improvement of photo-oxidation activity: role of oxygen on visible light photocatalytic activity.,2017,206: 319–327.
[29] ZHAO W, LI Y, ZHAO P,Insights into the photocatalysis mechanism of the novel 2D/3D Z-Scheme g-C3N4/SnS2heterojunction photocatalysts with excellent photocatalytic performances.,2021, 402: 123711.
[30] YI J J, SHE X J, SONG Y H,Solvothermal synthesis of metallic 1T-WS2: a supporting co-catalyst on carbon nitride nanosheets toward photocatalytic hydrogen evolution.,2018, 335: 282–289.
[31] YANG Q, CHEN C C, ZHANG Q Y,Molecular engineering of supramolecular precursor to modulate g-C3N4for boosting photocatalytic hydrogen evolution.,2020, 164: 337–348.
[32] HOU T T, XIAO Y, CUI P X,Operando oxygen vacancies for enhanced activity and stability toward nitrogen photofixation.,2019, 9(43): 1902319.
[33] SUN Z J, CHEN H L, ZHANG L,Enhanced photocatalytic H2production on cadmium sulfide photocatalysts using nickel nitride as a novel cocatalyst.,2016, 4(34): 13289–13295.
[34] QI W L, LIU S Q, LI F,Prussian blue derived Fe2N for efficiently improving the photocatalytic hydrogen evolution activity of g-C3N4nanosheets.,2019, 9(10): 2571–2577.
[35] TIAN N, ZHANG Y H, LI X W,Precursor-reforming protocol to 3D mesoporous g-C3N4established by ultrathin self-doped nanosheets for superior hydrogen evolution.,2017, 38: 72–81.
[36] LI Y H, GU M L, ZHANG X M,2D g-C3N4for advancement of photo-generated carrier dynamics: status and challenges.,2020, 41: 270–303.
[37] XIA P, CHENG B, JIANG J,Localized π-conjugated structure and EPR investigation of g-C3N4photocatalyst.,2019, 487: 335–342.
[38] WANG Y, CHEN L, CHEN C,Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation.,2019, 254: 283–291.
[39] LI K, WANG L, CHEN Z,Photocatalytic hydrogen evolution under ambient conditions on polymeric carbon nitride/ donor-π-acceptor organic molecule heterostructures.,2020, 30(43): 2005106.
0D/2D CoN/g-C3N4Composites: Structure and Photocatalytic Performance for Hydrogen Production
CHEN Hanxiang1, ZHOU Min1, MO Zhao2, YI Jianjian3, LI Huaming2, XU Hui2
(1. School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China; 2. School of the Institute for Energy Research, Jiangsu University, Zhenjiang 212013, China; 3. College of Environmental Science and Engineering, Yangzhou University, Yangzhou 225127, China)
In the photocatalytic hydrogen production reaction, the introduction of cocatalyst to promote the rapid transfer of photogenerated electrons is an effective way to improve the photocatalytic activity. At present, the most efficient cocatalysts are mainly precious metals, greatly restricting the use of this method. In this study, influence of the close interface between non-noble metal cocatalyst CoN and g-C3N40D/2D on the performance of photocatalytic hydrogen production was explored. The results showed that the support of non-noble metal cocatalyst CoN can effectively improve the photocatalytic hydrogen production of 2D g-C3N4, and the support amount also has an positive effect on its activity. The compact 0D/2D interface is favorable for the rapid transmission of photogenerated electrons. Photocatalytic efficiency of 10% CoN/2D g-C3N4composite reached 403.6 μmol·g–1·h–1, 20 times of that of 2D g-C3N4monomer, which was comparable to that of noble metal cocatalyst. As a hydrogen evolution cocatalyst, loaded CoN can significantly promote the charge transfer process, thus greatly improving the activity of photocatalytic hydrogen evolution.
non-noble metal; CoN; cocatalyst; photocatalysis; hydrogen production
1000-324X(2022)09-1001-08
10.15541/jim20210806
TQ174
A
2021-12-30;
2022-04-27;
2022-05-05
国家自然科学基金(22108110); 江苏省杰出青年科学家基金(BK20190045); 江苏省研究生科研创新计划(KYCX21_ 3391); 中国博士后基金(2021M691305); 江苏省博士后基金(2021K079A)
National Natural Science Foundation of China (22108110); Jiangsu Funds for Distinguished Young Scientists (BK20190045); Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_3391); China Postdoctoral Science Foundation (2021M691305); Jiangsu Province Postdoctoral Science Foundation (2021K079A)
陈瀚翔(1993–), 男, 博士研究生. E-mail: 2350505633@qq.com
CHEN Hanxiang (1993–), male, PhD candidate. E-mail: 2350505633@qq.com
许晖, 教授. E-mail: xh@ujs.edu.cn
XU Hui, professor. E-mail: xh@ujs.edu.cn
